Abstract
Background
Social impairments are a hallmark feature of schizophrenia and are a key predictor of functional disability. Deficits in social information processing likely underlie social impairment; however, this relationship is understudied. We previously demonstrated that patients with schizophrenia fail to habituate to neutral faces, providing evidence for an alteration in basic social information processing. It remains unknown whether patients with schizophrenia also show deficits in processing of more complex social information. Out-group bias provides an excellent opportunity to test complex social information processing because the bias requires basic face processing skills, the ability to discriminate between groups, as well as the ability to categorize oneself into a salient social group.
Methods
Study participants were 23 patients with schizophrenia and 21 controls. Using functional magnetic resonance imaging, habituation of response to 120 seconds of repeated presentations of faces was assessed in participants who viewed either same-gender faces or opposite-gender faces. The interaction between face gender (same/opposite) and group was examined in three key regions: amygdala, hippocampus, and visual cortex. Social impairment was measured using the PANSS and correlations between social impairment and out-group effect (main effect of face type) were performed in patients.
Results
Patients with schizophrenia had aberrant neural responses to opposite-gender faces (interaction, p < .05 corrected). Healthy controls showed an immediate heightened response to opposite-gender faces relative to same-gender faces; but in patients this effect was substantially delayed (~ 70 seconds). In patients with schizophrenia, the out-group bias was significantly correlated with social impairment. Patients with no social impairment showed a heightened neural response to opposite-gender faces after 30 seconds, whereas patients with mild-moderate social impairment failed to ever show a heightened response.
Conclusion
Alterations in neural responses during out-group processing predicted degree of social impairment in patients with schizophrenia; thus, neural responses to opposite-gender faces may provide a novel measure for studies of treatment response and disease outcome.
Keywords: schizophrenia, fMRI, habituation, social functioning
1. Introduction
Deficits in the processing of social information are paramount in schizophrenia and are thought to underlie the observable deficits in social impairment. A growing body of research demonstrates that patients with schizophrenia have deficits in social information processing (Lee et al., 2013). For example, patients have a well-established deficit in emotion perception, typically demonstrated as an impaired ability to recognize or label emotional facial expressions (Chan et al., 2010), which is accompanied by reduced activation in several brain regions implicated in social and emotional information processing (Li et al., 2010). However, emotional faces confound emotion processing with processing of the social information inherent in faces. To directly investigate neural processing of a socially relevant stimulus in patients with schizophrenia, we recently measured neural response to repeated presentation of neutral faces (Williams et al., 2013).
Decreased responding to repeated presentations of a stimulus over time, or habituation, is one of the most basic learning processes and provides an ideal opportunity to observe social information processing. In controls, neural responses to repeated faces habituated over time; however, patients failed to show habituation. Importantly, the habituation deficits were specific to faces as they were not observed when participants viewed neutral objects, providing compelling evidence for a basic deficit in social information processing. Failure to habituate may represent a trans-diagnostic biomarker for social impairments as other groups characterized by social deficits, including autism and high social anxiety, also show habituation deficits to social stimuli (Blackford et al., 2013; Kleinhans et al., 2009; Swartz et al., 2013).
Human faces convey a multitude of information critical for social interactions. In addition to emotion, faces provide salient information about identity, race, and gender. This additional information is used to quickly categorize others according to salient social groups. An interesting aspect of both race and gender is that humans show “out-group” biases; that is, behaviors and neural responses that differ when viewing someone that differs from oneself on either race (Hart et al., 2000; Olsson et al., 2005) or gender (Shapiro and Penrod, 1986; Shaw and Skolnick, 1994; Wright and Sladden, 2003). For gender bias, recognition memory is lower for out-group faces relative to in-group faces (Shaw and Skolnick, 1994).
The out-group phenomenon suggests that face properties such as gender elicit a level of social information processing that goes beyond basic face processing. Out-group bias likely requires several abilities, including the ability to discriminate between groups (Dunham et al., 2013) and to categorize oneself in to a salient social group (Turner et al., 1987). Patients with schizophrenia have intact gender discrimination (Bediou et al., 2005) but models of schizophrenia (i.e. ipseity-disturbance model) propose that patients with schizophrenia lack a consciousness of self (Nelson et al., 2014), which may produce deficits in self-categorization and social impairments. The neutral faces employed in our previous study did not vary in their race (all Caucasian), but did vary in gender, such that participants viewed faces of either the same or opposite gender to themselves. This provides a unique opportunity to test for differences in the neural processing of complex social information in schizophrenia and the impact on social functioning.
In the present study, we analyzed fMRI habituation data with a focus on gender out-group effects in schizophrenia. We hypothesized that patients with schizophrenia would fail to display the typical neural pattern of out-group bias—delayed habituation to opposite-gender faces. We further predicted that within patients, deficits in social information processing would predict social impairment.
2. Methods
This study is a novel analysis of a data set previously used to examine neural habituation in schizophrenia. Here we examine the role of face gender, which was not previously analyzed, on habituation in healthy controls and patients with schizophrenia, and incorporate measures of social impairment.
2.1 Participants
Participants were 25 patients with schizophrenia (schizophrenia n = 18; schizoaffective disorder n=7) and 23 healthy controls. Patients were recruited from an academic medical center inpatient unit and outpatient clinics. Healthy controls were recruited from the local community using advertisements. Diagnosis was determined using the Structured Clinical Interview for DSM-IV. Patients with schizoaffective disorder met criteria A, B, and C for schizophrenia and clinical variables did not differ between the two patient subgroups. Participants were excluded for: history of drug or alcohol dependence, substance abuse in the past 6 months, head injury, significant medical or neurological illness, and/or uncorrected vision deficits.
All participants completed the National Adult Reading Test as a measure of premorbid IQ. Patients with schizophrenia were also assessed with the Positive and Negative Syndrome Scale (PANSS), the Hamilton Depression Rating Scale, and the Young Mania Rating Scale. To assess social impairment we used two items from the PANSS: 1) active social avoidance defined as diminished social involvement associated with unwarranted fear, hostility or distrust; and 2) passive social withdrawal defined as diminished interest and initiative in social interactions. Whether these two items represent distinct or shared constructs remains unclear as some studies find they are distinct (Hansen et al., 2013) and others show they load on a common factor (Van den Oord et al., 2006). In our sample the two items were modestly correlated (r = .48, p = .02), therefore we analyzed them separately. Both items showed sufficient range for correlation analyses: social withdrawal (minimum = 1, maximum = 6) and social avoidance (minimum = 1, maximum = 7). This research was conducted in accordance with the Vanderbilt Human Research Protection Program and all participants provided written informed consent. Participants received financial compensation.
2.2. fMRI Task
Habituation to faces was assessed using fMRI and a repeated faces task (also known as repetition suppression or functional magnetic resonance adaptation). In this task, participants reviewed one 2-minute run of the same face. The run consisted of 120 face presentations (500-ms presentation, 500-ms inter stimulus interval). A fixation cross was presented for 10-s at the beginning of the run and 20-s at the end of the run to provide a baseline. Stimuli were black and white faces with neutral expressions obtained from standard stimulus sets (Gur et al., 2001; Lundqvist et al., 1998; Minear and Park, 2004; Tottenham et al., 2009). Face gender was counterbalanced across participant gender so that half of the participants saw a face that was the same gender and half saw a face that was an opposite gender. Stimuli were presented using Eprime 2.0.Eye-movements were monitored during the MRI session to ensure participants were awake and fixating the screen. To promote and quantify attention during the task, small versions of the faces (25% of original size) were presented on 10% of the trials and detected by participants with a button press. A brief pre-scan training was used to familiarize participants with the task. Participants were excluded for detecting less than 66% of targets (2 controls, 2 patients with schizophrenia).
2.3 Final Analysis Sample Characteristics
Participant characteristics are presented in Table 1. Of the 23 patients with schizophrenia, 12 (6 female) viewed a same gender face and 11 (5 female) viewed an opposite gender face. In the sample of 21 controls, 10 (5 female) viewed a same gender face and 11 (5 female) viewed an opposite gender face. There were no significant differences in age or race by group, face gender, nor the group x face gender interaction (all p > .30). The two groups of schizophrenia patients viewing either same or opposite gender faces did not differ in education, parent education, IQ, PANSS total, PANSS active social avoidance, PANSS passive social withdrawal, depression, mania, or chlorpromazine equivalent dose.
Table 1.
Participant Characteristics
| Schizophrenia N = 23 |
Control N = 21 |
|||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Age | 43.4 | 11.8 | 42.4 | 10.0 |
| Education, years* | 13.7 | 2.5 | 15.8 | 2.3 |
| Parental education, years | 13.2 | 2.7 | 13.4 | 2.4 |
| IQ/NART score | 106.2 | 8.5 | 109.3 | 8.3 |
| PANSS-total | 51.4 | 13.0 | ||
| PANSS-Passive Social Withdrawal | 2.4 | 1.5 | ||
| PANSS-Active Social Avoidance | 2.4 | 1.7 | ||
| Chlorpromazine equivalent dose | 521.1 | 261.8 | ||
| Hamilton Depression Rating Scale | 3.4 | 3.5 | ||
| Young Mania Rating Scale | 1.0 | 2.3 | ||
|
| ||||
| % | N | % | N | |
|
| ||||
| Sex (% female) | 48 | 11 | 47 | 10 |
| Race (%black/white/other) | 43/57/0 | 10/13/0 | 33/62/5 | 7/13/1 |
Note: PANSS = Positive and Negative Syndrome Scale,
controls > patients, p < .05
2.4. MRI Data Acquisition
Anatomical and echoplanar images (EPI) were collected on a 3T Philips (Achieva) scanner. High-resolution anatomical images were acquired with the following parameters: 256 mm field of view, 170 slices, and 1 mm slice thickness. EPI images were acquired using a sequence optimized for the medial temporal lobe: repetition time = 2000 milliseconds, echo time = 28 milliseconds, 38 3 mm axial slices (no gap, titled 15° relative to anterior commissure-posterior commissure), interleaved acquisition, voxel size = 3×3×3, field of view = 240 mm, flip angle = 90°).
2.5. MRI Data Processing and Analyses
Analyses were performed in SPM8. Functional data were motion corrected, normalized (EPI template) and smoothed (FWHM 5 mm). All participants had acceptable motion (< 3 mm in any direction). At the individual participant level, habituation was modeled with a symmetrical linear regressor with a slope of −1 to capture voxels with a gradual signal decrease across the run. Small image targets were modeled as separate events of 0 s duration and were not included in further analyses.
To test for a group difference in the out-group bias, we performed a second-level analysis of variance in SPM8. The between-subjects factors were group (SZ/CTL) and face type (Same-gender/Opposite-gender). A main effect of face type signifies greater habituation to same-gender faces relative to opposite-gender faces—the out-group effect. The interaction of group and face type indicates a group difference in the out-group effect. Significant clusters were identified using F tests. To illustrate significant group differences in the out-group effect (habituation differences in same-gender vs other-gender conditions), we modeled the BOLD signal time course as 10 s blocks in SPM8 and used MarsBar(Brett et al., 2002) to extract percent signal change from each of the blocks for each subject.
Next, to determine whether social impairment was associated with an out-group effect, we performed a full factorial analysis in patients only. The variables included face type (Same-gender/Opposite-gender) and social impairment scores (social avoidance and social withdrawal, performed separately). For this analysis, the interaction of face type x social impairment score indicated a significant association between social impairment and the out-group bias (different rate of habituation by face type). Significant clusters were again identified using F tests. To illustrate the size and direction of the findings from these analyses, we extracted the average beta value (representing habituation slope, from the subject-level analyses) for each participant and performed post-hoc correlation analyses. Analyses were performed separately for each face type condition to determine the degree of contribution from habituation to same-gender faces relative to habituation to opposite-gender faces on social impairment measures. Finally, to better illustrate the pattern of habituation over time, percent signal change was extracted from 10 s bins (see above) and plotted separately for low social impairment (PANSS score of 1, n=10) and mild-moderate social impairment (PANSS scores of 2-6, n=13).
Based on previous research findings, we performed analyses within three regions of interest (ROI): the hippocampus, the amygdala, and the visual cortex. Because hippocampal volume differences are common in patients with schizophrenia, we constructed a study-specific hippocampal ROI using hippocampal tracings. Individual ROIs were coregistered to the participants’ original structural image and normalized to Montreal Neurological Institute (MNI) space using the EPI template. Normalized, binary ROIs were averaged across participants and the bilateral hippocampi were defined as voxels that included the hippocampus for at least 50% of the participants. The amygdala ROI was based on a manual tracing of the study average brain by an expert (JUB)(Clauss et al., 2014). The primary visual cortex ROI was defined as the occipital cortex region based on the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002).Within each a priori region, we tested for significant voxel-wise differences. Type I error correction was provided by a cluster-threshold adjustment method based on Monte-Carlo simulations with our ROI masks (AlphaSim, http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). With a voxel p-value of 0.05, the following cluster sizes provide a corrected family-wise error rate of α = 0.05: left hippocampus k = 10, right hippocampus k = 12, left amygdala k = 11, right amygdala k = 12, visual cortex k = 38.
3. Results
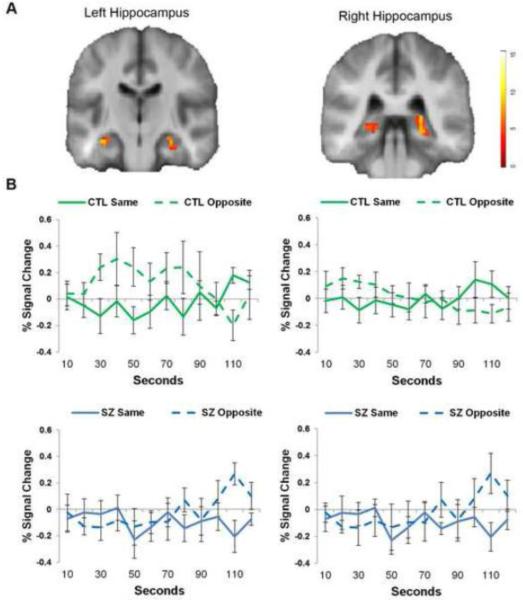
To determine whether patients with schizophrenia showed deficits in out-group responding, we tested for an interaction between group and face type (same-gender/opposite-gender). A significant interaction of group by out-group effect was observed in all three of the a priori regions of interest (see Figure 1, Supplemental Table 1). To determine if the three regions showed relevant differences, we performed a post-hoc repeated measures ANOVA on the percent signal change values for each region. The three regions showed similar patterns for the effects of group (p = .19), face type (p = .80), and the group by face type interaction (p = .91), suggesting that the effect is similar across the regions.
Figure 1. Schizophrenia is associated with a significantly delayed response to opposite-gender faces.
A. Clusters showing significant group x face type interaction (p < .05, corrected) are shown for the left hippocampus (A, top row) and right hippocampus (B, top row), illustrated on a group average brain. B. Percent signal change is plotted over time for healthy controls (first row) and patients (second row) by same-gender faces (solid lines) and opposite-gender faces (dotted lines).
The control participants showed a significant out-group effect that was apparent within the first 0 - 30 seconds. This pattern was best characterized by a heightened response to opposite-gender faces that eventually habituated after 70-90 seconds and no response to same-gender faces (similar to baseline). Relative to controls, patients had a substantially delayed out-group effects. Patients showed a relatively flat response to both same-gender and opposite-gender faces for the first 70-80 seconds, after which a significant increase in response to opposite-faces emerged.
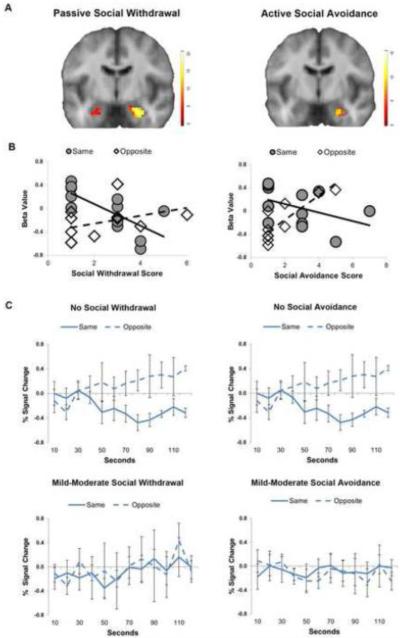
Next, we tested whether the neural out-group effect correlated with social impairment. Passive social withdrawal was significantly correlated with the out-group effect in all three regions of interest: the amygdala, hippocampus, and visual cortex (Figure 2A, Supplemental Table 2). Active social avoidance was significantly correlated with out-group bias in the hippocampus and visual cortex (Figure 2A, Supplemental Table 2), with substantial overlap with the regions found for social withdrawal. Across the clusters, passive social withdrawal was negatively correlated with neural response to same-gender faces and active social withdrawal was positively correlated with neural response to opposite-gender faces (Figure 2B, Table 2). Comparing patients with and without social impairment revealed two distinct patterns (Figure 2C). Patients with no social impairment had a delayed outgroup effect that didn’t emerge until 30-50 seconds and did not habituate. Patients with social impairment failed to show an out-group effect, even after 120 seconds.
Figure 2. Out-group response predicts social impairment in schizophrenia.
A. Social impairment was significantly correlated with out-group bias p < .05, corrected). Significant clusters are illustrated on a group average brain. B. Social impairment was positively correlated with response to same-gender faces and negatively correlated with response to opposite-gender faces. C. Patients with no social impairment demonstrate a delayed out-group bias that fails to habituate, whereas patients with social impairment fail to show an out-group bias. Percent signal change is plotted over time for patients with low social impairment (top row) and high social impairment (bottom row).
Table 2.
Correlations between social impairment and habituation to faces
| Passive Social Withdrawal | ||||
|
| ||||
| Same-gender Faces | Opposite-gender faces | |||
|
| ||||
| r | p | r | p | |
|
|
||||
| Amygdala (R) | −.85 | .001 | .33 | .32 |
| Hippocampus (L) | −.73 | .007 | .39 | .24 |
| Hippocampus (R) | −.86 | .03 | .49 | .12 |
| Visual Cortex (R) | −.58 | .05 | .63 | .04 |
|
| ||||
| Active Social Avoidance | ||||
|
| ||||
| Same-gender Faces | Opposite-gender faces | |||
|
| ||||
| r | p | r | p | |
|
|
||||
| Hippocampus (L) | .10 | .76 | −.71 | .02 |
| Hippocampus (R) | −.43 | .18 | .87 | .01 |
| Visual Cortex (L) | −.52 | .09 | .67 | .03 |
| Visual Cortex (L) | −.34 | .29 | .82 | .002 |
| Visual Cortex (R) | −.33 | .29 | .78 | .01 |
4. Discussion
The major findings of this study are that patients with schizophrenia have aberrant neural responses to out-group faces and that the degree of out-group bias is associated with social impairment. Controls show a rapid, heightened response to opposite-gender faces that habituates over time. Within patients with schizophrenia, degree of out-group bias correlated with social impairment. Low levels of social impairment were associated with a delayed response to opposite-gender faces that emerged after 30-50 seconds and failed to habituate. High levels of social impairment were associated with a failure to respond to opposite-gender faces. These findings enhance our understanding of the neural mechanisms underlying social impairment in schizophrenia and point to deficits in the processing of complex social information.
Out-group biases serve an important social function and are likely to have an evolutionary basis. The ability to allocate and sustain attention to a person who is different from oneself is adaptive and may serve purposes of either threat detection or reproductive partner evaluation. A robust gender out-group effect was observed in controls, consistent with previous findings in behavioral studies (Shapiro and Penrod, 1986; Shaw and Skolnick, 1994; Wright and Sladden, 2003). Controls did not show an elevated response to same-gender faces, even initially; however, habituation is often rapid and likely occurred within the first 10 seconds (Yamaguchi et al., 2004). In the schizophrenia group we found two patterns that distinguished patients with low social impairment from patients with high social impairment. The low social impairment group was characterized by an initial delay followed by sustained activation of the amygdala, hippocampus, and visual cortex, consistent with a heightened social perception of threat (Green and Phillips, 2004) and the failure to habituate observed in previous studies (Holt et al., 2005; Williams et al., 2013). In contrast, the high social impairment group was characterized by a failure to respond to opposite-gender, faces even after 120 face presentations. Thus, schizophrenia was associated with altered neural responses during complex social information processing, with two distinct subtypes of patients characterized by social impairment and pattern of neural responses.
The deficits in out-group bias were observed across a network of brain regions involved in social and emotional processing: the amygdala; hippocampus; and visual cortex. How might altered function in these brain regions contribute to social impairment? The out-group bias is fundamentally dependent on the abilities to discriminate between and categorize the faces as same-gender versus opposite-gender, and to categorize oneself into a salient social group (or assign meaning to the opposite-gender faces). The visual cortex and amygdala are key components of a face processing system (Haxby et al., 2000) involved in early processing and sensory gating of incoming visual signals; too much or too little visual information will hamper discrimination. The hippocampus performs match-mismatch computations (Blackford et al., 2010; Fried et al., 1997; Kumaran and Maguire, 2009) that are necessary for the creation of an out-group bias. Finally, the amygdala and hippocampus interact to retrieve the memories necessary for self-categorization and for attributing salience to members of other social groups. Critical for self-categorization is the self, which is thought to form through interactions between a person’s unique biological profile and social interactions over time. The ipseity-disturbance model of schizophrenia suggests that the self-disturbance leads to social impairments (Mishara et al., 2014; Nelson et al., 2014; Sass and Parnas, 1998). Thus, we propose that neural alterations in the amygdala, hippocampus, and visual cortex impact the processing of complex social information—demonstrated here as out-group bias deficits—and contribute to social impairments.
Our study has several limitations. First, we studied only chronic, medicated patients. It will be important to confirm our findings in the early stages of psychosis. Second, we assessed social impairment with the PANSS at a single point in time. While the PANSS has robust stability over time (Bryson et al., 1999) and a stable factor structure across the course of the disease (Lançon et al., 2000), additional measures would have allowed for a more detailed assessment of social impairment. Finally, it should be noted that the within-cell (group x face type) sample sizes were relatively small; therefore, these results should be considered preliminary and interpreted with caution.
In conclusion, we found that patients with schizophrenia show aberrant neural responses to opposite-gender faces. Importantly, patients with social impairment differed from patients without social impairment, demonstrating that meaningful biological differences may underlie these two subtypes of patients. Thus, neural responses to opposite-gender faces may be a useful measure in studies of treatment response and disease outcome in schizophrenia.
Supplementary Material
Acknowledgements
The authors thank Kristan Armstrong and Dr. Maureen McHugo for their assistance and feedback.
Role of the Funding Source
This work was supported by the National Institute of Mental Health (R01 MH070560 to SH; K01 MH083052 to JUB), and the National Center for Research Resources (grant UL1 RR024975-01, now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06, to LEW). The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
References
- Bediou B, Krolak-salmon P, Saoud M, Henaff M, Burt M, Dalery J. Face expression and sex recognition in schizophrenia and depression. Can. J. Psychiatry. 2005;50:525–533. doi: 10.1177/070674370505000905. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc. Cogn. Affect. Neurosci. 2013;8:143–50. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the amygdala in novelty detection. Neuroimage. 2010;50:1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] Neuroimage. 2002;16:s497. [Google Scholar]
- Bryson G, Bell M, Greig T, Kaplan E. Internal consistency, temporal stability and neuropsychological correlates of three cognitive components of the Positive and Negative Syndrome Scale (PANSS) Schizophr. Res. 1999;38:27–35. doi: 10.1016/s0920-9964(99)00004-3. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Li H, Cheung EFC, Gong Q-Y. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–90. doi: 10.1016/j.psychres.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Seay AL, Vanderklok R, Avery S, Cao A, Cowan RL, Benningfield MM, Blackford JU. Structural and functional bases of inhibited temperament. Soc. Cogn. Affect. Neurosci. 2014;9(12):2049–2058. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham Y, Chen EE, Banaji MR. Two signatures of implicit intergroup attitudes: developmental invariance and early enculturation. Psychol. Sci. 2013;24:860–8. doi: 10.1177/0956797612463081. [DOI] [PubMed] [Google Scholar]
- Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci. Biobehav. Rev. 2004;28:333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Hansen CF, Torgalsbøen A-K, Røssberg JI, Romm KL, Andreassen OA, Bell MD, Melle I. Object relations, reality testing, and social withdrawal in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 2013;201:222–5. doi: 10.1097/NMD.0b013e3182848ae0. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–5. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Haxby J, Hoffman E, Gobbini M. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, Ditman T, Welsh RC, Heckers S. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol. Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends Cogn. Sci. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lançon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophr. Res. 2000;42:231–239. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am. J. Psychiatry. 2013;170:334–41. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chan RCK, McAlonan GM, Gong Q. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr. Bull. 2010;36:1029–39. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces -KDEF. 1998 [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav. Res. Methods Instruments Comput. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Lysaker PH, Schwartz MA. Self-disturbances in schizophrenia: history, phenomenology, and relevant findings from research on metacognition. Schizophr. Bull. 2014;40:5–12. doi: 10.1093/schbul/sbt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Parnas J, Sass L. Disturbance of minimal self (ipseity) in schizophrenia: clarification and current status. Schizophr. Bull. 2014;40:479–82. doi: 10.1093/schbul/sbu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ebert JP, Banaji MR, Phelps EA. The role of social groups in the persistence of learned fear. Science (80-. ) 2005;309:785–7. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr. Bull. 1998;29:427–444. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- Shapiro PN, Penrod S. Meta-analysis of facial identification studies. Psychol. Bull. 1986;100:139–156. [Google Scholar]
- Shaw J, Skolnick P. Sex differences, weapon focus, and eyewitness reliability. J. Soc. Psychol. 1994;134:413–420. doi: 10.1080/00224545.1994.9712191. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JC, Hogg MA, Oakes PJ, Reicher SD, Wetherell MS. Rediscovering the social group: A self-categorization theory. Basil Blackwell; Cambridge, MA: 1987. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van den Oord EJCG, Rujescu D, Robles JR, Giegling I, Birrell C, Bukszár J, Murrelle L, Möller H-J, Middleton L, Muglia P. Factor structure and external validity of the PANSS revisited. Schizophr. Res. 2006;82:213–23. doi: 10.1016/j.schres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Williams LE, Blackford JU, Luksik A, Gauthier I, Heckers S. Reduced habituation in patients with schizophrenia. Schizophr. Res. 2013;151:124–132. doi: 10.1016/j.schres.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DB, Sladden B. An own gender bias and the importance of hair in face recognition. Acta Psychol. (Amst) 2003;114:101–114. doi: 10.1016/s0001-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J. Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.