Abstract
OBJECTIVE
To evaluate the impact of exercise training (ET) on metabolic parameters among participants with type 2 diabetes mellitus (T2DM) who do not improve their cardiorespiratory fitness (CRF) with training.
RESEARCH DESIGN AND METHODS
We studied participants with T2DM participating in the Health Benefits of Aerobic and Resistance Training in Individuals With Type 2 Diabetes (HART-D) trial who were randomized to a control group or one of three supervised ET groups for 9 months. Fitness response to ET was defined as a change in measured peak absolute oxygen uptake (ΔVO2peak, in liters per minute) from baseline to follow-up. ET participants were classified based on ΔVO2peak into fitness responders (ΔVO2peak ≥5%) and nonresponders (ΔVO2peak <5%), and changes in metabolic profiles were compared across control, fitness responder, and fitness nonresponder groups.
RESULTS
A total of 202 participants (mean age 57.1 ± 7.9 years, 63% women) were included. Among the exercise groups (n = 161), there was substantial heterogeneity in ΔVO2peak; 57% had some improvement in CRF (ΔVO2peak >0), with only 36.6% having a ≥5% increase in VO2peak. Both fitness responders and nonresponders (respectively) had significant improvements in hemoglobin A1c and measures of adiposity (ΔHbA1c: −0.26% [95% CI −0.5 to −0.01] and −0.26% [−0.45 to −0.08]; Δwaist circumference: −2.6 cm [−3.7 to −1.5] and −1.8 cm [−2.6 to −1.0]; Δbody fat: −1.07% [−1.5 to −0.62] and −0.75% [−1.09 to −0.41]). No significant differences were observed in the degree of change of these metabolic parameters between fitness responders and nonresponders. Control group participants had no significant changes in any of these metabolic parameters.
CONCLUSIONS
ET is associated with significant improvements in metabolic parameters irrespective of improvement in cardiorespiratory fitness.
Introduction
Higher levels of cardiorespiratory fitness are associated with improved long-term cardiovascular outcomes among patients with type 2 diabetes mellitus (T2DM) (1). Exercise training in patients with T2DM has been shown to improve cardiorespiratory fitness, glycemic control, and other metabolic parameters (2,3). However, the interrelationship between training-related changes in cardiorespiratory fitness and improvement in glycemic control and other metabolic parameters is not well understood. Previous studies (4,5) have observed significant heterogeneity in the magnitude of change in measures of cardiorespiratory fitness in response to exercise training. Recent analyses from the Dose Response to Exercise Training in Women (DREW) trial (6) showed that 30% of the study participants had no improvement in cardiorespiratory fitness after 6 months of supervised, moderate-intensity exercise training. It is unclear whether the metabolic benefits of exercise training are limited to those patients who improve their cardiorespiratory fitness. In this study, we examined the impact of exercise training on hemoglobin A1c (HbA1c) levels and other metabolic parameters among fitness nonresponder and responder participants in the Health Benefits of Aerobic and Resistance Training in Individuals With Type 2 Diabetes (HART-D) study. We hypothesized that exercise training would be associated with significant improvements in glycemic control in fitness responders as well as nonresponders.
Research Design and Methods
Study Design and Participants
The current study was performed as a secondary analysis of the HART-D trial. The full design and methodology of the HART-D study has been published previously (2). Briefly, the HART-D study was a 9-month randomized, controlled exercise-training trial comparing the effects of different modalities of exercise training on HbA1c levels in sedentary participants with T2DM. Exclusion criteria for the study included a BMI >48 kg/m2, age <30 or >75 years, blood pressure ≥160/100 mmHg, fasting triglyceride levels ≥500 mg/dL, use of insulin pump, urine protein levels >100 mg/dL, history of stroke, and advanced neuropathy or retinopathy or any serious medical condition that prevented adherence to the study protocol or the ability to exercise safely. Written informed consent was obtained from all participants prior to screening. The study protocol was reviewed and approved annually by the Pennington Biomedical Research Center institutional review board. The University of Texas Southwestern Medical Center institutional review board also approved the present substudy.
The HART-D study recruited 262 participants who were randomized to one of four groups: 1) a nonexercise control group, 2) aerobic training only, 3) resistance training only, and 4) a combination of aerobic and resistance training. The nonexercise control group was offered weekly stretching and relaxation classes, and was asked to maintain their baseline activity levels during the 9-month study period. All training sessions were performed under staff supervision in an exercise-training laboratory.
Exercise Intervention
Aerobic Training
Participants exercised 3–5 days/week at an intensity of 50–80% of their maximum cardiorespiratory fitness for a total dose of 12 kcal/kg/week. The caloric dose was adjusted on a weekly basis based on the changes in body weight. American College of Sports Medicine equations were used to estimate caloric expenditure rates and, therefore, the time required per session (7).
Resistance Training
Participants exercised 3 days/week, with each session consisting of two sets of four upper-body exercises (chest press, lateral pull-down, military press, and seated row), three sets of three lower-body exercises (leg press, leg extension, and hamstring curl), and two sets of abdominal and back exercises. Each set consisted of 10–12 repetitions. The prescribed weight was increased when the participant was able to complete 12 repetitions of a final set of each exercise on two consecutive sessions.
Combination Training
Participants had aerobic exercise training at a dose of 10 kcal/kg/body wt/week and two sessions of resistance training per week, with each session consisting of one set of each of the aforementioned nine exercises. The resistance training sessions used a similar progressive resistance program, as described above. The training regimen for the combination training group was consistent with federal physical activity guidelines and ensured equal time commitment among all exercise groups.
Exercise Testing
Among the HART-D study participants, cardiorespiratory fitness was measured as peak absolute oxygen uptake (VO2peak, in liters per minute) at baseline and at study completion using a treadmill test (TMX425; Trackmaster Treadmills, Newton, KS) protocol, as previously described (2). Participants self-selected a walking pace at a level grade, and the grade increased by 2% every 2 min at a constant treadmill speed until they reached volitional exhaustion. Breath-by-breath respiratory gases were measured using a TrueOne 2400 Metabolic Measurement Cart (Parvo Medics, Salt Lake City, UT). The same treadmill speed was used at the baseline and the end-of-study testing.
Outcome Assessment
Baseline and post-training blood testing was performed after at least a 10-h fast. HbA1c level was analyzed with a UniCel DxC 600 Pro (Beckman Coulter, Brea, CA). HbA1c level was also assessed at the monthly visits with the diabetes educator using a finger-stick sample analyzed by an automated glycosylated hemoglobin analyzer (DCA 2000; Bayer, Dublin, Ireland). Both HbA1c assays are certified under the National Glycohemoglobin Standardization Program. Weight was measured on a GSE 450 electronic scale, and height was measured with a standard stadiometer. Waist circumference (WC) was measured to the nearest 0.1 cm just above the iliac crest while the subject was at minimal expiration. Body composition was measured by dual-energy X-ray absorptiometry with a QDR 4500/A whole-body scanner (Hologic, Bedford, MA). Diabetes medication type and dosage were assessed by detailed questionnaire with visual confirmation of prescription bottles. Participants were categorized as having increased use of, decreased use of, or no change in use of diabetes medications based on baseline and follow-up medication dosages.
Statistical Analysis
For the present analysis, we have included all control participants and all exercise-training participants who had fitness test data available at baseline and follow-up, and had >70% adherence to exercise training. Cardiorespiratory fitness response was assessed as the change in measured VO2peak (ΔVO2peak, in liters per minute) from baseline to follow-up. For the present analysis, a ≥5% increase in VO2peak from baseline to follow-up was defined as a clinically meaningful cardiorespiratory fitness response (8,9), and the exercise-training participants were categorized as fitness nonresponders (<5% increase in VO2peak) or fitness responders (≥5% increase in VO2peak). Baseline clinical, demographic, and anthropometric characteristics were compared among the study groups (control, fitness responders, and fitness nonresponders) using the Wilcoxon rank-sum trend test for all continuous variables and the χ2 trend test for categorical variables.
The primary outcome for the present analyses was change in HbA1c levels from baseline to completion of training. Changes in HbA1c levels, anthropometric measures, and exercise test parameters were assessed among the study groups using linear mixed-effect models for repeated measures over time. The models were adjusted for baseline values, age, sex, race/ethnicity, and duration of diabetes. For HbA1c measurements, monthly data were available, whereas exercise test parameters and anthropometric measures were available only at baseline and trial completion. For participants with missing HbA1c data at the 9-month follow-up, the change in HbA1c level was calculated based on the difference in finger-stick–measured HbA1c levels at baseline and at the last recorded monthly visit. Results are presented as least squares–adjusted means with 95% CIs. Sensitivity analyses were performed among exercise-training participants after excluding resistance training–only participants to compare the changes in metabolic parameters with exercise training between fitness responder and nonresponder groups.
The association between continuous changes in fitness and change in HbA1c levels from baseline to post-training was further characterized using multivariable adjusted linear regression analysis adjusted for age, sex, race/ethnicity, baseline HbA1c level, diabetes duration, baseline fitness, baseline BMI, change in fitness, and change in the percentage of body fat.
Concomitant reductions in hypoglycemic medication use and reductions in HbA1c levels were assessed as an additional secondary outcome using a composite dichotomous outcome variable. Individuals who decreased diabetes medication or reduced HbA1c level by ≥0.5% without increasing their use of medications were defined as successfully achieving the HbA1c-diabetes medication composite outcome. Multivariable adjusted logistic regression analysis models were constructed to determine the likelihood of achieving the composite secondary outcome in the study participants with exercise training after adjustment for age, sex, race/ethnicity, baseline HbA1c level, diabetes duration, baseline fitness, baseline BMI, change in fitness, and change in the percentage of body fat. All statistical analyses were performed using SAS for Windows (release 9.2; SAS Institute, Inc., Cary, NC).
Results
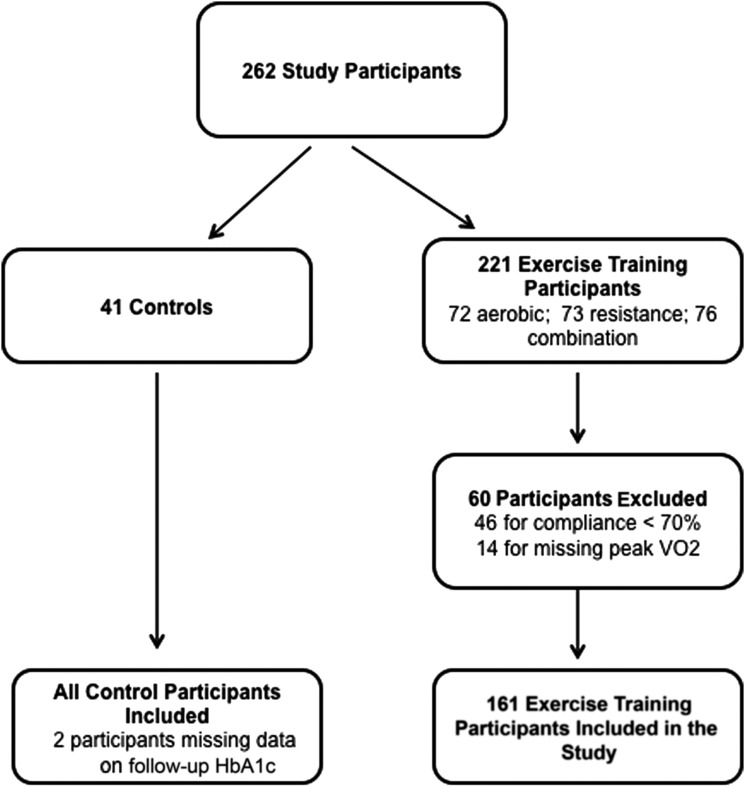
A total of 202 participants (mean age 57.1 ± 7.9 years, women 62.9%) were included (Fig. 1). All exercise-training participants had baseline and 9-month follow-up HbA1c levels available. Follow-up HbA1c levels were missing in two control participants. We observed a significant heterogeneity in ΔVO2peak in response to comparable doses of exercise training, with 43% of exercise-training participants experiencing no improvement in fitness after exercise training. Furthermore, only 36.6% of exercise-training participants had a clinically meaningful fitness response (ΔVO2peak ≥5%) with exercise training.
Figure 1.
Patient selection for the current study. All control participants and exercise-training participants with available baseline and follow-up peak oxygen uptake (in liters per minute) data and >70% adherence to exercise prescription were included in the current study.
Baseline clinical and demographic characteristics are presented in Table 1. Fitness responders were younger and had a greater proportion of combination training than fitness nonresponders. The proportion of fitness responders was similar in the aerobic training–only (31.3%) and resistance training–only (33.9%) groups.
Table 1.
Comparison of baseline characteristics of participants across the study groups defined by cardiorespiratory fitness response to exercise intervention
| Variable | Control (n = 41) | Fitness nonresponder (n = 102) | Fitness responder (n = 59) | P value* |
|---|---|---|---|---|
| Age, years | 58.6 (8.3) | 57.6 (7.7) | 55.2 (8.1) | 0.03 |
| Men, % | 31.7 | 41.2 | 33.9 | 0.40 |
| African Americans, % | 41.5 | 37.2 | 42.3 | 0.61 |
| BMI, kg/m2 | 34.8 (6.2) | 34.1 (5.6) | 34.3 (5.9) | 0.79 |
| WC, cm | 110.6 (14.4) | 111.1 (12.8) | 111.0 (13.1) | 0.89 |
| Body fat, % | 38.5 (7.0) | 37.1 (7.7) | 37.9 (7.0) | 0.45 |
| Systolic BP, mmHg | 127 (14) | 127 (13) | 125 (13) | 0.35 |
| Diastolic BP, mmHg | 76 (8) | 74 (8) | 75 (9) | 0.76 |
| Resting HR, bpm | 84 (13) | 81 (15) | 84 (12) | 0.31 |
| HbA1c | 0.74 | |||
| % | 7.7 (1.5) | 7.2 (1.1) | 7.2 (1.1) | |
| mmol/mol | 61 | 55 | 55 | |
| VO2peak, L/min | 1.79 (0.5) | 1.89 (0.48) | 1.88 (0.6) | 0.57 |
| Exercise groups, % | 0.332 | |||
| Aerobic | NA | 34.3 | 27.1 | |
| Resistance | NA | 36.2 | 32.2 | |
| Aerobic + resistance | NA | 29.4 | 40.7 | |
| Baseline RER (peak exercise) | 1.14 (0.08) | 1.15 (0.09) | 1.13 (0.09) | 0.19 |
| Exercise SBPpeak, mmHg | 192 (26) | 194 (29) | 194 (21) | 0.76 |
| Diabetes duration, years | 7.2 (5.2) | 7.3 (5.8) | 7.6 (6.2) | 0.91 |
| Insulin use, % | 17.1 | 18.6 | 15.2 | 0.67 |
Data are presented as mean (SD), except as noted. BP, blood pressure; HR, heart rate; NA, not applicable; RER, respiratory exchange ratio; SBPpeak, peak systolic blood pressure.
*Fitness responder vs. nonresponder.
Changes in selected exercise test parameters from baseline to the end of the trial among the fitness responders and nonresponders are compared in Table 2. Fitness responders had a significantly greater increase in exercise time, peak exercise heart rate, peak exercise blood pressure, and maximum exercise heart rate reserve than fitness nonresponders. These findings validate the criteria for a clinically meaningful fitness response (≥5% increase in VO2peak) that was used in the current study. Change in total caloric intake from baseline to follow-up was not significantly different among the control, fitness responder, and fitness nonresponder groups (median change in total daily caloric intake: control group −135.1 kcal, range −376.4 to 235.2 kcal; fitness responder group −149.2 kcal, range −564.5 to 82.4 kcal); fitness nonresponder group −134.4 kcal, range −330.1 to 107 kcal; P = 0.44).
Table 2.
Change in selected exercise test parameters from baseline to post training among exercise-training participants
| Variable | Fitness nonresponder (n = 102) | Fitness responder (n = 59) | P value* |
|---|---|---|---|
| ΔVO2peak, L/min | −0.07 (−0.1 to 0.04) | 0.24 (0.20–0.28) | <0.001 |
| ΔHeart ratepeak, bpm | −4.8 (−7.2 to −2.4) | 3.3 (0.2–6.5) | <0.001 |
| ΔExercise time, min | 0.86 (0.39 to 1.33) | 3.2 (2.57–3.80) | <0.001 |
| Δ% Predicted heart rate reserve† | −4.7 (−7.8 to −1.6) | 5.1 (1.0–9.1) | 0.0002 |
| ΔExercise SBPpeak, mmHg | −0.9 (−6.9 to 7.5) | 11 (5.3–16.7) | 0.02 |
Values are expressed as fitted mean (95% CI) derived from linear mixed models that are adjusted for baseline value, age, sex, duration of diabetes, and race/ethnicity. SBPpeak, peak systolic blood pressure.
*Fitness responder vs. nonresponder.
†% Predicted heart rate reserve = [(peak exercise heart rate − resting heart rate)/(estimated maximum heart rate − resting heart rate)] × 100% where estimated maximum heart rate = 220 − age (years).
Figure 2 depicts the monthly mean HbA1c levels across the control, fitness responder, and fitness nonresponder groups. In multivariable models adjusting for age, sex, race/ethnicity, diabetes duration, and baseline HbA1c level, compared with the control group, mean HbA1c levels measured over time were significantly lower for both the fitness responder group (P = 0.01) and the fitness nonresponder group (P = 0.003). However, there was no significant difference between the fitness responder and fitness nonresponder groups for the trends in monthly mean HbA1c levels (P = 0.25).
Figure 2.
Monthly mean HbA1c levels across the study groups. The data are represented as fitted means derived from a linear mixed model that included the covariables age, sex, race/ethnicity, diabetes duration, and baseline HbA1c level. The error bars represent SEs. The group effect was significant for comparison of trends in monthly mean HbA1c levels between control vs. responder (P = 0.014), as well as control vs. nonresponder groups (P = 0.0026), but not for responder vs. nonresponder (P = 0.254).
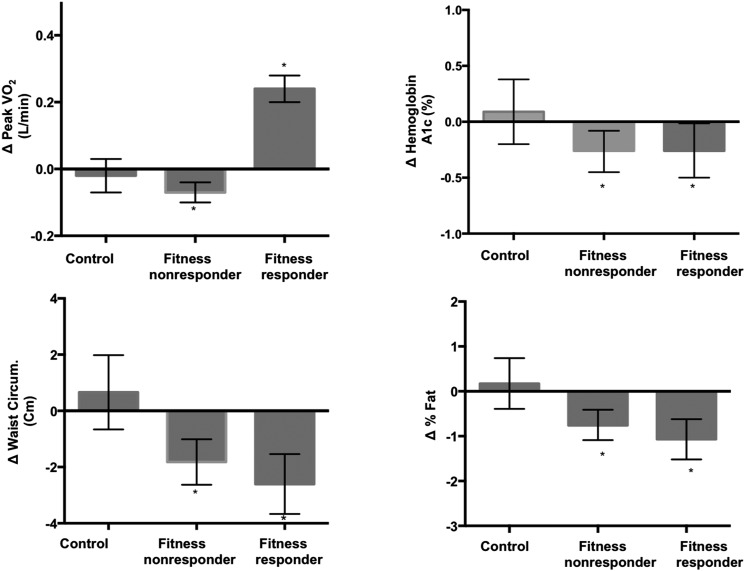
Changes in VO2peak, HbA1c levels, and anthropometric measures from baseline to the end of the trial within the study groups are shown in Fig. 3. While only fitness responders had a significant improvement in VO2peak, both fitness responders and nonresponders experienced significant reductions in HbA1c level from baseline to final assessment (fitness responders ΔHbA1c −0.26% [95% CI −0.5 to −0.01], fitness nonresponders ΔHbA1c −0.26% [95% CI −0.45 to −0.08]). Similarly, anthropometric measures such as WC and percentage of body fat improved significantly with exercise training among fitness responders (ΔWC −2.6 cm [95% CI −3.7 to −1.5], Δbody fat −1.07% [95% CI −1.5 to −0.62]) as well as nonresponders (ΔWC −1.8 cm [95% CI −2.6 to −1.0], Δbody fat −0.75% [95% CI −1.09 to −0.41]). Furthermore, the absolute change in HbA1c levels and anthropometric measures with exercise training was not significantly different among fitness responders versus nonresponders. Similar improvements in HbA1c levels, WC, and percentage of body fat were observed among fitness responders and nonresponders from baseline to follow-up on sensitivity analyses after excluding resistance training–only participants (Table 3).
Figure 3.
Comparisons of change in VO2peak, HbA1c level, and anthropometric measures from baseline to trial completion among control, fitness responder, and fitness nonresponder groups. The values are expressed as fitted means, and all are adjusted for baseline value, age, sex, duration of diabetes, and race/ethnicity. *P < 0.05 for change from baseline to end of trial within a particular study group. Circum., circumference.
Table 3.
Changes in metabolic parameters from baseline to follow-up among fitness responders and nonresponders with and without resistance training participants
| Variable | Sensitivity analysis (excluding resistance-training group) |
Primary analysis (all exercise-training groups) |
||
|---|---|---|---|---|
| Responders (N = 40) | Nonresponders (N = 65) | Responders (N = 59) | Nonresponders (N = 102) | |
| ΔVO2peak, L/min | 0.26 (0.21–0.30) | −0.06 (−0.10 to −0.03) | 0.24 (0.20–0.28) | −0.07 (−0.1 to –0.04) |
| ΔHbA1c,% | −0.30 (−0.63 to 0.01) | −0.29 (−0.54 to −0.01) | −0.26 (−0.5 to −0.01) | −0.26 (−0.45 to −0.08) |
| ΔWC, cm | −2.39 (−3.64 to −1.13) | −2.05 (−3.03 to −1.07) | −2.6 (−3.7 to −1.5) | −1.8 (−2.6 to −1.0) |
| Δ% Body fat | −0.85 (−1.4 to −0.29) | −0.69 (−1.12 to −0.25) | −1.07 (−1.5 to −0.62) | −0.75 (−1.09 to −0.41) |
Values are expressed as fitted mean (95% CI) derived from linear mixed models that are adjusted for baseline value, age, sex, duration of diabetes, and race/ethnicity.
In adjusted linear regression analyses, exercise training, baseline HbA1c level, and change in the percentage of body fat were identified to be significant predictors of change in HbA1c level (Table 4). Change in fitness was not a significant determinant of change in HbA1c level in adjusted analysis (standardized β-estimate = −0.13, P = 0.69).
Table 4.
Predictors of change in HbA1c among the study participants
| Participant characteristics | Standardized estimate | P value |
|---|---|---|
| Baseline HbA1c | −0.43 | <0.001 |
| Exercise training (Y/N) | −0.42 | 0.03 |
| Δ% Body fat | 0.08 | 0.04 |
Model was adjusted for age, sex, race/ethnicity, baseline HbA1c level, diabetes duration, lean body mass, baseline VO2peak, baseline BMI, ΔVO2peak, medication change, exercise training, and change in percentage of body fat. N, no; Y, yes.
The proportion of individuals who achieved the composite outcome of either decreasing glucose-lowering medication or reducing HbA1c level by ≥0.5% without increasing the use of medications was numerically greater in the exercise-training groups compared with the control group (36% vs. 20.5%; P = 0.08). In multivariable adjusted logistic regression analysis, exercise training was associated with significantly greater odds of achieving the composite glycemic outcome (odds ratio 3.0 [95% CI 1.04–9.2]). Among exercise-training participants, the proportion of participants who achieved the diabetes medication-HbA1c composite outcome was not significantly different between fitness nonresponders and responders (33% vs. 41%, P = 0.4).
Conclusions
We observed several important findings. First, a substantial proportion of participants with T2DM have no significant improvement in cardiorespiratory fitness in response to exercise training. Second, exercise training is associated with significant improvement in glycemic control among both fitness responders and fitness nonresponders. Third, exercise training–related improvement in glycemic control among fitness nonresponders is associated with a significant reduction in central obesity and percentage of body fat. Taken together, these findings suggest that exercise training improves metabolic parameters in patients with T2DM independent of changes in cardiorespiratory fitness.
The prevalence of fitness nonresponse among T2DM patients observed in the current study is greater than that previously reported among healthy adults (6). This is particularly significant since the dose of exercise used in the current study was similar to that recommended by the National Institutes of Health (10–12 kcal/kg/week) (10). The high fitness nonresponse rate observed among the exercise-training participants could be related to multiple factors. Patients with diabetes have increased prevalence of chronotropic incompetence, left ventricular hypertrophy, and adverse remodeling, all of which are associated with a greater risk for fitness nonresponse (11,12). Also, previous studies (13,14) have shown that patients with diabetes have slowed muscle perfusion kinetics and slowed oxidative phosphorylation that could lead to a relatively blunted improvement in cardiorespiratory fitness with exercise training.
Our study findings have important clinical implications for exercise counseling provided to sedentary middle-aged adults with T2DM. The current public health recommendations (10) suggest that such individuals should accumulate a minimum of 30 min of moderate-intensity physical activity on most days of a week with a goal to improve cardiorespiratory fitness. However, it may not be feasible to achieve this target in a significant proportion of patients with coexisting conditions such as obesity, older age, and cardiovascular disease (15). The current study suggests that improvements in metabolic parameters are observed among exercise-training participants independent of their change in cardiorespiratory fitness. This highlights the importance of sustained exercise training even in individuals who do not demonstrate improvement in measures of cardiorespiratory fitness. Furthermore, this study suggests that exercise-training programs should target and assess for improvement in metabolic parameters such as glycemic control, WC, and percentage of body fat among individuals with T2DM.
Response to exercise training has been traditionally expressed in terms of improvement in cardiorespiratory fitness (4,16,17). However, physiological adaptation to exercise training is a complex and heterogeneous process affecting multiple organ systems (18). Favorable effects of exercise on cardiac output and peripheral oxygen use mediate training-related improvement in cardiorespiratory fitness (19,20). Nonresponsiveness of cardiorespiratory fitness represents a failure of just one potential adaptation to exercise training. It is plausible that fitness-nonresponsive participants undergo favorable adaptations in other physiological pathways independent of fitness change. Small longitudinal studies (21,22) from the 1980s reported a lack of association between the magnitude of improvement in VO2peak and aerobic performance among recreationally active participants who underwent short-term endurance training. More recently, Vollaard et al. (23) showed significant adaptations associated with metabolic control and aerobic performance even among exercise-training participants who had minimal improvements in VO2peak after training (low responders). However, these prior studies assessed participants without T2DM or other metabolic disorders. The current study provides further evidence in support of this notion among participants with T2DM, and shows that fitness nonresponders have significant improvement in metabolic parameters such as HbA1c level and adiposity, changes that are not significantly different from those in fitness responders. Thus, lack of improvement in one specific phenotype, specifically in VO2peak, does not negate other potential benefits of exercise and highlights the importance of assessing training response in a broader clinical context.
Recent studies (24–26) have identified fitness improvement in response to exercise training as a significant predictor of improvement in glycemic control among participants with T2DM. However, these studies used estimated METs, derived from the maximal speed and grade reached during a treadmill test, as a measure of fitness. The impact of changes in VO2peak, the gold standard measure of cardiorespiratory fitness, on metabolic outcomes in exercise-training trials is not well understood. While some studies (24,27,28) have identified an increase in VO2peak as a significant predictor of improvement in HbA1c levels, others have failed to observe this association. Brennan et al. (27) evaluated the association between changes in VO2peak and insulin sensitivity among 60 participants with T2DM who underwent aerobic training for 3–4 months, and reported that exercise-induced changes in VO2peak do not mediate changes in insulin sensitivity. In the current study, we have confirmed this lack of association between changes in VO2peak and improvement in HbA1c in a much larger study population with a significantly longer duration of exercise training. In contrast, Larose et al. (28) showed that change in VO2peak was a significant predictor of HbA1c change in response to exercise training. This discrepancy between the study findings could be due to the different parameters used as a measure of fitness change in the two studies. While change in VO2peak (in liters per minute) was used as the measure of change in fitness in the current study, Larose et al. (28) used the change in oxygen uptake scaled to body mass (in liters per kilogram per minute) as the measure of change in fitness. A significant reduction in body weight with exercise training was observed by Larose et al. (28), and, as the unit of indexation, influenced the estimated VO2peak independent of changes in absolute VO2 (in liters per minute) not scaled to weight. In that study, weight changes were significantly associated with improvement in VO2peak scaled to body mass as well as with HbA1c, and could explain the observed association between changes in fitness (oxygen uptake scaled to body mass) and glycemic control. Furthermore, there were significant differences in the analytical approach (per group in Larose et al. [28] vs. combined in HART-D) and dose of exercise training (lower dose of training in the HART-D study) used in the two studies that could also have contributed toward the discrepant study findings.
The lack of association between change in cardiorespiratory fitness and improvement in glycemic control is likely due to differences in the underlying adaptive mechanisms that result from exercise training. Improvement in cardiorespiratory fitness in response to exercise training is associated with central cardiovascular adaptations, physiological cardiac remodeling, and improvement in stroke volume (20,29,30). In contrast, training-associated changes in glycemic control are more related to improvement in insulin sensitivity secondary to peripheral adaptations in the adipose and skeletal muscle tissue (31,32). Previous studies (26,33,34) have identified changes in measures of central adiposity as significant predictors of improvement in HbA1c and glycemic control. Similarly, the present observations suggest that reduction in the percentage of body fat is associated with significant improvement in HbA1c, though whether it is causal or even contributory remains unclear. Supporting a contributory role, it is notable that the magnitude of reduction in WC and the percentage of body fat with training was similar among fitness responders and nonresponders, which were associated in both groups with demonstrable improvements in glucose metrics.
Our study has important limitations. First, the participants of the HART-D study had relatively well-controlled diabetes with an average HbA1c level of 7.6. It is possible that among individuals with worse glycemic control, fitness responders may have a greater improvement in HbA1c level with exercise training compared with nonresponders. Second, we do not have a standard measure of insulin resistance that could have helped us to better understand the mechanism of improvement in glycemic control among fitness nonresponders. Third, we used a cutoff of ≥5% change in VO2peak as a clinically meaningful fitness response based on previous studies and consensus in the literature. The threshold for meaningful improvement in fitness in these sedentary patients with T2DM could be different. However, we observed similar results using a more lenient definition for fitness improvement (change in VO2peak >0 L/min, data not shown). Furthermore, we did not observe any association between continuous change in fitness and change in HbA1c level in linear regression analysis, suggesting that our observations are insensitive to the threshold of fitness response. Finally, a food frequency questionnaire was used at baseline and follow-up to assess changes in diet, which limits our ability to identify changes in caloric intake and diet composition.
In conclusion, exercise training is associated with significant improvement in glycemic control and measures of adiposity among participants with T2DM independent of their change in cardiorespiratory fitness. Further studies are needed to confirm the mechanisms by which training improves metabolic parameters among fitness nonresponders.
Article Information
Funding. The HART-D Study (NCT00458133) was supported by grant DK-068298 from the National Institutes of Health.
Duality of Interest. D.K.M. reports receiving honoraria for trial leadership and consultation with GlaxoSmithKline, The Medicines Company, Takeda Pharmaceuticals, Novo Nordisk, Orexigen, Cubist, Janssen, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Merck, Regeneron, Lexicon, and Eisai. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.P. contributed to conceptualization of the study idea, the analysis plan, and manuscript preparation. D.L.S., I.J.N., S.N.B., N.J., and C.P.E. contributed to the review and editing of the manuscript. D.K.M. contributed to the review and preparation of the manuscript. C.R.A. contributed to the data analysis. J.D.B. contributed to conceptualization of the study idea, the analysis plan, and manuscript preparation; had full access to all data in the study; and had final responsibility for the decision to submit for publication. T.S.C. contributed to the review of the manuscript and was the primary investigator for the HART-D study. All authors have read and have agreed to the manuscript as written. J.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association Scientific Sessions 2014, Chicago, IL, 15–19 November 2014.
References
- 1.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med 2005;165:2114–2120 [DOI] [PubMed] [Google Scholar]
- 2.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006;29:2518–2527 [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 1999;87:1003–1008 [DOI] [PubMed] [Google Scholar]
- 5.Hautala AJ, Kiviniemi AM, Mäkikallio TH, et al. Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 2006;96:535–542 [DOI] [PubMed] [Google Scholar]
- 6.Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc 2009;41:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Sports Medicine ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA, Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 8.Fleg JL, Piña IL, Balady GJ, et al. Assessment of functional capacity in clinical and research applications: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation 2000;102:1591–1597 [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 2010;3:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health Physical activity and cardiovascular health. JAMA 1996;276:241–246 [PubMed] [Google Scholar]
- 11.Schmid JP, Zurek M, Saner H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur J Prev Cardiol 2013;20:585–592 [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, Ayers C, Blair S, et al. Cardiac determinants of heterogeneity in fitness change in response to moderate intensity aerobic exercise training: the DREW study. J Am Coll Cardiol 2015;65:1057–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007;30:2880–2885 [DOI] [PubMed] [Google Scholar]
- 14.Geary K, Knaub LA, Schauer IE, et al. Targeting mitochondria to restore failed adaptation to exercise in diabetes. Biochem Soc Trans 2014;42:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int 2009;24:416–427 [DOI] [PubMed] [Google Scholar]
- 16.Kohrt WM, Malley MT, Coggan AR, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol (1985) 1991;71:2004–2011 [DOI] [PubMed] [Google Scholar]
- 17.Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000;32:70–84 [DOI] [PubMed] [Google Scholar]
- 18.Booth FW, Laye MJ. The future: genes, physical activity and health. Acta Physiol (Oxf) 2010;199:549–556 [DOI] [PubMed] [Google Scholar]
- 19.Zoladz JA, Korzeniewski B, Grassi B. Training-induced acceleration of oxygen uptake kinetics in skeletal muscle: the underlying mechanisms. J Physiol Pharmacol 2006;57(Suppl. 10):67–84 [PubMed] [Google Scholar]
- 20.Rowland T. Echocardiography and circulatory response to progressive endurance exercise. Sports Med 2008;38:541–551 [DOI] [PubMed] [Google Scholar]
- 21.Hardman AE, Williams C, Wootton SA. The influence of short-term endurance training on maximum oxygen uptake, submaximum endurance and the ability to perform brief, maximal exercise. J Sports Sci 1986;4:109–116 [DOI] [PubMed] [Google Scholar]
- 22.Ramsbottom R, Williams C, Fleming N, Nute ML. Training induced physiological and metabolic changes associated with improvements in running performance. Br J Sports Med 1989;23:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollaard NB, Constantin-Teodosiu D, Fredriksson K, et al. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J Appl Physiol (1985) 2009;106:1479–1486 [DOI] [PubMed] [Google Scholar]
- 24.Balducci S, Zanuso S, Cardelli P, et al.. ; Italian Diabetes Exercise Study (IDES) Investigators. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care 2012;35:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakicic JM, Egan CM, Fabricatore AN, et al.; Look AHEAD Research Group . Four-year change in cardiorespiratory fitness and influence on glycemic control in adults with type 2 diabetes in a randomized trial: the Look AHEAD Trial. Diabetes Care 2013;36:1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sénéchal M, Swift DL, Johannsen NM, et al. Changes in body fat distribution and fitness are associated with changes in hemoglobin A1c after 9 months of exercise training: results from the HART-D study. Diabetes Care 2013;36:2843–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan AM, Lam M, Stotz P, Hudson R, Ross R. Exercise-induced improvement in insulin sensitivity is not mediated by change in cardiorespiratory fitness. Diabetes Care 2014;37:e95–e97 [DOI] [PubMed] [Google Scholar]
- 28.Larose J, Sigal RJ, Khandwala F, Prud'homme D, Boule NG, Kenny GP; Diabetes Aerobic and Resistance Exercise (DARE) Trial Investigators . Associations between physical fitness and HbA1(c) in type 2 diabetes mellitus. Diabetologia 2011;54:93–102 [DOI] [PubMed] [Google Scholar]
- 29.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation 2010;122:1637–1648 [DOI] [PubMed] [Google Scholar]
- 30.Fajans SS. Early stages of diabetes: definitions and present concepts. Adv Exp Med Biol 1979;119:7–11 [DOI] [PubMed] [Google Scholar]
- 31.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 2007;85:662–677 [DOI] [PubMed] [Google Scholar]
- 32.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther 2008;88:1279–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care 2012;35:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335–2341 [DOI] [PubMed] [Google Scholar]