Abstract
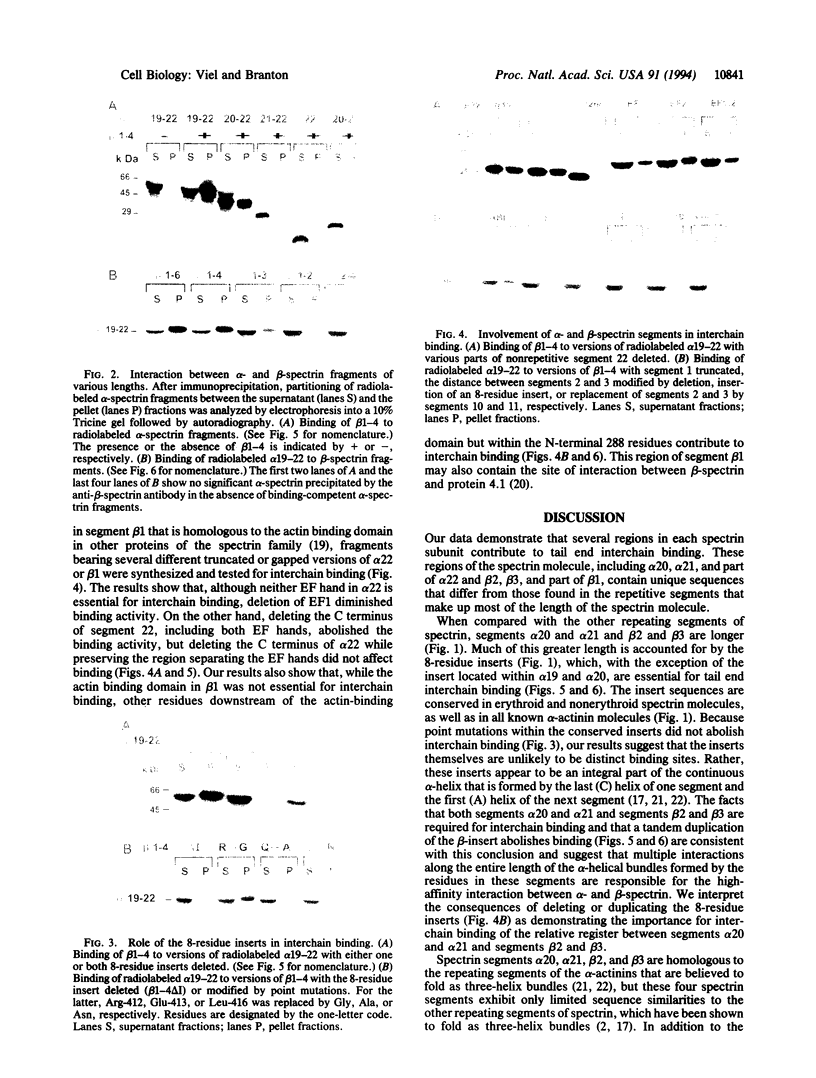
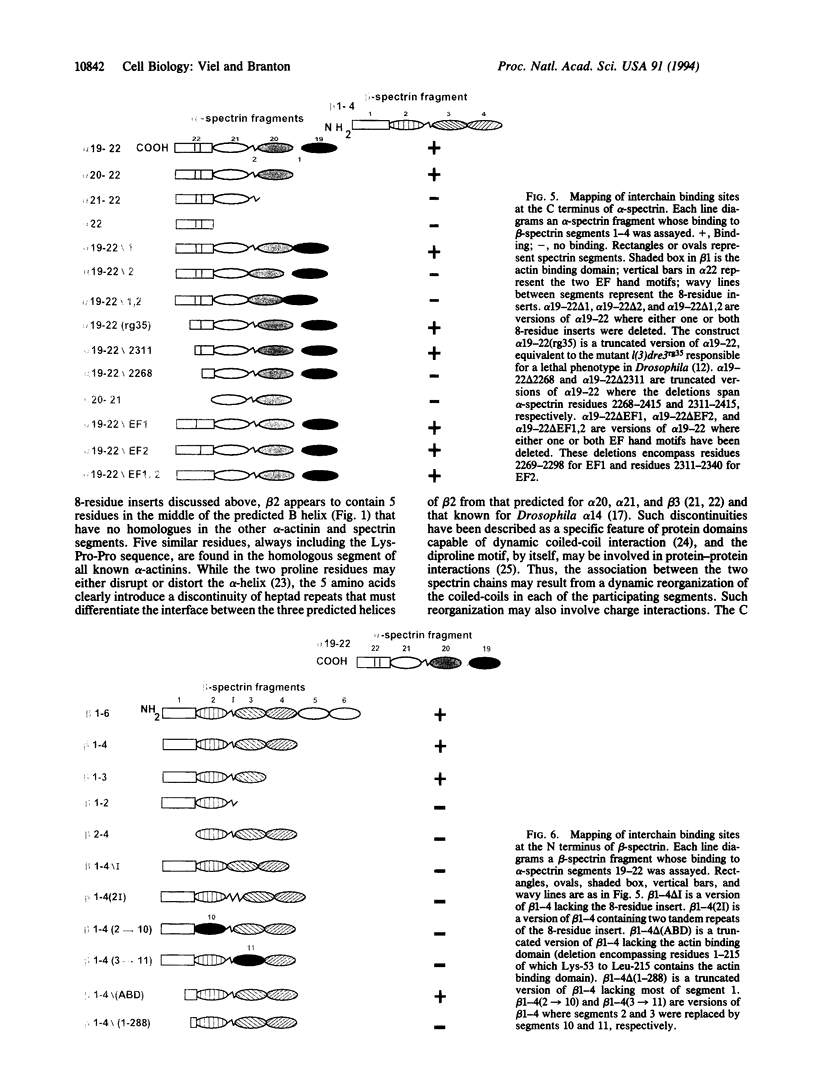
Spectrin's function as an actin-crosslinking protein and membrane skeleton component involves the tail end of the molecule, where multiple interactions between two spectrin chains and between these chains and other proteins give rise to complexes that form membrane skeleton network junctions. To determine whether the sequences that contribute to interchain binding can be distinguished from sequences that are involved in other spectrin tail end functions, we mapped the regions in each Drosophila spectrin chain that are required for interchain binding in vitro. Segments 20 and 21 of the alpha chain and 2 and 3 of the beta chain are required for binding. Binding appears to be very dependent on the lateral register of segments in the two apposed chains. Domains of the nonrepetitive segments, 22 of alpha chain and 1 of beta chain, are also involved in associating the two chains. Required sequences within these nonrepetitive segments are interspersed within domains that are known to be involved in associations with other structural proteins, such as actin, and regulatory components, such as protein 4.1 and calcium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzhubei A. A., Sternberg M. J. Left-handed polyproline II helices commonly occur in globular proteins. J Mol Biol. 1993 Jan 20;229(2):472–493. doi: 10.1006/jmbi.1993.1047. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Tse W. T., Lux S. E., Forget B. G. Beta spectrin kissimmee: a spectrin variant associated with autosomal dominant hereditary spherocytosis and defective binding to protein 4.1. J Clin Invest. 1993 Aug;92(2):612–616. doi: 10.1172/JCI116628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Brandin E., Lue R. A., Winograd E., Branton D. The complete sequence of Drosophila beta-spectrin reveals supra-motifs comprising eight 106-residue segments. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6187–6191. doi: 10.1073/pnas.89.13.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Tyler J. M., Branton D. Spectrin-actin associations studied by electron microscopy of shadowed preparations. Cell. 1980 Oct;21(3):875–883. doi: 10.1016/0092-8674(80)90451-1. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Fishkind D. J., Mooseker M. S., Morrow J. S. Functional diversity among spectrin isoforms. Cell Motil Cytoskeleton. 1989;12(4):225–247. doi: 10.1002/cm.970120405. [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Sillman A. L., Bar-Zvi D., Goldstein L. S., Branton D. The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989 Nov;109(5):2197–2205. doi: 10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R. Structure and evolution of the actin crosslinking proteins. Bioessays. 1991 May;13(5):219–226. doi: 10.1002/bies.950130504. [DOI] [PubMed] [Google Scholar]
- Fishkind D. J., Bonder E. M., Begg D. A. Isolation and characterization of sea urchin egg spectrin: calcium modulation of the spectrin-actin interaction. Cell Motil Cytoskeleton. 1987;7(4):304–314. doi: 10.1002/cm.970070403. [DOI] [PubMed] [Google Scholar]
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karinch A. M., Zimmer W. E., Goodman S. R. The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11833–11840. [PubMed] [Google Scholar]
- Lee J. K., Coyne R. S., Dubreuil R. R., Goldstein L. S., Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993 Dec;123(6 Pt 2):1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Oas T. G., Endow S. A. Springs and hinges: dynamic coiled coils and discontinuities. Trends Biochem Sci. 1994 Feb;19(2):51–54. doi: 10.1016/0968-0004(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Dixon T. W., Cohen C. Analysis of the three-alpha-helix motif in the spectrin superfamily of proteins. Biophys J. 1992 Apr;61(4):858–867. doi: 10.1016/S0006-3495(92)81893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Weglarz L., DeSilva T. M. Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. J Biol Chem. 1992 Jul 25;267(21):14775–14782. [PubMed] [Google Scholar]
- Williamson M. P. The structure and function of proline-rich regions in proteins. Biochem J. 1994 Jan 15;297(Pt 2):249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Forget B. G. Erythroid and nonerythroid spectrins. Blood. 1993 Jun 15;81(12):3173–3185. [PubMed] [Google Scholar]
- Winograd E., Hume D., Branton D. Phasing the conformational unit of spectrin. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10788–10791. doi: 10.1073/pnas.88.23.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Hofmann A., Köppel B., Schleicher M., Noegel A. A. The Ca(2+)-binding domains in non-muscle type alpha-actinin: biochemical and genetic analysis. J Cell Biol. 1993 May;121(3):599–606. doi: 10.1083/jcb.121.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Winograd E., Viel A., Cronin T., Harrison S. C., Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993 Dec 24;262(5142):2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]
- Yoshino H., Minari O. Characterization of the lateral interaction between human erythrocyte spectrin subunits. J Biochem. 1991 Oct;110(4):553–558. doi: 10.1093/oxfordjournals.jbchem.a123618. [DOI] [PubMed] [Google Scholar]