Compared to 3TC/ZDV+EFV (N=519), participants randomized to FTC/TDF+EFV (N=526) experienced significantly greater increases in weight, mid-arm, mid-thigh, waist, and hip circumferences, and no lipoatrophy cases. 38-42% of participants in both arms were overweight/obese at 144 weeks.
Keywords: anthropomorphics, antiretroviral therapy, highly active, HIV, lipodystrophy, obesity
Abstract
Background. Existing data on anthropomorphic changes in resource-limited settings primarily come from observational or cross-sectional studies. Data from randomized clinical trials are needed to inform treatment decisions in these areas of the world.
Methods. The AIDS Clinical Trials Group Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study was a prospective, randomized evaluation of the efficacy of emtricitabine/tenofovir + efavirenz (FTC/TDF + EFV) vs lamivudine/zidovudine + efavirenz (3TC/ZDV + EFV) for the initial treatment of human immunodeficiency virus (HIV)-1-infected individuals from resource-diverse settings. Changes in anthropomorphic measures were analyzed using mixed-effect models for repeated measurements, using all available measurements at weeks 48, 96, and 144. Intent-to-treat results are presented; as-treated results were similar.
Results. Five hundred twenty-six participants were randomized to FTC/TDF + EFV, and 519 participants were randomized to 3TC/ZDV + EFV. Significantly greater increases from baseline to week 144 were seen among those randomized to FTC/TDF + EFV vs 3TC/ZDV + EFV in all measures except waist-to-hip ratio, with the following mean changes: weight, 4.8 vs 3.0 kg; body mass index, 1.8 vs 1.1 kg/m2; mid-arm, 1.7 vs 0.7 cm; waist, 5.2 vs 4.3 cm; hip, 3.8 vs 1.4 cm; and mid-thigh circumference, 3.1 vs 0.9 cm. There were 7 clinical diagnoses of lipoatrophy in the 3TC/ZDV + EFV arm compared with none in the FTC/TDF + EFV arm. The proportion of overweight or obese participants increased from 25% (week 0) to 42% (week 144) for FTC/TDF + EFV and from 26% to 38% for 3TC/ZDV + EFV.
Conclusions. Our findings support first-line use of FTC/TDF + EFV in resource-limited settings and emphasize the need for interventions to limit weight gain among overweight or obese HIV-infected participants in all settings.
The 2013 World Health Organization (WHO) guidelines recommend an initial antiretroviral regimen of once-daily tenofovir (TDF) with lamivudine (3TC) or emtricitabine (FTC) plus efavirenz (EFV) because of high efficacy and fewer side effects compared with zidovudine (ZDV)-containing regimens [1]. Despite these recommendations, 13 of 58 (22%) low- or medium-income focus countries have not adopted TDF + 3TC (or FTC) + EFV as the preferred first-line antiretroviral regimen and ZDV- or stavudine (d4T)-containing regimens are still used [2].
Changes in anthropomorphic measures occur with antiretroviral therapy (ART), including loss of subcutaneous fat (lipoatrophy) and an accumulation of central fat (lipohypertrophy) [3, 4]. In a randomized clinical trial conducted in high-income countries with a majority of male participants, a regimen of TDF + FTC + EFV had more favorable effects on subcutaneous fat compared with a regimen of ZDV/3TC + EFV[5–7]. Anthropomorphic changes in resource-limited settings could differ from what has been observed in resource-rich settings because of differences in race and gender, access to care, physical activity, and diet. Existing data on anthropomorphic changes in resource-limited settings primarily come from observational studies or studies of d4T [8–13], and additional data from randomized clinical trials are needed to inform treatment decisions in these areas of the world. The goal of the current study was to investigate the effect of randomly assigned treatment with coformulated FTC/TDF + EFV versus coformulated 3TC/ZDV +EFV on anthropomorphic changes in a multinational clinical trial; these 2 regimens had similar effects on a composite outcome of virologic failure, disease progression, or death [14].
METHODS
The AIDS Clinical Trials Group (ACTG) Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS; ClinicalTrials.gov NCT00084136) study was a phase IV, prospective, randomized, open-label evaluation of the efficacy of once-daily protease inhibitor and once-daily nonnucleoside reverse-transcriptase inhibitor antiretroviral combinations for the initial treatment of human immunodeficiency virus (HIV)-1-infected individuals from 9 countries: Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, United States, and Zimbabwe. Details and primary outcomes of this study have been previously reported [14]. Enrollment occurred between May 2005 and July 2007, and participants were observed to April or May 2010. The participants were ≥18 years old, had documented HIV-1 infection, had received no more than 7 days of cumulative prior ART (prior use of single-dose nevirapine or ZDV for any duration to prevent mother-to-child transmission of HIV was allowed), and had a CD4 cell count <300 cells/µL within 90 days prior to entry into the study.
The results presented here are for a prespecified secondary objective concerning the difference in changes in anthropometric measures after starting 3TC/ZDV + EFV versus FTC/TDF + EFV. The Data and Safety Monitoring Board recommended stopping treatment early with a third regimen (didanosine-EC +FTC + atazanavir) due to inferiority; these participants are not included here. Anthropometric measurements including weight and mid-arm, mid-thigh, waist, and hip circumferences were evaluated at entry (immediately before starting ART) and at weeks 48, 96, and 144 (within ±6 weeks); height was measured at entry only. Body circumferences were measured using a standard protocol including 3 measurements at each visit; the median of the measurements was used in analyses. Body mass index (BMI) was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), and overweight or obese (≥25 kg/m2) [15]. Waist circumference >94 cm for males or >80 cm for females and waist-to-hip ratio (WHR) ≥0.90 for males and ≥ 0.85 for females were considered “high-risk” markers of metabolic complications by WHO standards [15]. Lipoatrophy was first noted by a physician, with fat changes confirmed by the study participant.
Written informed consent was obtained from all participants, and the human experimentation guidelines of the US Department of Health and Human Services were followed. The study was approved by local Ethics Committees at each participating institution.
Changes in anthropomorphic measures were analyzed using mixed-effect models for repeated measurements, ignoring any change in ART and using all available measurements at weeks 48, 96, and 144. Additional as-treated analyses were conducted with similar results. Analyses of whether the difference between treatments varied by sex or by baseline BMI category were conducted by including subgroup by treatment interaction terms in the models. A P value of < .05 was considered statistically significant. No adjustment was made for multiple comparisons.
RESULTS
One thousand forty-five subjects were randomized to receive FTC/TDF + EFV (n = 526) or 3TC/ZDV + EFV (n = 519). At entry, median age was 34 years, and 46% of participants were female (Table 1). Median CD4 count and HIV-1 RNA were 167 cells/µL and 5.0 log10 copies/mL, respectively. At entry, 9% of participants were underweight and 26% were overweight or obese; 22% had high-risk waist circumferences and 38% had high-risk WHR. Weight measurements were available for 973, 941, and 902 subjects at weeks 48, 96, and 144, respectively. Differences in the BMI categories by country are shown in Table 2. Thirty-five subjects died, 106 were lost to follow-up before week 144, and 2 were in follow-up at week 144 but did not have weight measurements. A small number of subjects did not have body circumference measurements at each visit (up to 8%), usually because of lack of time during the clinic visit.
Table 1.
Baseline Characteristics of the Study Participants
| Overall N = 1045 | FTC/TDF + EFV N = 526 | 3TC/ZDV + EFV N = 519 | |
|---|---|---|---|
| Gendera | |||
| Male | 562 (54) | 284 (54) | 278 (54) |
| Female | 483 (46) | 242 (46) | 241 (46) |
| Age (years)b | 34 (29–41) | 34 (29–41) | 34 (29–40) |
| Countrya | |||
| Brazil | 155 (15) | 76 (14) | 79 (15) |
| Haiti | 68 (7) | 33 (6) | 35 (7) |
| India | 169 (16) | 88 (17) | 81 (16) |
| Malawi | 147 (14) | 73 (14) | 74 (14) |
| Peru | 86 (8) | 44 (8) | 42 (8) |
| South Africa | 140 (13) | 70 (13) | 70 (13) |
| Thailand | 67 (6) | 35 (7) | 32 (6) |
| United States | 140 (13) | 70 (13) | 70 (13) |
| Zimbabwe | 73 (7) | 37 (7) | 36 (7) |
| CD4+ count (cells/µL)b | 167 (89–228) | 162 (86–221) | 169 (92–237) |
| HIV-1 RNA (log10 copies/mL)b | 5.0 (4.6–5.4) | 5.0 (4.5–5.5) | 5.0 (4.6–5.4) |
| AIDS diagnosis (current or prior)a | 113 (11) | 55 (10) | 58 (11) |
| Weight (kg)c | 62.9 (14.0) | 62.3 (13.8) | 63.4 (14.2) |
| BMI (kg/m2)c,d | 23.2 (4.4) | 23.0 (4.4) | 23.4 (4.5) |
| Underweighta | 91 (9) | 54 (10) | 37 (7) |
| Normala | 680 (65) | 340 (65) | 340 (66) |
| Overweight or obesea | 274 (26) | 132 (25) | 142 (27) |
| Mid-arm Circumference (cm)c | 28.0 (4.0) | 27.8 (4.0) | 28.2 (4.0) |
| Mid-thigh Circumference (cm)c | 47.9 (6.6) | 47.5 (6.3) | 48.3 (6.8) |
| Waist Circumference (cm)c | 79.5 (10.2) | 79.1 (10.3) | 79.9 (10.1) |
| High-risk circumferencea,e | 222 (22) | 107 (21) | 115 (23) |
| Hip circumference (cm)c | 92.5 (10.2) | 92.0 (10.0) | 93.0 (10.3) |
| Waist-to-Hip Ratioc | 0.86 (0.08) | 0.86 (0.07) | 0.86 (0.08) |
| High-risk ratioa,f | 387 (38) | 195 (38) | 192 (38) |
Abbreviations: AIDS, acquired immune deficiency syndrome; BMI, body mass index; EFV, efavirenz; FTC, emtricitabine; TDF, tenofovir; ZDV, zidovudine; 3TC, lamivudine.
a N (%).
b Median (25th and 75th percentile).
c Mean (standard deviation).
d BMI categories: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight or obese (≥25.0 kg/m2).
e High-risk waist circumference defined as >94 cm for male or >80 cm for female.
f High-risk waist-to-hip ratio defined as ≥0.90 for male or ≥0.85 for female.
Table 2.
Frequency and Percentage of Underweight, Normal Weight, and Overweight/Obese Participants at Baseline
| Country | BMI <18.5 kg/m2 |
BMI 18.5–24.9 kg/m2 |
BMI ≥25 kg/m2 |
|---|---|---|---|
| Brazil | 9 (6%) | 90 (58%) | 56 (36%) |
| Haiti | 12 (18%) | 49 (72%) | 7 (10%) |
| India | 40 (24%) | 109 (65%) | 20 (12%) |
| Malawi | 14 (10%) | 112 (76%) | 21 (14%) |
| Peru | 0 (0) | 62 (72%) | 24 (28%) |
| South Africa | 3 (2%) | 81 (58%) | 56 (40%) |
| Thailand | 7 (10%) | 48 (72%) | 12 (18%) |
| United States | 3 (2%) | 71 (51%) | 66 (47%) |
| Zimbabwe | 3 (4%) | 58 (79%) | 12 (16%) |
Abbreviations: BMI, body mass index.
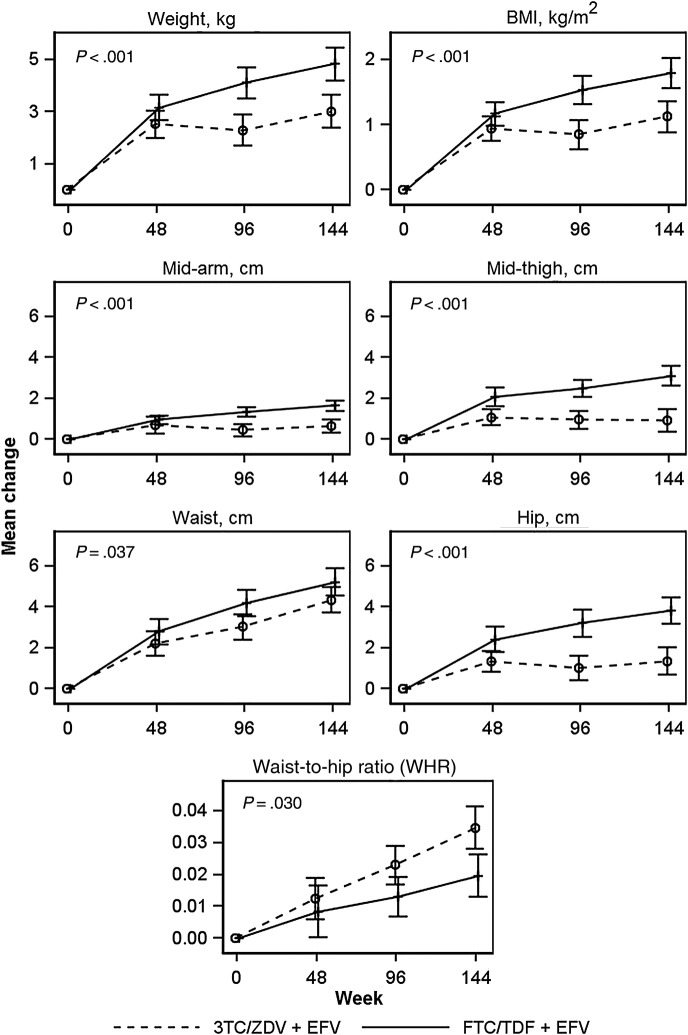
All anthropomorphic measures increased significantly from baseline to week 48, 96, and 144 in both study arms (Figure 1). Significantly greater increases from baseline to week 144 were seen among those randomized to FTC/TDF + EFV compared with 3TC/ZDV + EFV in all measures except WHR, with the following mean changes: weight, 4.8 vs 3.0 kg; BMI, 1.8 vs 1.1 kg/m2; mid-arm circumference, 1.7 vs 0.7 cm; waist circumference, 5.2 vs 4.3 cm; hip circumference, 3.8 vs 1.4 cm; and mid-thigh circumference, 3.1 vs 0.9 cm (Figure 1). Participants in the FTC/TDF + EFV arm had significantly less gain in WHR from baseline to week 144 than in the 3TC/ZDV + EFV arm (WHR, 0.021 vs 0.029; Figure 1).
Figure 1.
Mean changes in anthropomorphic measures by randomized treatment from week 0 to week 144. P values represent repeated measures analyses across all study weeks, and bars are 95% confidence intervals.
Among subjects observed to 144 weeks, the proportion of overweight or obese participants increased from 25% (week 0) to 42% (week 144) for FTC/TDF + EFV and from 26% to 38% for 3TC/ZDV + EFV. Among participants with a normal or underweight BMI at baseline, 25% in the FTC/TDF + EFV and 18% in the 3TC/ZDV + EFV arm became overweight or obese by 144 weeks. Among participants with a normal baseline waist circumference, 24% and 19% of FTC/TDF + EFV and 3TC/ZDV + EFV participants, respectively, developed a high-risk waist circumference by week 144; 33% and 46% of FTC/TDF + EFV and 3TC/ZDV + EFV participants, respectively, developed a high-risk WHR. There were no clinical diagnoses of lipoatrophy in the FTC/TDF + EFV arm and 7 in the 3TC/ZDV + EFV arm.
The difference in mean BMI change between treatment arms did not vary significantly among baseline BMI categories (repeated measures interaction, P = .49). Overall, underweight participants showed increases in BMI by week 144 (mean 2.0 kg/m2 and 1.7 kg/m2 for FTC/TDF + EFV and 3TC/ZDV + EFV, respectively), as did overweight participants (1.8 and 0.6 kg/m2, respectively), and participants with normal baseline BMI (1.8 and 1.3 kg/m2, respectively). The difference between treatments in mean changes in waist circumference varied by sex (repeated measures interaction, P = .038); men assigned to FTC/TDF +EFV had a significantly greater increase in waist circumference compared with men assigned to 3TC/ZDV + EFV, whereas the mean change in women was similar for the 2 treatment arms. No significant sex interactions were detected in other anthropomorphic measures. In addition, there was no significant evidence that differences between treatments varied by country.
DISCUSSION
In this study, we present results from the first analysis of changes in weight and anthropomorphic measures in a randomized, controlled trial of ART initiation in resource-diverse settings. Similar to findings in resource-rich settings [5–7], FTC/TDF +EFV and 3TC/ZDV + EFV were both associated with mean gains in body weight, likely representing a return-to-health. Furthermore, assignment to FTC/TDF + EFV produced significantly greater mean gains in weight, BMI, and waist, hip, mid-arm, and mid-thigh circumference compared with 3TC/ZDV + EFV. A greater waist circumference increase was found among men assigned to FTC/TDF + EFV compared with 3TC/ZDV + EFV. Although this result is intriguing, we are uncertain whether a biologic mechanism exists that might explain this finding (and the lack of a difference between men and women for other anthropomorphic outcomes).
All clinical diagnoses of lipoatrophy were in the 3TC/ZDV +EFV arm, and the minimal mean increase in mid-arm or mid-thigh circumference in this arm by week 144 suggests the presence of additional subclinical lipoatrophy, as has been previously shown (5, 16). The AIDS Clinical Trials Group Study A5142 demonstrated that 96 weeks of EFV and TDF combined with 3TC or FTC resulted in smaller losses in extremity fat (by dual-energy x-ray absorptiometry [DEXA]) than EFV and either ZDV or d4T with 3TC or FTC [16]. Likewise, in Gilead 934, where a subset of participants also had DXA (N = 86), the FTC/TDF + EFV arm had significantly greater gain in limb fat compared with the 3TC/ZDV + EFV arm after 144 weeks [5]. The greater gain in mean WHR among those randomized to 3TC/ZDV + EFV in the present study may represent a smaller gain of subcutaneous fat in the hips with a proportionately greater accumulation of visceral fat in the waist, although this is speculation without a computed tomography (CT) image to verify the fat location. The anthropomorphic changes observed here are consistent with both lipoatrophy and lipohypertrophy and are associated with adverse metabolic effects including heightened cardiovascular risk beyond that observed with obesity alone [17–19]. Furthermore, both smaller limb and larger waist circumference have been associated with increased mortality among persons infected with HIV [20].
Another notable finding of our study is the large proportion of participants who were overweight or obese within both study arms. Worldwide, more than 35% of adults are overweight and more than 11% are obese, with many resource-limited countries experiencing the burden of both obesity and undernutrition simultaneously [21]. Although it is traditionally considered a disease of wasting, persons with HIV are also experiencing an increasing prevalence of obesity with up to 65% of HIV-infected persons in the United States overweight or obese [22–27]. Over one quarter of participants in our study were overweight or obese at entry, even with a median CD4 count <200 and 11% with a current or prior acquired immune deficiency syndrome (AIDS) diagnosis; approximately 40% of participants were overweight or obese by week 144. These findings are consistent with prior observational studies on anthropomorphic changes in some resource-limited settings [28]. A retrospective study of HIV-infected participants in Brazil demonstrated an increase in the percentage of overweight or obese persons from 36% to 44% over a period of 1–7 years [29]. Among South African HIV-infected participants initiating ART, the proportion of overweight or obese participants increased from 33% to 58% at 12 months [30]. It is interesting to note that, although the self-perception of a central fat gain has been associated with ART nonadherence in HIV-infected US women [31], weight gain was viewed favorably in a South African cohort as a marker of prosperity and good health [28, 30]. Persistent discrimination against persons infected with HIV and a strong cultural belief that underweight body type is associated with HIV may drive obesity among individuals infected with HIV, whereas intentional obesity limits the stigma associated with HIV. Indeed, among women in South Africa, 70% of women associated an underweight body type with HIV/AIDS, whereas none of the women associated an overweight body type with HIV/AIDS [32]. Given that a large study of body composition and HIV in the United States demonstrated that increased central fat was associated with greater 5-year mortality [20], the importance of obesity prevention and treatment in resource-limited settings should be emphasized in treatment guidelines. Research on interventions to limit weight gain among overweight or obese HIV-infected persons initiating ART in diverse settings is needed.
The main strength of our study is the randomized assignment of ART, permitting a direct comparison of the 2 regimens without the bias of regimen availability, provider choice, or personal preference. Randomization resulted in similar demographics between treatment arms [14] and, therefore, limited residual confounding. The choice of 2 ART regimens that are commonly used in resource-limited settings together with the diversity of our study population, including geographic, sex, race or ethnicity, socioeconomic, and nutritional variation, adds to the generalizability of our findings. It is important to note that the entry criteria for our clinical trial could have resulted in enrollment of a study population that is not representative of all HIV-infected persons in resource-limited settings, and it may have excluded participants with fewer resources and greater food insecurity. Participants may have been healthier or judged likely to be more compliant with therapy than HIV-infected individuals who did not enroll. At the time our study was conducted, we were unable to obtain imaging by DEXA or CT imaging for visceral fat at most of the study sites, thus our findings are based on anthropomorphic measurements. Prior studies have demonstrated the usefulness of anthropomorphic measurements in the clinical diagnosis of lipoatrophy or lipohypertrophy [33–35]. Waist circumference or WHR performed similarly to magnetic resonance imaging-measured regional adipose tissue in predicting metabolic syndrome among both HIV-infected and -uninfected persons [36]; however, these associations can vary by race or ethnicity and gender [15]. Physical activity and diet were not collected and therefore could not be incorporated into this analysis.
CONCLUSIONS
In summary, initiation of ART with FTC/TDF + EFV was associated with greater gains in nearly all anthropomorphic measures compared with 3TC/ZDV + EFV. The significantly greater gain in WHR and minimal increase in mid-arm and mid-thigh circumference in the 3TC/ZDV + EFV arm provides evidence that anthropomorphic changes previously demonstrated in studies conducted in the United States and European countries [5] also occur in more globally diverse populations. Loss of subcutaneous fat can be associated with adverse metabolic outcomes, poor adherence, and reduced quality of life [19, 37, 38], thus the anthropomorphic changes observed in our study support current World Health Organization recommendations favoring FTC/TDF + EFV over 3TC/ZDV + EFV as the initial antiretroviral regimen in resource-limited settings. Because uptake of these ART recommendations in many low- and middle-income countries has been low [2], we anticipate that the results presented here will help to promote wider implementation in these settings.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. In addition, K. M. E. received support by the National Institute of Aging of the National Institutes of Health under Award Number K23AG050260. Gilead Sciences, Inc. provided emtricitabine, tenofovir-DF, emtricitabine/tenofovir-DF, and financial support for purchase of other study drugs. GlaxoSmithKline provided lamivudine, zidovudine, and lamivudine/zidovudine.
Potential conflicts of interest. M. D. H. serves on data monitoring committees for Boehringer Ingelheim, Pfizer, and Tibotec. L. S. serves on a data monitoring committee for Pfizer. A. G. has received research funding from the Gilead Foundation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at: www.who.int/hiv/pub/guidelines/arv2013/download/en/ Accessed 13 October 2014.
- 2.World Health Organization. Global update on the health sector response to HIV. Available at: www.who.int/hiv/pub/progressreports/update2014/en/ Accessed 13 October 2014.
- 3.McComsey GA, Kitch D, Sax PE et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis 2011; 53:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shikuma CM, Zackin R, Sattler F et al. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis 2004; 39:1223–30. [DOI] [PubMed] [Google Scholar]
- 5.Arribas JR, Pozniak AL, Gallant JE et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr 2008; 47:74–8. [DOI] [PubMed] [Google Scholar]
- 6.Pozniak AL, Gallant JE, DeJesus E et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immune Defic Syndr 2006; 43:535–40. [DOI] [PubMed] [Google Scholar]
- 7.Gallant JE, DeJesus E, Arribas JR et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354:251–60. [DOI] [PubMed] [Google Scholar]
- 8.Thompson V, Medard B, Taseera K et al. Regional anthropometry changes in antiretroviral-naive persons initiating a Zidovudine-containing regimen in Mbarara, Uganda. AIDS Res Hum Retroviruses 2011; 27:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudfeld CR, Isanaka S, Mugusi FM et al. Weight change at 1 mo of antiretroviral therapy and its association with subsequent mortality, morbidity, and CD4 T cell reconstitution in a Tanzanian HIV-infected adult cohort. Am J Clin Nutr 2013; 97:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George JA, Venter WD, Van Deventer HE, Crowther NJ. A longitudinal study of the changes in body fat and metabolic parameters in a South African population of HIV-positive patients receiving an antiretroviral therapeutic regimen containing stavudine. AIDS Res Hum Retroviruses 2009; 25:771–81. [DOI] [PubMed] [Google Scholar]
- 11.Innes S, Cotton MF, Haubrich R et al. High prevalence of lipoatrophy in pre-pubertal South African children on antiretroviral therapy: a cross-sectional study. BMC Pediatr 2012; 12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menezes CN, Crowther NJ, Duarte R et al. A randomized clinical trial comparing metabolic parameters after 48 weeks of standard- and low-dose stavudine therapy and tenofovir disoproxil fumarate therapy in HIV-infected South African patients. HIV Med 2014; 15:3–12. [DOI] [PubMed] [Google Scholar]
- 13.Ludy MJ, Hendricks K, Houser R et al. Body composition in adults infected with human immunodeficiency virus in Khon Kaen, Thailand. Am J Trop Med Hyg 2005; 73:815–9. [PubMed] [Google Scholar]
- 14.Campbell TB, Smeaton LM, Kumarasamy N et al. efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr 2010; 64:2–5. [DOI] [PubMed] [Google Scholar]
- 16.Haubrich RH, Riddler SA, DiRienzo AG et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009; 23:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallon PW, Wand H, Law M et al. Buffalo hump seen in HIV-associated lipodystrophy is associated with hyperinsulinemia but not dyslipidemia. J Acquir Immune Defic Syndr 2005; 38:156–62. [DOI] [PubMed] [Google Scholar]
- 18.Koethe JR, Hulgan T, Niswender K. Adipose tissue and immune function: a review of evidence relevant to HIV infection. J Infect Dis 2013; 208:1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond E, McKinnon E, Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: prevalence and metabolic consequences. Clin Infect Dis 2010; 51:591–9. [DOI] [PubMed] [Google Scholar]
- 20.Scherzer R, Heymsfield SB, Lee D et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS 2011; 25:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO global strategy on diet, physical activity and health: Fact sheet on obesity and overweight, No 311. Available at: www.who.int/mediacentre/factsheets/fs311/en/ Accessed 13 October 2014.
- 22.Crum-Cianflone N, Roediger MP, Eberly L et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boodram B, Plankey MW, Cox C et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women's Interagency HIV Study. AIDS Patient Care STDS 2009; 23:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crum-Cianflone N, Tejidor R, Medina S et al. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS 2008; 22:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchacz K, Baker RK, Palella FJ Jr et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013; 18:65–75. [DOI] [PubMed] [Google Scholar]
- 26.Wand H, Ramjee G. High prevalence of obesity among women who enrolled in HIV prevention trials in KwaZulu-Natal, South Africa: healthy diet and life style messages should be integrated into HIV prevention programs. BMC Public Health 2013; 13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontbonne A, Cournil A, Cames C et al. Anthropometric characteristics and cardiometabolic risk factors in a sample of urban-dwelling adults in Senegal. Diabetes Metab 2011; 37:52–8. [DOI] [PubMed] [Google Scholar]
- 28.Ali MK, Magee MJ, Dave JA et al. HIV and metabolic, body, and bone disorders: what we know from low- and middle-income countries. J Acquir Immune Defic Syndr 2014; 67(Suppl 1):S27–39. [DOI] [PubMed] [Google Scholar]
- 29.Maia Leite LH, De Mattos Marinho Sampaio AB. Progression to overweight, obesity and associated factors after antiretroviral therapy initiation among Brazilian persons with HIV/AIDS. Nutr Hosp 2010; 25:635–40. [PubMed] [Google Scholar]
- 30.Hurley E, Coutsoudis A, Giddy J et al. Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting. S Afr Med J 2011; 101:645–50. [PubMed] [Google Scholar]
- 31.Plankey M, Bacchetti P, Jin C et al. Self-perception of body fat changes and HAART adherence in the Women's Interagency HIV Study. AIDS Behav 2009; 13:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matoti-Mvalo T, Puoane T. Perceptions of body size and its association with HIV/AIDS. S Afr J Clin Nutr 2011; 24:40–5. [Google Scholar]
- 33.Gerrior J, Kantaros J, Coakley E et al. The fat redistribution syndrome in patients infected with HIV: measurements of body shape abnormalities. J Am Diet Assoc 2001; 101:1175–80. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson DL, Knox T, Spiegelman D et al. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis 2005; 40:1837–45. [DOI] [PubMed] [Google Scholar]
- 35.Innes S, Schulte-Kemna E, Cotton MF et al. Biceps skin-fold thickness may detect and predict early lipoatrophy in HIV-infected children. Pediatr Infect Dis J 2013; 32:e254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherzer R, Shen W, Bacchetti P et al. Simple anthropometric measures correlate with metabolic risk indicators as strongly as magnetic resonance imaging-measured adipose tissue depots in both HIV-infected and control subjects. Am J Clin Nutr 2008; 87:1809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crane HM, Grunfeld C, Harrington RD et al. Lipoatrophy among HIV-infected patients is associated with higher levels of depression than lipohypertrophy. HIV Med 2008; 9:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guaraldi G, Murri R, Orlando G et al. Lipodystrophy and quality of life of HIV-infected persons. AIDS Reviews 2008; 10:152–61. [PubMed] [Google Scholar]