Abstract
Background:
The expression of podoplanin is up-regulated in a number of different human cancers, including squamous cell carcinoma of the oral cavity and its relationship with tumor invasion raises the possibility that podoplanin expression could be used as a biomarker for diagnosis and prognosis.
Aim:
The aim of the present study is to evaluate the expression of podoplanin in different grades of squamous cell carcinoma (SCC) and to correlate the expression of podoplanin with relevant clinical features such as age, sex, site and associated habits.
Materials and Methods:
Retrospective study was carried on formalin fixed, paraffin embedded blocks of oral SCC (OSCC) from the archives of Department of Oral and Maxillofacial Pathology, Vishnu Dental College, Bhimavaram. Thirty diagnosed cases were included, of which 10 were well-differentiated SCC (WDSCC) (n = 10), 10 moderately DSCC and 10 poorly DSCC. Demographics including age, sex, gender and associated habit history, were recorded. Immunohistochemical staining was done with podoplanin anti D2–40 antibody, for all the cases of OSCC and assessed qualitatively. The data obtained were tabulated and subjected to statistical analysis.
Results:
In the present study, 27 cases of SCC showed podoplanin expression and remaining three cases showed no expression. The scoring criterion suggested by Yuan et al. was followed for semi-quantitative assessment. OSCC, seven cases presented weak expression (Immunoreactive score [IRS] 0–3), 15 presented moderate expression (IRS Score 4–7) and 5 presented high expression (IRS Score > 8). The assessment of podoplanin expression in the cytoplasm, the membrane and the cytoplasm and membrane (both) of tumor cells showed overall high positivity in the cytoplasmic followed by both and the membrane.
Conclusion:
Podoplanin could be a potent biomarker in assessing the cytoplasm/membrane staining of tumor cells. Furthermore, a high level of podoplanin expression is suggestive of high frequency of lymph node metastasis and immature status in the differentiation process of OSCC.
Keywords: Epithelial mesenchymal transition, Immunohistochemistry, Podoplanin, Squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a devastating disease involving dysregulation of numerous pathways linked to cellular differentiation, cell cycle control, epithelial mesenchyme interaction, apoptosis, angiogenesis, and metastasis.[1] Abnormal proliferation of cell results from the accumulation of multiple genetic alterations influenced by genetic predisposition as well as by environmental influences, including tobacco, alcohol, chronic inflammation and viral infections. HNSCC is the sixth most common cancer accounting over 500,000 new cases annually worldwide that includes sites in the oral cavity, pharynx and larynx. Oral SCC (OSCC) is the most common malignant tumor of the HN regions and accounts for the two-thirds of the HNSCC cases occurring in developing countries.[2,3]
The potentially malignant disorders such as leukoplakia, erythroplakia, palatal changes associated with reverse smoking, oral submucous fibrosis, lichen planus, syphilitic glossitis, and sideropenic dysphagia have a high risk of malignant transformation, on average about 1% of oral lesions transform into cancer annually. Knowledge in genomic and basic research have made ease in understanding the molecular process governing tumor formation and progression.[1] The important factor which shows a direct impact on prognosis and therapeutic strategy in various types of cancer is that of lymph node status. Electron microscopic studies have revealed that the lymphatic spread of cancer cells will show the poor prognosis. Understanding the lymphatic system at the molecular level showed some loophole 15 years back, due to lack of lymphatic epithelium markers. The introduction of the markers that is, LYVE I, Prox1 and podoplanin has explored the lymphatic vessels present in the tumor and peritumoral areas, and the prognosis of various malignancies such as malignant melanoma, SCC of the HN, breast cancer and gastric adenocarcinoma.[4]
Podoplanin is a 38-KDa type-I transmembrane glycoprotein consisting of 162 amino acids, nine of which form the intracellular domain. The extracellular domain is highly O glycosylated with sialic acid, α-2, 3 linked to galactose, forming the main part of the protein carbohydrate moieties. In normal human tissue podoplanin is expressed in kidney podocytes, alveolar type-1 cells, lymphatic endothelium, skeletal muscle, placenta, lung and heart, in the myofibroblasts of the breast and salivary glands, in the osteoblasts and mesothelial cells and in the basal layer of human epidermis. The expression of podoplanin is up-regulated in a number of different human cancers, including SCC of the oral cavity. In addition, recent experimental results have demonstrated that podoplanin mediates a pathway leading to collective cell migration and invasion in vivo and in vitro. The expression of podoplanin in human cancers and its relationship with tumor invasion raises the possibility that podoplanin expression could be used as a biomarker for diagnosis and prognosis.[1]
Podoplanin is specifically expressed in lymphatic endothelial cells but not in blood endothelial cells. OSCC s spread through the lymphatic route and podoplanin can be utilized for recognizing the lymphatic vessels in the tumor and peritumoral areas. The expression of podoplanin by the tumor cells can also provide a clue towards the tumor migration and progression. Hence, the present study aims to analyze and correlate the podoplanin expression in different grades of OSCC.
Materials and Methods
This study is an retrospective study, retrieved from the archives and the tissue blocks of squamous cell carcinoma. Also random selection is done based on the clinical and histopathological data required was carried on formalin fixed, paraffin embedded blocks of OSCC from the archives of Department of Oral and Maxillofacial Pathology, Vishnu Dental College, Bhimavaram. 30 diagnosed cases were included, of which 10 were well differentiated SCC (WDSCC) (n = 10), 10 moderately DSCC (MDSCC) and 10 were of poorly DSCC (PDSCC).
Demographics including age, sex, gender and associated habit history, were recorded for all the 30 cases of OSCC. Diagnosis for the selected cases was also established on hematoxylin and eosin stained sections. Immunohistochemical staining was done with podoplanin anti D2–40 antibody, for all the cases of OSCC.
Interpretation and counting
Presence of brown colored end product at the site of target antigen was considered as positive immunoreactivity. Cytoplasmic and/or membrane staining was considered as positive immunoreactivity. Lymphatics were considered as internal control. Ten representative areas were selected in each slide. Hundred cells were counted in 10 different fields so as to assess the percentage of podoplanin expression in the stained slides. Also, the brown stained cells of each representative area were differentiated based on the podoplanin expression in the cytoplasm, membrane or both (cytoplasm and membrane). Quantity scores of 0–5 were given if 0%, 1% to 10%, 11% to 30%, 31% to 50%, 51% to 80%, and 81% to 100% of the tumor cells showing positivity. The staining intensity was rated on a scale of 0–3, with 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The raw data were then converted to a German immunoreactive score (IRS) by multiplying the quantity and staining intensity scores. The final scores were put on the scale ranging from 0 to 15 as 0 to 3 = Weak, 4 to 7 = Moderate, >8 = Strong.
Results
Of 30 cases of OSCC, 19 were male, and 11 were female with an age range of 20–80 years with a mean age of 50 years. The predominant cases were observed in fourth to sixth decade of life. OSCC recorded were observed to affect all the sites of the oral mucosa with buccal mucosa being the predominant site followed by alveolar mucosa, tongue, palatal mucosa. The least affected site includes retromolar trigone, floor of the mouth and vestibule.
The related history of the tobacco habit in smokeless or smoking form was recorded. Another habit including alcohol was also assessed. Present study showed 19 cases with a history of smoking, nine cases with alcohol habit, seven with pan chewing and remaining eight did not reveal any habit history [Figure 1]. Podoplanin was highly positive for the lymphatic vessels within the tumor tissue and were considered as internal control. The expression of podoplanin in the tumor cells were observed in the cytoplasm, the membrane and the cytoplasm/membrane (both) and these varied expressions by the tumor cells in different grades of carcinoma were suggestive of prognostic importance [Figures 2, 3 and 4].
Figure 1.
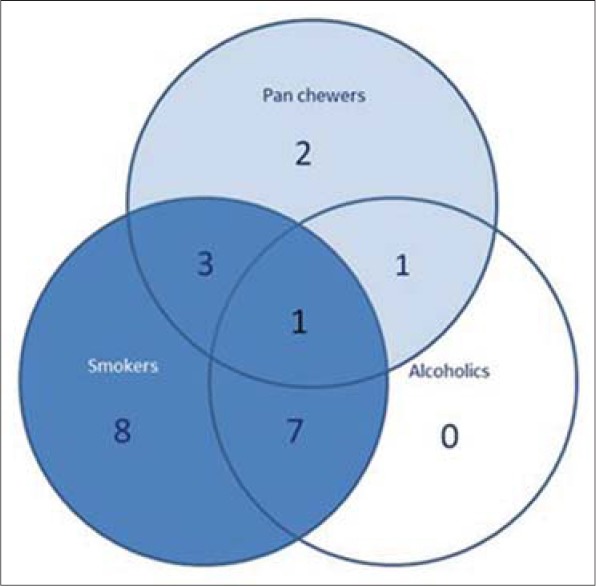
Pie chart showing number of cases and their association with habit
Figure 2.
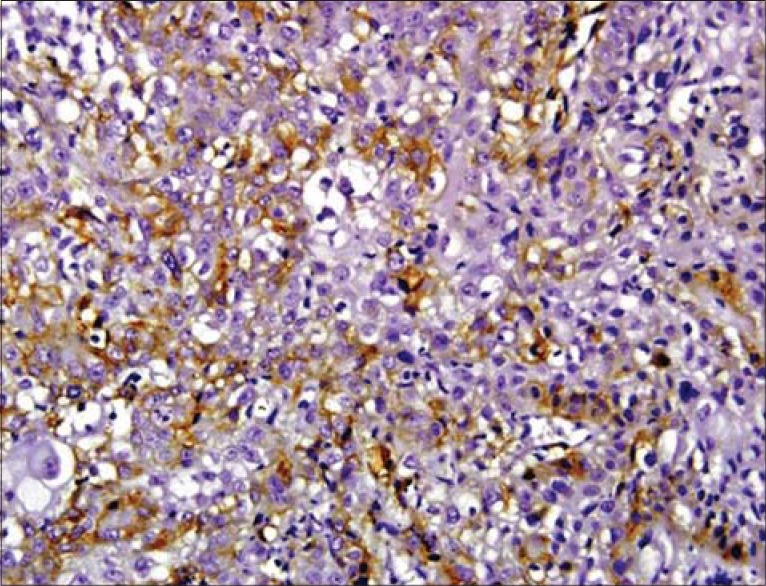
Podoplanin expression in the cytoplasm of tumor cells
Figure 3.
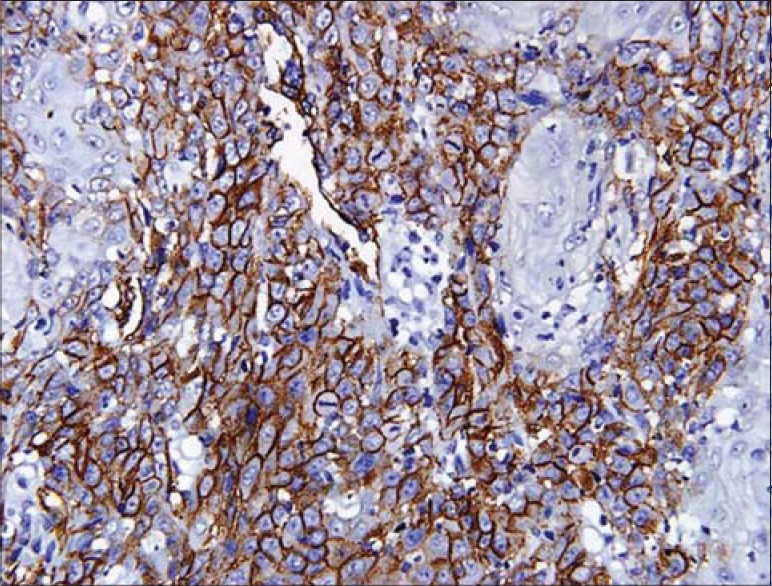
Podoplanin expression in the membrane of tumor cells
Figure 4.
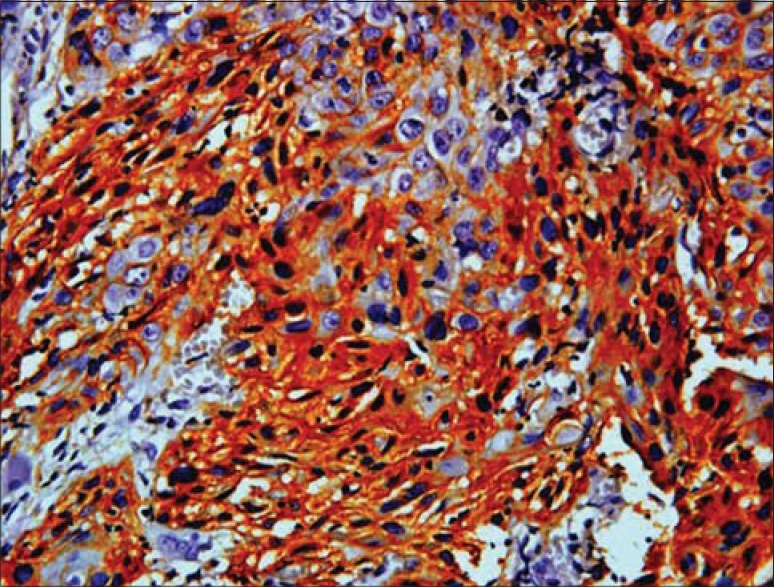
Podoplanin expression in both (cytoplasm/membrane) of tumor cells
Immunostaining of podoplanin was assessed in all the grades of SCC. WDSCC showed positivity in nine cases, MDSCC in all the 10 cases and PDSCC in eight cases. Three cases, one in WDSCC and two in PDSCC showed negative staining. Podoplanin expression in the cytoplasm was highest in PDSCC (71%) followed by WDSCC (56.5%) and then by MDSCC (53.86%). Expression in membrane was also more in PDSCC (6.7%) then in WDSCC (5.1%) and least in MDSCC (1.31%). The variability in the expression of the podaplanin in the cytoplasm and membrane (both) was observed, which revealed highest in MDSCC (44.79%), followed by WDSCC (38.4%) and least in PDSCC (22.2%).
The expressions of podoplanin by tumor cells were observed to be focal and diffuse [Figures 5 and 6]. The focal expression was observed in the tumor nests of WDSCC whereas diffuse expression was observed in MDSCC and PDSCC. IRS scoring of MDSCC showed three cases as strong, four as moderate and three as weak whereas PDSCC showed one as strong, five as moderate and two as weak. WDSCC revealed one as strong, six as moderate and two as weak IRS score [Graph 1].
Figure 5.
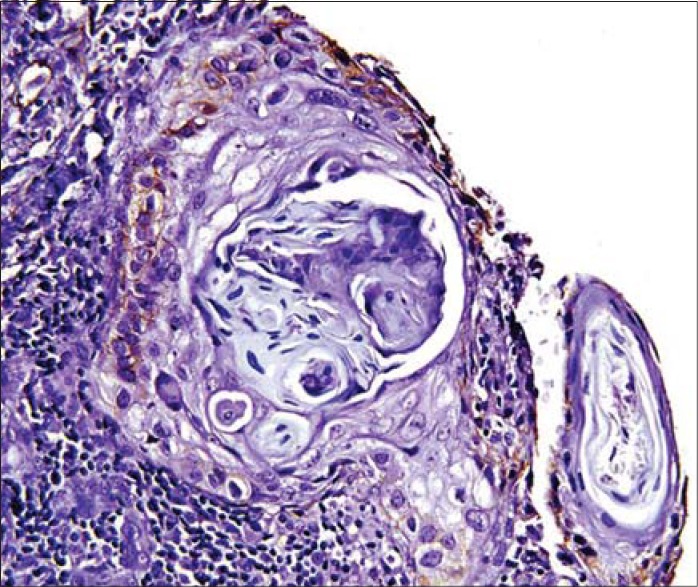
Podoplanin positivity in the periphery of the tumor cells
Figure 6.
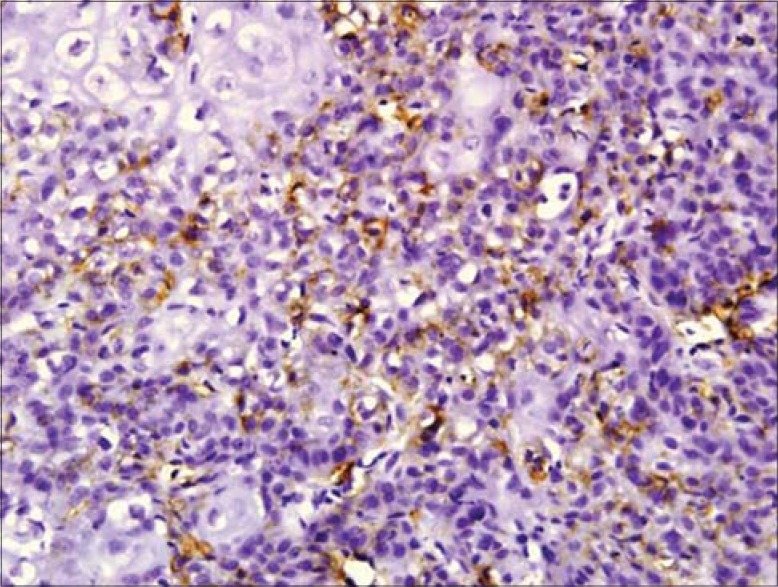
Diffuse podoplanin positivity in MDSCC and PDSCC
Graph 1.
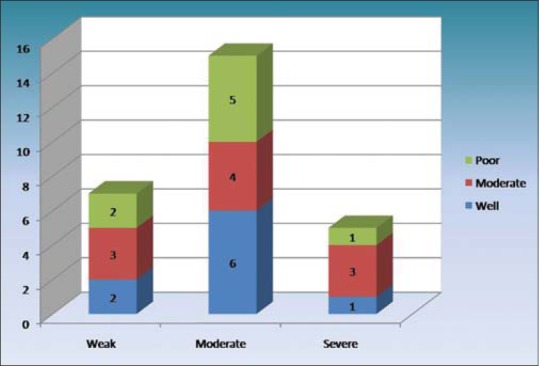
IRS scoring in well, moderate and severe squamous cell carcinomas
The correlation of habit with the IRS scoring of OSCC revealed major number of cases (15) with moderate scoring out of which 13 cases were associated with habit. Seven cases were with a weak score all associated with habit. Five cases showed strong scores out of which three were associated with habit. Pearson's correlation of IRS with sex and habit were not statistically significant.
Discussion
Oral cancer development is a multistep process with accretion of genetic, epigenetic and metabolic alterations resulting from exposure to carcinogens. Metastatic spread to regional lymphnodes through the lymphatic system is considered one of the major pathways by which SCC disseminates.[1,5,6]
The incidence of OSCC is approximately 2–10/100,000 population/year that could be due to the environmental differences or life style or habits such as betel quid, snuff dipping or reverse smoking. Tobacco in various forms is regarded as the main cause of oral cancer. Apart from tobacco the other associated factors include alcohol, ultraviolet light, Vitamin A and C deficiency, immuno-compromised patients, viral infections and to the least extent trauma. OSCC shows a male predominance with 2:1 ratio except for the carcinoma arising on the vermillion border of the lower lip and with the wide age range of fourth to seventh decades.[7] The present study observed occurrence of OSCC predominantly in male with a peak incidence in fifth to seventh decades and buccal mucosa as the most common site followed by alveolar mucosa, tongue and palatal mucosa. 22 cases of OSCC were associated with a history of smoking, alcohol and pan chewing. Eight cases presented without adverse habits such as tobacco and alcohol, which could be attributed to the combined effect of genetic, epigenetic and environmental factors.
Podoplanin is a mucin like transmembrane glycoprotein that is expressed in lymphatic endothelium and various normal human tissues.[8] The podoplanin expression was found in tumor cells of various types of cancer such as vascular tumors, malignant mesothelioma, central nervous system tumors and SCC. The presence of this protein in tumor cells is helpful for pathological diagnosis and is reported to be expressed in aggressive tumors with higher invasive and metastatic potential.[9,10] The present study assessed the expression of podoplanin in different grades of OSCC as this heterogeneous malignancy is characterized by the increased proliferation of the tumor cells which initiates cellular migration and invasion.
Podoplanin expression shows two patterns (a) diffused expression in the tumor cells and (b) focal expression in proliferating periphery with no expression in the central areas of the tumor cell nests.[9] The hierarchical distribution pattern of podoplanin was observed within the tumor nests of WDSCC. The podoplanin positive tumor cells appear to be more localized to the periphery of the tumor nests whereas the central area appeared negative.[11,12] The expression at the periphery of the tumor cells suggests its higher proliferative and self-renewal capacity, whereas the central cells are suggestive of terminal differentiation of tumor cells resulting from maturation and/or degenerative changes. These findings were in correlation with various authors including Martín Villar et al.[11] (2005), Shimada et al.[12] (2009), Carvalho et al.[13] (2010), Rodrigo et al.[1] (2010), Margaritescu et al.[14] (2010) and Chuang et al.[10] (2013). The podoplanin expression pattern was different in MDSCC and PDSCC when compared to WDSCC. Most of the cases of MDSCC showed a diffuse pattern except for one which shows focal expression whereas all the cases of PDSCC showed diffuse expression. The diffuse expression of podoplanin in MDSCC and PDSCC suggests some cytoskeletal alterations within the tumor cells thereby suggesting increased cellular migration and a role in carcinogenesis.[5] This finding is in contrast to Rodrigo et al. who showed highly significant podoplanin expression in well differentiated tumors when compared to poorly differentiated tumors and Franchi et al. who reported no staining of tumor cells in their study from HNSCC.[1,15]
Of 30 cases of SCC, three cases lacked podoplanin expression, and 27 showed expression that was scored by the criteria suggested by Yuan et al.[2] The overexpression of podoplanin was observed in MDSCC with three cases showing high IRS score. High levels of podoplanin may be suggestive of high frequency for lymph node metastasis than those which expressed a lower level of podoplanin. The difference in the scoring of podoplanin expression was not statistically significant probably because of small sample size and technique sensitivity.
An attempt has been made to assess the habit relationship and podoplanin expression in OSCC. The finding of which suggested that the habit alone may not cause cellular changes instead it may act as an accelerating factor in the tumor progression. Apart from the habit other factors like genetic, epigenetic, transcriptional and post-transcriptional mechanisms may co-exist so as to promote tumorigenesis.[2,16]
Schacht et al. in his study showed predominant cytoplasmic podoplanin positivity in PDSCC, enhanced membrane podoplanin expression in MDSCC and no expression in WDSCC. Our study is in slight correlation with Schacht et al. in the cytoplasmic podoplanin expression which was high in PDSCC followed by WDSCC and then MDSCC.[17] The diverse podoplanin expression in the cytoplasm, the membrane and the cytoplasm and membrane (both) of tumor cells of SCC cases may show enhanced propensity for tumor progression. The present study also showed both the cytoplasmic and membrane positivity being higher in all the grades of SCC when compared to the only membrane positivity and this high expression could be attributed to the expression of podoplanin at the protein and mRNA levels.[18] Overall podoplanin expression was high in MDSCC when compared to PDSCC and WDSCC thereby reflecting the immature status in the differentiation process of SCC.
The limitations drawn from the present study is that the expression of podoplanin was studied exclusively on the tumor cells with a limited number of OSCC cases. The localization of podoplanin expression also raised the question whether it was truly induced by the tumor cells or by factors secreted from stromal cells, as the tumor cells present close to surrounding stromal cells and the tumor microenvironment interactions might collectively plays a decisive role in tumor progression.
In the present study, the podoplanin expression was studied mainly in the tumor cell nests without involving the invasing front and observed that the podoplanin expression was highly expressed by the epithelial tumor cells along with the stroma surrounding the tumor cells. This finding is in correlation with Astarita et al.[19] and Yamanashi et al.[20] On the other hand we also stress that podoplanin expression alone cannot be sufficient for tumor progression because many lesions exhibited the protein expression only in the basal cell layers.[11,21] Hence, to assess the prognostic role of podoplanin in different grades of SCC needs further investigation with large sample size and considering the staining pattern so as to assess its accurate role in the morbidity and mortality.
Acknowledgment
We would like to acknowledge the management, principal and vice principal for allowing us to carry out this project in this esteemed institution. We would also like to acknowledge Mr. Satyanarayana, Laboratory Technician for his assistance in sectioning and segregating the slides for the research study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rodrigo JP, García-Carracedo D, González MV, Mancebo G, Fresno MF, García-Pedrero J. Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol Cancer. 2010;9:48. doi: 10.1186/1476-4598-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–9. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]
- 3.Lingen MW, Pinto A, Mendes RA, Franchini R, Czerninski R, Tilakaratne WM, et al. Genetics/epigenetics of oral premalignancy: Current status and future research. Oral Dis. 2011;17(Suppl 1):7–22. doi: 10.1111/j.1601-0825.2011.01789.x. [DOI] [PubMed] [Google Scholar]
- 4.Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997–3006. [PubMed] [Google Scholar]
- 5.Longatto Filho A, Oliveira TG, Pinheiro C, de Carvalho MB, Curioni OA, Mercante AM, et al. How useful is the assessment of lymphatic vascular density in oral carcinoma prognosis? World J Surg Oncol. 2007;5:140. doi: 10.1186/1477-7819-5-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreppel M, Drebber U, Wedemeyer I, Eich HT, Backhaus T, Zöller JE, et al. Podoplanin expression predicts prognosis in patients with oral squamous cell carcinoma treated with neoadjuvant radiochemotherapy. Oral Oncol. 2011;47:873–8. doi: 10.1016/j.oraloncology.2011.06.508. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran R. 7th ed. Elsevier publications; 2012. Benign and malignant tumors of the oral cavity. Shaffer's Text Book of Oral Pathology; pp. 101–25. [Google Scholar]
- 8.Wang Y, Sun J, Gu Y, Zhao S, Groome LJ, Alexander JS. D2-40/podoplanin expression in the human placenta. Placenta. 2011;32:27–32. doi: 10.1016/j.placenta.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartuli FN, Luciani F, Caddeo F, Compagni S, Piva P, Ottria L, et al. Podoplanin in the development and progression of oral cavity cancer: A preliminary study. Oral Implantol (Rome) 2012;5:33–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH, Tseng CK, et al. Tumor cell expression of podoplanin correlates with nodal metastasis in esophageal squamous cell carcinoma. Histol Histopathol. 2009;24:1021–7. doi: 10.14670/HH-24.1021. [DOI] [PubMed] [Google Scholar]
- 11.Martín-Villar E, Scholl FG, Gamallo C, Yurrita MM, Muñoz-Guerra M, Cruces J, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 12.Shimada Y, Ishii G, Nagai K, Atsumi N, Fujii S, Yamada A, et al. Expression of podoplanin, CD44, and p63 in squamous cell carcinoma of the lung. Cancer Sci. 2009;100:2054–9. doi: 10.1111/j.1349-7006.2009.01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho FM, Zaganelli FL, Almeida BG, Goes JC, Baracat EC, Carvalho JP. Prognostic value of podoplanin expression in intratumoral stroma and neoplastic cells of uterine cervical carcinomas. Clinics (Sao Paulo) 2010;65:1279–83. doi: 10.1590/S1807-59322010001200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margaritescu C, Raica M, Pirici D, Simionescu C, Mogoanta L, Stinga AC, et al. Podoplanin expression in tumor-free resection margins of oral squamous cell carcinomas: An immunohistochemical and fractal analysis study. Histol Histopathol. 2010;25:701–11. doi: 10.14670/HH-25.701. [DOI] [PubMed] [Google Scholar]
- 15.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: A morphometric study with clinical correlations. Cancer. 2004;101:973–8. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 16.González-Alva P, Tanaka A, Oku Y, Miyazaki Y, Okamoto E, Fujinami M, et al. Enhanced expression of podoplanin in ameloblastomas. J Oral Pathol Med. 2010;39:103–9. doi: 10.1111/j.1600-0714.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 17.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–21. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Ogose A, Kawashima H, Hotta T, Ariizumi T, Li G, et al. High-level expression of podoplanin in benign and malignant soft tissue tumors: Immunohistochemical and quantitative real-time RT-PCR analysis. Oncol Rep. 2011;25:599–607. doi: 10.3892/or.2011.1141. [DOI] [PubMed] [Google Scholar]
- 19.Astarita JL, Acton SE, Turley SJ. Front Immunol. Podoplanin; 2012. Emerging functions in development, the immune system, and cancer; p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, et al. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77:53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 21.Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo T, Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]


