Abstract
OBJECTIVE
Impaired glucose tolerance (IGT) through to type 2 diabetes is thought to confer a continuum of risk for neuropathy. Identification of subjects at high risk of developing type 2 diabetes and, hence, worsening neuropathy would allow identification and risk stratification for more aggressive management.
RESEARCH DESIGN AND METHODS
Thirty subjects with IGT and 17 age-matched control subjects underwent an oral glucose tolerance test, assessment of neuropathic symptoms and deficits, quantitative sensory testing, neurophysiology, skin biopsy, and corneal confocal microscopy (CCM) to quantify corneal nerve fiber density (CNFD), branch density (CNBD), and fiber length (CNFL) at baseline and annually for 3 years.
RESULTS
Ten subjects who developed type 2 diabetes had a significantly lower CNFD (P = 0.003), CNBD (P = 0.04), and CNFL (P = 0.04) compared with control subjects at baseline and a further reduction in CNFL (P = 0.006), intraepidermal nerve fiber density (IENFD) (P = 0.02), and mean dendritic length (MDL) (P = 0.02) over 3 years. Fifteen subjects who remained IGT and 5 subjects who returned to normal glucose tolerance had no significant baseline abnormality on CCM or IENFD but had a lower MDL (P < 0.0001) compared with control subjects. The IGT subjects showed a significant decrease in IENFD (P = 0.02) but no change in MDL or CCM over 3 years. Those who returned to NGT showed an increase in CNFD (P = 0.05), CNBD (P = 0.04), and CNFL (P = 0.05), but a decrease in IENFD (P = 0.02), over 3 years.
CONCLUSIONS
CCM and skin biopsy detect a small-fiber neuropathy in subjects with IGT who develop type 2 diabetes and also show a dynamic worsening or improvement in corneal and intraepidermal nerve morphology in relation to change in glucose tolerance status.
Introduction
The International Diabetes Federation states that there are currently 316 million people with impaired glucose tolerance (IGT), which will increase to 471 million people by 2035 (1). There is considerable debate as to whether these subjects should be considered to have a medical problem (2). However, in subjects with IGT, the risk of developing type 2 diabetes ranges from 3.6 to 8.7% per year (3). Furthermore, IGT is also independently associated with the traditional microvascular complications of diabetes, including retinopathy, microalbuminuria, and neuropathy (4). There appears to be a good rationale for identifying subjects with IGT, but there are limited data identifying subjects with IGT who may be at greatest risk for developing diabetes and its complications.
In relation to neuropathy, the specific focus of this study, the UK Prospective Diabetes Study, showed that at the time of diagnosis of type 2 diabetes, 5–7% of patients already had neuropathy (5), and longitudinal data from the Rochester cohort have shown that duration and severity of exposure to hyperglycemia are related to the severity of neuropathy (6). In a recent study of patients with ∼2 years of type 2 diabetes, there was also evidence of a significant neuropathy (7). However, there is debate as to whether IGT is associated with neuropathy, with some studies showing evidence of neuropathy (8–12), while others do not (13–15). We recently showed that a significant small-fiber neuropathy occurred in 40.5% of 37 subjects with IGT (16). Interestingly, a recent study evaluating electrochemical sweat conductance, a proxy for small-fiber neuropathy, has shown that healthy subjects with an abnormal response have a significantly increased odds ratio for the development of IGT over 2 years (17). Of relevance, lifestyle modification has been shown to improve intraepidermal nerve fiber density (IENFD) (11) and to improve after chemical axotomy (18). We previously showed an improvement in corneal nerve morphology after an improvement in glycemic control, lipids, and blood pressure (19) after simultaneous pancreas and kidney transplantation (20) and more recently in patients on continuous subcutaneous insulin infusion (21), suggesting a dynamic regenerative capacity of the small fibers in relation to metabolic change. We have undertaken a longitudinal study in subjects with IGT to assess whether baseline and follow-up measures of neuropathy, particularly small-fiber neuropathy, relate to changes in glucose tolerance over 3 years.
Research Design and Methods
Selection of Patients
We assessed 30 subjects with IGT based on an oral glucose tolerance test (OGTT) (2-h glucose 7.8–11.1 mmol) at Central Manchester and Manchester Children’s University Hospital and 17 health control subjects. Exclusion criteria were any history of neuropathy due to a nondiabetic cause and any history of ocular pathology or systemic disease with corneal involvement. This study was approved by the Central Manchester Research and Ethics Committee, and written informed consent was obtained from all subjects prior to participation. This research adhered to the tenets of the Declaration of Helsinki.
Assessment of Neuropathy
All study participants underwent assessment at baseline and 12, 24, and 36 months. Participants underwent assessment of BMI, blood pressure, OGTT, HbA1c, lipid profile (total cholesterol, LDL, HDL, and triglycerides), albumin-to-creatinine excretion ratio, and estimated glomerular filtration rate (eGFR). Symptoms of diabetic peripheral neuropathy were assessed using the Neuropathy Symptom Profile (NSP). Neurological deficits were evaluated using the simplified neuropathy disability score (NDS), which is comprised of vibration perception, pinprick, temperature sensation, and presence or absence of ankle reflexes. Vibration perception threshold (VPT) was tested using a Neurothesiometer (Horwell; Scientific Laboratory Supplies, Wilfrod, Nottingham, U.K.). Cold (CT) and warm (WT) thresholds were established on the dorsolateral aspect of the left foot (S1) using the TSA-II NeuroSensory Analyzer (Medoc, Ramat-Yishai, Israel).
Electro-diagnostic studies were undertaken using a Dantec “Keypoint” system (Dantec Dynamics, Bristol, U.K.) equipped with a DISA temperature regulator to keep limb temperature constantly between 32 and 35°C. Sural sensory nerve amplitude (SNAP), sural sensory nerve conduction velocity (SNCV), and peroneal motor nerve conduction velocity (PMNCV) and amplitude (PMNA) were assessed by a consultant neurophysiologist.
Skin Biopsy
A 3-mm punch skin biopsy was taken from the dorsum of the foot, ∼2 cm above the second metatarsal head under local anesthesia (1% lidocaine). Sections (50 μm) were stained using anti-human PGP 9.5 antibody (Abcam, Cambridge, U.K.), and nerve fibers were demonstrated using SG chromogen (Vector Laboratories, Peterborough, U.K.). IENFD was quantified in accordance with established criteria and expressed as no. per millimeter (22). Twenty Z-stack images per case were taken using a Zeiss AxioImager M2 microscope, and mean dendritic length (MDL) (length of IENF from piercing the dermo-epidermal junction to its terminal in the epidermis) was manually traced and quantified using the ImagePro 6.2 program (MediaCybernetics, Marlow, U.K.).
Corneal Confocal Microscopy
Patients underwent examination with the corneal confocal microscopy (CCM) (Heidelberg Retinal Tomograph III Rostock Cornea Module; Heidelberg Engineering, Heidelberg, Germany) as per our previously established protocol (23). Six nonoverlapping images/patient from the center of the cornea were selected and quantified in a masked fashion. Three corneal nerve parameters were quantified: corneal nerve fiber density (CNFD), the total number of major nerves per square millimeter of corneal tissue; corneal nerve branch density (CNBD), the number of branches emanating from all major nerve trunks per square millimeter of corneal tissue; and corneal nerve fiber length (CNFL), the total length of all nerve fibers and branches (millimeter per square millimeter) within the area of corneal tissue. Analysis of the images was done using purposefully designed automated software called ACCmetrics (24).
Statistical Analysis
Analysis was carried out on SPSS for Mac (version 19.0; IBM Corporation, Armonk, NY). All data are expressed as means ± SEM. The data were tested for normality by using the Shapiro Wilk Normality test and by visualizing the histogram and normal Q-Q plot. To assess within- and between-group differences, we used one-way ANOVA (nonparametric Kruskal-Wallis). A significant P value was considered to be <0.05 (post hoc Tukey).
Results
Baseline
The clinical characteristics are summarized in Table 1. The control and IGT subjects were age matched (62.3 ± 1.8 vs. 60 ± 2.1 years, P = 0.2). Subjects with IGT had a significantly higher HbA1c (42.7 ± 0.9 vs. 38.3 ± 0.7 mmol/mol, P < 0.0001) and BMI (32.0 ± 1.0 vs. 27.6 ± 0.9 kg/m2, P = 0.01) and lower HDL (1.2 ± 0.1 vs. 1.7 ± 0.1 mmol/L, P = 0.03) but comparable total cholesterol, triglycerides, eGFR, and blood pressure compared with control subjects.
Table 1.
Clinical and metabolic parameters and neuropathy assessment in control subjects and subjects with IGT at baseline and follow-up
| Control (N = 17) | Baseline (N = 30) | P* | 12 months | 24 months | 36 months | P† | |
|---|---|---|---|---|---|---|---|
| Age (years) | 62.3 ± 1.8 | 60 ± 2.1 | NS | ||||
| BMI (kg/m2) | 27.6 ± 0.9 | 32.0 ± 1.0 | 0.01 | 31.2 ± 1.1 | 31.0 ± 1.2 | 33.0 ± 1.3 | NS |
| HbA1c (mmol/mol) | 38.3 ± 0.7 | 42.7 ± 0.9 | <0.0001 | 44.0 ± 1.2 | 43.0 ± 1.5 | 44.2 ± 2.0 | NS |
| Cholesterol (mmol/L) | 5.4 ± 0.2 | 4.8 ± 0.2 | NS | 4.8 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 | NS |
| HDL (mmol/L) | 1.7 ± 0.1 | 1.2 ± 0.1 | 0.03 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | NS |
| Triglycerides (mmol/L) | 1.8 ± 0.2 | 2.2 ± 0.3 | NS | 2.2 ± 0.3 | 2.0 ± 0.5 | 1.8 ± 0.3 | NS |
| LDL (mmol/L) | 3.0 ± 0.2 | 2.6 ± 0.2 | NS | 2.6 ± 0.2 | 2.3 ± 0.3 | 2.5 ± 0.2 | NS |
| eGFR (mL/min/1.73 m2) | 82.4 ± 2.0 | 79.1 ± 3.0 | NS | 78.8 ± 3.4 | 74.9 ± 3.5 | 74.3 ± 4.3 | 0.03 |
| Blood pressure (mmHg) | 136 ± 4.3/ 75.9 ± 2.5 | 129.2 ± 3.4/ 72.9 ± 2.1 | NS | 131.3 ± 12.6/ 69.0 ± 3.8 | 129.0 ± 3.9/ 72.7 ± 2.2 | 129.3 ± 3.4/ 75.4 ± 2.3 | NS |
| NSP (/10) | 0.3 ± 0.1 | 3.4 ± 0.7 | <0.0001 | 2.78 ± 0.7 | 3.5 ± 0.8 | 2.8 ± 0.6 | NS |
| NDS (/10) | 1.1 ± 0.3 | 2.9 ± 0.5 | 0.03 | 3.6 ± 0.6 | 2.8 ± 0.5 | 2.4 ± 0.6 | NS |
| VPT (V) | 8.4 ± 1.5 | 16.2 ± 2.1 | 0.02 | 17.7 ± 2.4 | 18.7 ± 2.7 | 16.8 ± 2.1 | NS |
| CT (°C) | 27.9 ± 1.1 | 25.5 ± 1.4 | NS | 25.3 ± 1.1 | 23.7 ± 1.8 | 24.9 ± 0.9 | NS |
| WT (°C) | 39.5 ± 1.1 | 39.8 ± 1.0 | NS | 40.2 ± 0.8 | 41.9 ± 0.9 | 40.4 ± 0.7 | NS |
| NS | |||||||
| SNCV (m/s) | 49.0 ± 1.1 | 49.7 ± 1.4 | NS | 47.4 ± 1.3 | 47.4 ± 1.3 | 46.6 ± 1.3 | 0.007 |
| SNAP (μV) | 15.3 ± 1.8 | 11.3 ± 1.2 | NS | 11.8 ± 1.8 | 11.4 ± 1.9 | 10.7 ± 1.7 | NS |
| PMNCV (m/s) | 46.5 ± 1.0 | 45.1 ± 0.8 | NS | 44.4 ± 0.8 | 44.9 ± 0.8 | 44.7 ± 0.9 | |
| PMNA (mV) | 5.2 ± 0.4 | 4.4 ± 0.4 | NS | 3.7 ± 0.3 | 3.3 ± 0.3 | 3.9 ± 0.3 | NS |
| IENFD (no./mm) | 8.5 ± 0.6 | 6.4 ± 0.8 | NS | 6.5 ± 4.1 | NA | 3.2 ± 0.8 | 0.02 |
| MDL (μm) | 63.0 ± 4.2 | 25.1 ± 1.6 | <0.0001 | 24.6 ± 2.7 | NA | 22.9 ± 3.1 | NS |
| CNFD (no./mm2) | 30.7 ± 1.5 | 24.4 ± 1.3 | <0.0001 | 22.6 ± 1.5 | 27.4 ± 1.5 | 24.4 ± 1.2 | NS |
| CNBD (no./mm2) | 37.0 ± 2.7 | 33.8 ± 2.9 | NS | 34.5 ± 3.5 | 34.9 ± 3.4 | 33.6 ± 2.8 | NS |
| CNFL (mm/mm2) | 20.4 ± 3.14 | 15.3 ± 0.6 | 0.0004 | 14.9 ± 0.8 | 16.4 ± 0.8 | 14.5 ± 0.6 | NS |
Data are means ± SEM. NA, not assessed; NS, not significant. All symbols represent statistically significant differences.
*P value IGT baseline vs. control;
†IGT baseline vs. 36 months.
The IGT group had a significantly higher NSP (3.4 ± 0.7 vs. 0.3 ± 0.1, P < 0.0001), NDS (2.9 ± 0.5 vs. 1.1 ± 0.3, P = 0.03), and VPT (16.2 ± 2.1 vs. 8.4 ± 1.5, P = 0.02) compared with the control group. There was no significant difference in SNCV and PMNCV and amplitude between subjects with IGT and the control subjects.
There was no difference in IENFD; however, MDL was significantly lower in the IGT group compared with control subjects (25.1 ± 1.6 vs. 63.0 ± 4.2 μm, P < 0.0001). CNFD (24.4 ± 1.3 vs. 30.7 ± 1.5 no./mm2, P < 0.0001) and CNFL (15.3 ± 0.6 vs. 20.4 ± 3.14 mm/mm2, P = 0.004) were significantly lower, but there was no difference in CNBD between subjects with IGT and control subjects.
There was no correlation between HDL and CCM measures at baseline (CNFL r = 0.2, P = 0.2; CNBD r = 0.2, P = 0.1; and CNFD r = 0.2, P = 0.3).
Longitudinal Assessments
Control subjects showed no significant change in metabolic parameters or neuropathy measures over 3 years (repeat skin biopsy not performed in control subjects). In subjects with IGT, BMI, HbA1c, lipids, and blood pressure remained stable, and there was a small but significant reduction in eGFR (79.1 ± 3.0 vs. 74.3 ± 4.3 mL/min/1.73 m2, P = 0.03) over 3 years (Table 1). The longitudinal data for the neuropathy assessments are presented in Table 1. There was no significant change in NSP, NDS, VPT, or thermal thresholds. There was a significant reduction in SNCV (49.7 ± 1.4 vs. 46.6 ± 1.3 m/s, P = 0.007) and IENFD (6.4 ± 0.8 vs. 3.2 ± 0.8 no./mm, P = 0.02), but no change in MDL or CCM measures, from baseline to 36 months.
Change in Neuropathy Measures in Relation to Change in Glucose Tolerance
All subjects with IGT underwent an annual OGTT over 36 months, 10 developed type 2 diabetes, 15 remained with IGT, and 5 regressed to normal glucose tolerance (NGT) (Table 2). Figure 1 shows CCM images from each group.
Table 2.
Neuropathy assessments at baseline and 36 months in subjects who reverted to NGT, remained with IGT, or developed type 2 diabetes at 36 months
| Control (N = 17) |
NGT (N = 5) |
IGT (N = 15) |
Type 2 diabetes (N = 10) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 36 months | Baseline | 36 months | Baseline | 36 months | Baseline | 36 months | |
| HbA1c (mmol/mmol) | 38.3 ± 0.7 | 37.7 ± 0.9 | 41.4 ± 2.3 | 40.5 ± 2.4 | 42.8 ± 1.2 | 42.3 ± 2.3 | 42.4 ± 1.0 | 50.3 ± 1.4∼ |
| NSP (/10) | 0.3 ± 0.1 | 0.4 ± 0.2 | 3.6 ± 1.4† | 1.8 ± 1.3 | 4.0 ± 1.3† | 4.2 ± 1.2 | 2.5 ± 0.9† | 3.0 ± 1.3 |
| NDS (/10) | 1.1 ± 0.3 | 1.4 ± 0.2 | 3.8 ± 2.0* | 4.8 ± 2.2 | 2.5 ± 0.6* | 2.3 ± 0.5 | 3.5 ± 1.4* | 1.6 ± 1.6 |
| VPT (V) | 8.4 ± 1.5 | 9.1 ± 1.8 | 19.3 ± 3.5* | 24.2 ± 7.1 | 13.5 ± 3.4* | 13.3 ± 2.6 | 16.9 ± 3.3* | 17.3 ± 3.5 |
| SNCV (m/s) | 49.0 ± 1.1 | 46.0 ± 1.6 | 47.9 ± 2.6 | 44.2 ± 3.2 | 50.8 ± 1.4 | 46.3 ± 1.9 | 50.1 ± 2.3 | 47.1 ± 2.0 |
| SNAP (μV) | 15.3 ± 1.8 | 13.2 ± 2.7 | 8.8 ± 2.6 | 6.3 ± 2.0 | 12.5 ± 1.5 | 11.3 ± 1.7 | 11.0 ± 2.5 | 12.0 ± 3.4 |
| PMNCV (m/s) | 46.5 ± 1.0 | 45.0 ± 1.4 | 44.1 ± 2.4 | 44.5 ± 2.2 | 45.9 ± 1.2 | 45.7 ± 2.5 | 44.7 ± 1.2 | 44.1 ± 1.0 |
| PMNA (mV) | 5.2 ± 0.4 | 5.3 ± 0.3 | 3.2 ± 0.6 | 3.2 ± 0.6 | 3.8 ± 0.4 | 3.8 ± 0.4 | 4.6 ± 0.6 | 4.2 ± 0.6 |
| CT (°C) | 27.9 ± 1.1 | 27.6 ± 0.4 | 22.9 ± 1.0 | 20.3 ± 3.9 | 29.2 ± 1.6 | 26.5 ± 0.6 | 24.8 ± 2.5 | 25.0 ± 1.4 |
| WT (°C) | 39.5 ± 1.1 | 38.8 ± 0.6 | 42.8 ± 1.9 | 41.5 ± 2.5 | 38.8 ± 1.7 | 40.1 ± 1.2 | 39.6 ± 1.1 | 40.1 ± 1.0 |
| IENFD (no./mm) | 8.5 ± 0.6 | 6.5 ± 1.1 | 3.0 ± 0.4∼ | 6.7 ± 1.1 | 2.8 ± 0.3∼ | 6.5 ± 1.2 | 3.9 ± 0.9∼ | |
| MDL (μm) | 63.0 ± 4.2 | 25.1 ± 3.7† | 27.5 ± 4.2 | 27.9 ± 2.1† | 29.3 ± 3.8 | 21.9 ± 2.1† | 16.5 ± 0.3∼ | |
| CNFD (no./mm2) | 30.7 ± 1.5 | 29.7 ± 1.4 | 25.4 ± 1.9 | 29.8 ± 1.5* | 28.6 ± 1.5 | 27.9 ± 1.3 | 20.0 ± 2.2^ | 18.3 ± 1.7 |
| CNBD (no./mm2) | 37.0 ± 2.7 | 39.0 ± 3.2 | 29.3 ± 5.6 | 44.9 ± 6.2# | 38.8 ± 3.7 | 38.7 ± 3.1 | 25.6 ± 5.2# | 21.8 ± 3.9 |
| CNFL (mm/mm2) | 20.4 ± 3.14 | 19.2 ± 0.9 | 15.7 ± 1.3 | 17.2 ± 0.9* | 16.8 ± 0.8 | 16.2 ± 0.6 | 13.7 ± 1.2# | 11.8 ± 1.0+ |
Data are means ± SEM. All symbols represent statistically significant differences.
∼P = 0.02,
#P = 0.04,
*P = 0.05,
^P = 0.003,
+P = 0.0006,
†P < 0.0001, baseline vs. control or baseline vs. 36 months.
Figure 1.
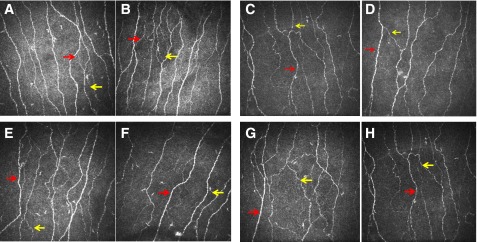
Corneal confocal images from control subject at baseline (A), control subject at follow-up (B), IGT subject who developed type 2 diabetes at baseline (C), IGT subject who developed type 2 diabetes at follow-up (D), IGT subject who remained IGT at baseline (E), IGT subject who remained IGT at follow-up (F), IGT subject who reverted to NGT at baseline (G), and IGT subject who reverted to NGT at follow-up (H). Red arrow, corneal nerve fiber; yellow arrow, corneal nerve branch.
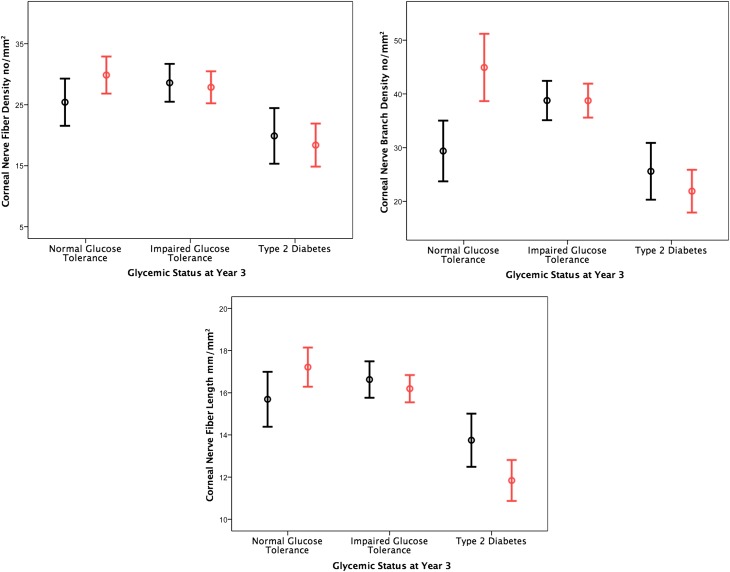
In the 10 subjects who developed type 2 diabetes, their baseline CNFD (20.0 ± 2.2 vs.30.7 ± 1.5 no./mm2, P = 0.003), CNBD (25.6 ± 5.2 vs. 37.0 ± 2.7 no./mm2, P = 0.04), and CNFL (13.7 ± 1.2 vs. 20.4 ± 3.2 mm/mm2, P = 0.04) were significantly lower compared with control subjects. Over 36 months, there was a significant increase in HbA1c (42.4 ± 1.0 vs. 50.3 ± 1.4 mmol/mol, P = 0.02) and a significant decrease in CNFL (13.7 ± 1.2 vs. 11.8 ± 1.0 mm/mm2, P = 0.006), MDL (21.9 ± 2.1 vs. 16.5 ± 0.32 μm, P = 0.02), and IENFD (6.5 ± 1.2 vs. 3.9 ± 0.9 no./mm, P = 0.002), with no significant change in any other measure of neuropathy (Fig. 2). Of the IGT subjects who had a significant (a CNFD value <2 SD below the mean for control subjects) reduction in CNFD at baseline, 87.5% developed type 2 diabetes and 12.5% remained IGT or reverted to NGT (P = 0.007). In subjects who had a significant (a CNFL value <2 SD below the mean for control subjects) reduction in CNFL, 100% developed type 2 diabetes (P < 0.0001).
Figure 2.
Change in corneal nerve fiber morphological parameters in subjects at baseline (black) and 36 months (red).
In the 15 IGT subjects who remained IGT, their baseline CNFD (28.6 ± 1.5 vs. 30.7 ± 1.5 no./mm2, P = 0.33), CNBD (38.8 ± 3.7 vs. 37.0 ± 2.7 no./mm2, P = 0.54), and CNFL (16.8 ± 0.8 vs. 20.4 ± 3.2 mm/mm2, P = 0.75) were comparable with control subjects. There was a significant reduction in IENFD (6.7 ± 1.1 vs. 2.8 ± 0.3 no./mm, P = 0.02) with no change in any measure of neuropathy over 36 months.
In the 5 subjects who became NGT, baseline CNFD (25.4 ± 1.9 vs. 30.7 ± 1.5 no./mm2, P = 0.06), CNBD (29.3 ± 5.6 vs. 37.0 ± 2.7 no./mm2, P = 0.07), and CNFL (15.7 ± 1.3 vs. 20.4 ± 3.2 mm/mm2, P = 0.24) did not differ from control subjects. However, there was a significant increase in CNFD (25.4 ± 1.9 vs. 29.8 ± 1.5 no./mm2 P = 0.05), CNBD (29.3 ± 5.6 vs. 44.9 ± 6.2 no./mm2, P = 0.04), and CNFL (15.7 ± 1.3 vs. 17.2 ± 0.9 mm/mm2, P = 0.05). There was a significant decrease in IENFD (6.5 ± 1.1 vs. 3.0 ± 0.4 no./mm, P = 0.02) with no significant change in any other measure of neuropathy over 36 months (Table 2 and Fig. 2).
Conclusions
The association between peripheral neuropathy (PN) and IGT remains controversial. Hughes et al. (25) found that in 50 consecutive subjects with PN and 50 consecutive control subjects, there was no significant difference in the prevalence of IGT, but in the PN group serum triglycerides were significantly higher. Fujimoto et al. (26) showed that subjects with IGT had comparable nerve conduction studies but had a greater prevalence of retinopathy and nephropathy compared with control subjects. More recently, Dyck et al. (27) showed that the frequency of PN was comparable in healthy subjects (1.7%) and subjects with impaired glycemia (2.0%) and was only increased in those with type 2 diabetes (7.8%). In a cohort of 393 subjects, Ziegler et al. (28) found that there was an increased prevalence of polyneuropathy in those with IGT (13%) compared with those with impaired fasting glycemia (11.3%) and control subjects (7.4%), although this was not significant. These findings may be attributed to the fact that neuropathy was diagnosed by assessing predominantly large fibers (13,29). Indeed, there are accruing data to suggest that there is an increased prevalence of painful symptoms (30–32) and evidence of a small-fiber neuropathy in subjects with IGT (10,11,16,32). Thus, small-fiber neuropathy may be the earliest change in the spectrum of PN, with injury beginning in the small myelinated Aδ and unmyelinated C fibers, which over time progresses to affect larger nerves (33).
While IENFD is accepted as the gold standard for quantifying IENF pathology, interestingly, Pittenger et al. (34) showed that MDL was reduced before IENFD in subjects with metabolic syndrome and may therefore be an early marker of sensory neuropathy. Our data support these findings, as MDL was significantly reduced, while IENFD was comparable in the IGT cohort compared with control subjects at baseline. Furthermore, MDL appears to be more responsive to changes in glucose tolerance status with a further worsening in only those IGT subjects who developed type 2 diabetes, while IENFD showed a reduction in all three groups.
In relation to causal factors, Pittenger et al. (34) also reported a correlation between PN and HDL cholesterol. In the current study, we show that HDL cholesterol was lower in the IGT group compared with the control subjects; however, this was not associated with lower CCM measures. In an 18 week open-label trial, Boyd at al. (35) showed that treatment with topiramate resulted in a significant improvement in MDL at the forearm and proximal leg and an increase in IENFD at the proximal leg.
Smith et al. (11) have shown that a 1-year diet and lifestyle intervention program leads to an increase in IENFD. However, the much larger Da Qing study showed that lifestyle intervention over 6 years reduced the incidence of severe retinopathy but had no impact on neuropathy, although the end point was monofilament insensitivity (36). More recently, a 6-month twice weekly individualized exercise program significantly improved the rate of cutaneous nerve regeneration in a capsaicin nerve ablation model (18). Our recent study in patients with type 1 diabetes undergoing simultaneous pancreas kidney transplantation showed that CCM can detect small-fiber regeneration as early as 6 months postsurgery (37). We have also shown that improvement in glycemia as well as blood pressure and lipids leads to corneal nerve regeneration (19). This leads to the notion that if there is an improvement in glycemia, then it may improve neuropathy. In the current study, we show that subjects with IGT have evidence of small-fiber neuropathy, as evidenced by a greater prevalence of painful symptoms and abnormalities in CCM as well as a reduction in MDL—in keeping with our recent study (16). However, we now show that patients who progress to type 2 diabetes have worse baseline corneal nerve morphology and MDL at a time when they are diagnosed with IGT. This is in keeping with a recent study showing that subjects with NGT, but with abnormal electrochemical sweat conductance, have a significantly increased odds ratio for the development of IGT (17). Furthermore, subjects who progressed to type 2 diabetes also showed a further significant reduction in CNFL and MDL. In subjects who remained with IGT, there was no baseline loss or any change over time. In subjects who reverted to NGT, the baseline CCM values did not differ significantly from control subjects, and indeed there was a significant increase in all CCM parameters. While this is a small study, the detailed quantification, particularly of the small fibers, provides insights into the dynamic relationship between small-fiber damage and repair in relation to overall glucose tolerance status.
We confirm the data from our previous study showing that small-fiber neuropathy, detected using CCM, is prevalent in subjects with IGT (16). More importantly, both CCM and MDL appear to be early and dynamic markers of small-fiber neuropathy, which may allow risk stratification of subjects with IGT who are likely to progress to type 2 diabetes.
Article Information
Acknowledgments. This research was facilitated by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network and undertaken in the National Institute for Health Research Wellcome Clinical Research Facility.
Funding. This research was funded by an award from the National Institutes of Health (R1-05991).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A. researched data, performed analysis, and wrote the manuscript. M.F., I.N.P., G.P., U.A., H.F., O.A., A.M., A.J.A., and W.J. researched data. A.J.M.B. reviewed the manuscript. M.T. researched data. M.J. researched data and reviewed the manuscript. R.A.M. designed the study, reviewed and revised the manuscript, and was principal investigator of the study. R.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, et al. IDF Diabetes Atlas. 2013
- 2.Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ 2014;349:g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes 2003;52:2867–2873 [DOI] [PubMed] [Google Scholar]
- 5.Manley S, Meyer L, Neil H, Ross I, Turner R, Holman R. UK Prospective Diabetes Study 6. Complications in newly diagnosed type 2 diabetic patients and their association with different clinical and biochemical risk factors. Diabetes Res 1990;13:1–11 [PubMed] [Google Scholar]
- 6.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993;43:817–824 [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D, Papanas N, Zhivov A, et al.; German Diabetes Study (GDS) Group . Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014;63:2454–2463 [DOI] [PubMed] [Google Scholar]
- 8.Putz Z, Tabák ÁG, Tóth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012;17(Suppl. 2):15–21 [DOI] [PubMed] [Google Scholar]
- 10.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology 2001;57:1701–1704 [DOI] [PubMed] [Google Scholar]
- 11.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29:1294–1299 [DOI] [PubMed] [Google Scholar]
- 12.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111 [DOI] [PubMed] [Google Scholar]
- 13.Dahlin LB, Thrainsdottir S, Cederlund R, et al. Vibrotactile sense in median and ulnar nerve innervated fingers of men with Type 2 diabetes, normal or impaired glucose tolerance. Diabet Med 2008;25:543–549 [DOI] [PubMed] [Google Scholar]
- 14.Sundkvist G, Dahlin LB, Nilsson H, et al. Sorbitol and myo-inositol levels and morphology of sural nerve in relation to peripheral nerve function and clinical neuropathy in men with diabetic, impaired, and normal glucose tolerance. Diabet Med 2000;17:259–268 [DOI] [PubMed] [Google Scholar]
- 15.Pourhamidi K, Dahlin LB, Englund E, Rolandsson O. No difference in small or large nerve fiber function between individuals with normal glucose tolerance and impaired glucose tolerance. Diabetes Care 2013;36:962–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care 2014;37:2643–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller G, Parfentyeva E, Olschewsky J, Bornstein SR, Schwarz PE. Assessment of small fiber neuropathy to predict future risk of type 2 diabetes. Prim Care Diabetes 2013;7:269–273 [DOI] [PubMed] [Google Scholar]
- 18.Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol 2015;77:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 2011;28:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013;62:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care 2015;38:e3–e4 [DOI] [PubMed] [Google Scholar]
- 22.Lauria G, Hsieh ST, Johansson O, et al.; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912, e44–9
- 23.Petropoulos IN, Alam U, Fadavi H, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci 2014;55:2071–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal 2011;15:738–747 [DOI] [PubMed] [Google Scholar]
- 25.Hughes RA, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004;127:1723–1730 [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes 1987;36:730–739 [DOI] [PubMed] [Google Scholar]
- 27.Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care 2012;35:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve 2007;36:536–541 [DOI] [PubMed] [Google Scholar]
- 30.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400 [DOI] [PubMed]
- 31.Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A; KORA Study Group . Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009;13:582–587 [DOI] [PubMed] [Google Scholar]
- 32.Rajabally YA. Neuropathy and impaired glucose tolerance: an updated review of the evidence. Acta Neurol Scand 2011;124:1–8 [DOI] [PubMed] [Google Scholar]
- 33.Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014;37:1418–1424 [DOI] [PubMed] [Google Scholar]
- 34.Pittenger GL, Mehrabyan A, Simmons K, et al. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord 2005;3:113–121 [DOI] [PubMed] [Google Scholar]
- 35.Boyd AL, Barlow PM, Pittenger GL, Simmons KF, Vinik AI. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2010;3:431 [DOI] [PMC free article] [PubMed]
- 36.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 37.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 2007;30:2608–2612 [DOI] [PubMed] [Google Scholar]