Abstract
Objectives
To determine the impact of tuberculosis (TB) treatment at the time of combination antiretroviral therapy (cART) initiation on virologic and CD4 count response to cART.
Methods
Systematic review and meta-analysis of studies reporting HIV RNA and CD4 count response, stratified by TB treatment status at cART initiation. Stratified random-effects and meta-regression analyses were used when possible.
Results
25 eligible cohort studies reported data on 49,578 (range 42-15,646) adults, of whom 8,826 (18%) were receiving TB treatment at cART initiation. 17 studies reported virologic response; 21 reported CD4 count response. The summarized random-effects relative risk (RRRE) of virologic suppression in those receiving vs. not receiving TB treatment at different time points following cART initiation was 1.06 (0.86-1.29) at 1-4 months, 0.91 (0.83-1.00) at 6 months, 0.99 (0.94-1.05) at 11-12 months, and 0.99 (0.77-1.28) at 18-48 months. The overall RRRE at 1-48 months was 0.97 (95% CI:0.92-1.03). Available data regarding the effect of TB treatment on virologic failure were heterogeneous and inconclusive (13 estimates). Differences in median CD4 count gain between those receiving vs. not receiving TB treatment ranged from -10 to 60 cells/μL (median 27) by 6 months (7 estimates) and -10 to 29 (median 6) by 11-12 months (5 estimates), though the heterogeneity of the response measures did not support meta-analysis.
Conclusions
Patients receiving TB treatment at cART initiation experience similar virologic suppression and CD4 count reconstitution as those not receiving TB treatment, reinforcing the need to start cART during TB treatment and allowing more confidence in clinical decision-making.
Keywords: HIV, tuberculosis, antiretroviral therapy, viral load, CD4 lymphocyte count, systematic review, adults
Introduction
Tuberculosis (TB) threatens the health of people living with HIV (PLWH). Globally in 2011, 13% of incident TB cases were co-infected with HIV and an estimated 0.4 million TB deaths occurred among PLWH [1]. Given the World Health Organization's 2010 recommendation that all PLWH with TB be initiated on combination antiretroviral therapy (cART), regardless of CD4 count [2], and the goal of 100% cART coverage of co-infected patients by 2015 [3], many individuals are initiating cART while concurrently on TB therapy. PLWH who are also being treated for TB may experience a differential response to cART due to drug-drug interactions [4, 5], an increased risk of drug toxicity [4, 5], immune reconstitution inflammatory syndrome [6], and the potential for lower adherence due to the high pill burden [5]. The effect of TB treatment and its associated potential challenges and complications regarding a patient's response to cART require careful evaluation.
We aimed to describe the impact of receiving TB treatment at the time of cART initiation on virologic and CD4 count response to cART among HIV-infected adults. In addition, we highlighted the various outcome measures used in the literature and make recommendations for some methodological standards that may ease future between-study comparisons.
Methods
Search strategy and selection criteria
To investigate the effect of TB treatment at time of cART initiation on virologic response and CD4 count response, we carried out a systematic and sensitive search using an a priori protocol developed according to PRISMA guidelines [7]. We searched PubMed and EMBASE, as well as abstract databases from the 2009 to 2012 Conferences on Retroviruses and Opportunistic Infections, International Union Against Tuberculosis and Lung Disease World Conferences on Lung Health, and International AIDS Society conferences. The search terms “HIV AND Tuberculosis AND (Viral Load OR CD4 lymphocyte count OR Mortality) AND Antiretroviral therapy” were used to identify relevant articles in PubMed and EMBASE. Searches were performed on January 29, 2013 and included original human subjects studies published since 1997 (the start of the cART era). Additional articles were identified from reference lists, reviews, and Web of Science citation lists.
H.M.S. and A.V.R. independently reviewed titles and abstracts of original studies retrieved by the search. H.M.S. reviewed full-text and references of selected articles. H.M.S. and M.R.P. independently abstracted study data from full reports; discrepancies were resolved by consensus among co-authors.
Studies were included if they reported HIV RNA and/or CD4 count response following cART initiation among antiretroviral treatment-naïve HIV-infected adults, stratified by TB treatment status at cART initiation. Studies with ≤5% antiretroviral-experienced patients or patients only previously exposed to a single intrapartum dose of nevirapine were also included. Studies of children <14 years of age were excluded. No additional exclusion criteria or language restrictions were imposed.
Data extraction
The following information, if available, was abstracted from each article: first author surname; publication year; study dates; geographic location; study design; clinical setting; sample size; number receiving and not receiving TB treatment at cART initiation; if TB treatment was the main exposure of interest; types of TB included; culture confirmation of TB cases; TB site; timing of TB treatment in relation to cART initiation; length of follow-up; proportion antiretroviral-naïve; percentage male; mean or median participant age; criteria for cART initiation; cART regimen; baseline median CD4 count and HIV RNA; HIV RNA outcome measure(s); CD4 count outcomes measure(s); covariate adjustment; exclusion criteria; proportion lost-to-follow-up; and how each study handled loss-to-follow-up, mortality, and regimen switching. For this purposes of this review, we abstracted results as presented in the specific studies according to their individual methods and assumptions.
Statistical analysis
Reported effect estimates over any length of time were abstracted. If only count data of those who experienced an outcome, stratified by TB treatment status, were reported, a risk ratio (RR) and 95% confidence interval (CI) were calculated. If a study reported an outcome only graphically, outcome values were visually estimated [8]. Standard error estimates were inferred from reported CIs by [ln(upper limit) – ln(lower limit)]/3.92 [9]. As we aimed to quantify virologic suppression, if a study reported on patients who failed to suppress, this information was converted to obtain data on suppression. For CD4 counts, if 2 of 3 of the following measures were reported, we calculated the third measure: mean baseline CD4 count, mean change in CD4 count from baseline, mean absolute CD4 count. We were unable to calculate the missing measures if only median CD4 counts were reported.
For virologic suppression, summarized relative risks were calculated using random-effects summarization with unconditional variances and the method of moments estimated between-study variance (τ2) [8]. As several studies reported estimates at multiple of time points, we used the estimate closest to the midpoint of each study's follow-up time to get an overall relative risk for virologic suppression. In addition, to examine short- and long-term virologic suppression, summary relative risks at 1-4 months, 6 months, 11-12 months and 18-48 months were calculated. The p-values for a standard chi-square homogeneity test statistic were used to assess overall consistency among the effect estimates across studies. τ2 was used to calculate 95% population effects intervals [10] (where 95% of populations are estimated to have their means), opposite effects proportions [11] (proportion of populations likely to experience a relative risk below unity), and 95% prediction intervals [11] (95% of these intervals will cover the true value estimated by a future study). Stratified and random-effects meta-regression analyses were used to calculate stratum-specific summary measures and 95% CIs, along with ratios of the stratum-specific RRs as described by Bassler [12].
Funnel plots of ln(virologic suppression relative risk) vs. the inverse-variance weight of studies were visually examined for asymmetry and statistically assessed using methods proposed by Begg [13] and Egger [14] and the trim-and-fill method [15]. STATA (version 12, Stata corporation, College Station, TX) was used for these analyses.
Results
Selected studies
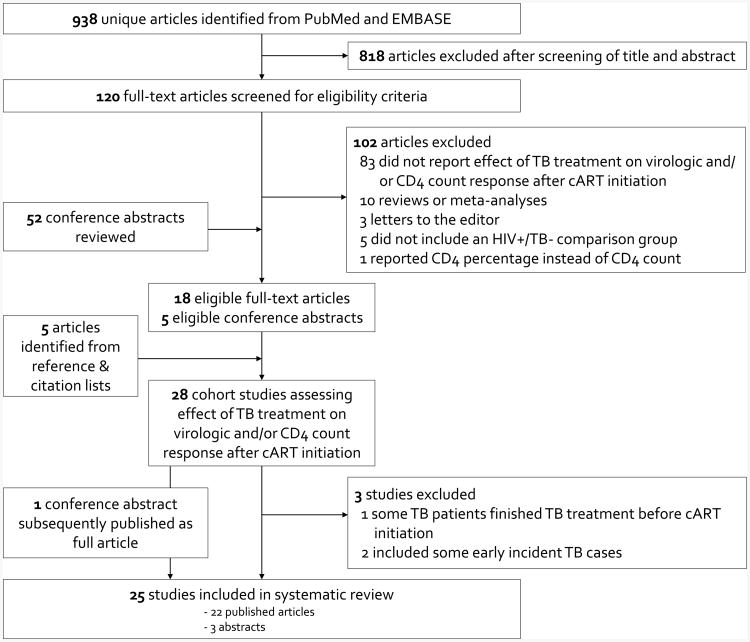
990 unique abstracts were reviewed: 795 from PubMed, 143 from EMBASE, and 52 from conference proceedings (Fig. 1). Of these, 120 full-text articles were selected for review. In total, 18 articles [16-33] and five conference abstracts [34-38] were eligible. Five additional articles met our inclusion criteria: two from reference lists [39, 40] and three from Web of Science citation searches [41-43]. One included abstract [34] was subsequently published as a full article [44]; only data from the full article was included. Three eligible studies were excluded: one because some patients included in the TB treatment-exposed group had completed TB treatment just prior to cART initiation [36], and two because they included some early incident TB cases in their TB treatment-exposed group [20, 31]. Articles reporting on the same study population, but at differing time points following cART initiation, were retained [26, 27, 39]. Similarly, we retained both reports of a Tanzanian cohort [22, 42].
Figure 1. Identification and selection of eligible studies.
Study and population characteristics
The 25 final studies provided data on 49,578 PLWH, of which 8,826 (18%) were receiving TB treatment at cART initiation. Selected study and population characteristics are displayed in Table 1. All were cohort studies; four reported virologic response, eight reported CD4 count response, and 13 reported both outcomes. While the majority of studies was based in Sub-Saharan Africa, nine included Asian populations [17, 23, 26, 27, 29, 32, 33, 38, 39] and three were from Europe or North America [19, 21, 28]. Most publications assessed response to cART, regardless of regimen type, though some reported estimates specific to nevirapine-[18, 26, 27, 30, 39] or efavirenz-based [18, 22, 25, 29, 30, 42] cART (see Supplemental Digital Content 1, which provides study-specific details on cART regimens). Though included studies used a variety of cART regimens, nevirapine was often used in combination with stavudine and efavirenz was often used in combination with zidovudine and lamivudine. All studies used cART initiation as the time origin, except one which began at the commencement of cART education and adherence sessions, with most patients starting cART a month or two later [44]. Two studies included women previously exposed to a single intrapartum dose of nevirapine [18, 40], and one study included 3% antiretroviral-experienced patients [17].
Table 1. Characteristics of 25 studies reporting the effect of tuberculosis treatment on virologic and/or CD4 count response to combination antiretroviral therapy among HIV-infected adults.
| Publication year |
Study | Geographic location |
Sample size |
TB treatment, N (%) |
Main exposure is TB |
Types of TB includeda | Study design |
cART regimenb |
Naïve, % |
Male, % |
Mean age, years |
Median baseline CD4 count, cells/μL |
Median baseline HIV RNA, log10 copies/mL |
Lost to follow- upc, % |
Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | Hung[23] | Taiwan | 276 | 46 (17) | Yes | Definitive, probable, or possible TB and on TB treatment at cART initiation | P | All | 100 | 92 | 33 | 73 | 5.4 | 7 | Both |
| 2004 | Patel[29] | India | 255 | 126 (49) | Yes | On TB treatment for definite or probable TB at cART initiation | P | EFV | 100 | 73 | 37 | 101 | NS | 0 | CD4 |
| 2006 | Breen[19] | England | 164 | 82 (50) | Yes | On TB treatment at cART initiation. TB diagnosed by culture, nucleic acid amplification, radiography, histology or clinical algorithm. | R | All | 100 | 52 | 35 | 77 | 5.1 | 0 | Both |
| 2006 | Manosuthi[39] | Thailand | 140 | 70 (50) | Yes | Receiving rifampicin for active TB ≥1 month prior to cART enrollment | P | NVP | 100 | 68 | 36 | 29 | 5.6 | 0 | HIV RNA |
| 2008 | Boulle (a)[18]d | South Africa | 1935 | 209 (11) | Yes | Concurrent TB treatment at cART initiation and for ≥14 days post cART initiation. TB diagnosed by microscopy, culture or clinical algorithm. | P | NVP | 100 | 21 | 31 | 110 | 5.0 | 6 | Both |
| 2008 | Boulle (b)[18]d | South Africa | 2035 | 1074 (53) | Yes | Concurrent TB treatment at cART initiation and for ≥14 days post cART initiation. TB diagnosed by microscopy, culture or clinical algorithm. | P | EFV | 100 | 40 | 33 | 78 | 5.2 | 6 | Both |
| 2008 | Manosuthi[26] | Thailand | 140 | 70 (50) | Yes | Receiving rifampicin for active TB ≥1 month prior to cART enrollment | P | NVP | 100 | 68 | 36 | 29 | 5.6 | 10 | Both |
| 2008 | Mussini[28] | Italy, Spain, England, Canada | 624 | 168 (27) | No | TB as initial AIDS diagnosis prior to cART initiation | P | All | 100 | 78 | 39 | 41 | 5.3 | NS | HIV RNA |
| 2008 | Sumantri[32] | Indonesia | 130 | 87 (67) | Yes | On TB treatment for pulmonary TB at cART initiation. TB was diagnosed via chest x-ray or microscopy. | P | All | 100 | 80 | 32 | 156 | NS | NS | Both |
| 2009 | Shipton[30] | Botswana | 310 | 155 (50) | Yes | On TB treatment at cART initiation | R | All | 100 | 40 | 36 | 79 | 5.8 | 64 | Both |
| 2010 | Boulle[40] | South Africa | 7323 | 2760 (38) | No | On TB treatment at cART initiation | P | All | 100 | 32 | 33 | 101 | 5.1 | 10 | Both |
| 2010 | Manosuthi[27] | Thailand | 140 | 70 (50) | Yes | Receiving rifampicin for active TB ≥1 month prior to cART enrollment | P | NVP | 100 | 68 | 36 | 31 | 5.6 | 11 | Both |
| 2010 | Tan[38] | Malaysia | 42 | 15 (36) | Yes | On TB treatment at cART initiation | P | All | 100 | NS | 41 | 30 | 5.0 | NS | Both |
| 2010 | Wanchu (a)[33]e | India | 104 | 52 (50) | Yes | Diagnosed with TB and started TB treatment 1 month prior to cART initiation. TB diagnosed by microscopy, radiography, clinical criteria or histology. | R | All | 100 | 69 | 35 | 155 | NS | NS | CD4 |
| 2010 | Wanchu (b)[33]e | India | 130 | 65 (50) | Yes | Diagnosed with TB and started TB treatment 1 month prior to cART initiation. TB diagnosed by microscopy, radiography, clinical criteria or histology. | R | All | 100 | 79 | 38 | 50 | NS | NS | CD4 |
| 2011 | Almeida[16] | Mozambique | 89 | 27 (30) | No | Confirmed or suspected TB at cART initiation | P | All | 100 | 45 | NS | NS | NS | 31 | HIV RNA |
| 2011 | Auld[41] | Mozambique | 2596 | 267 (10) | No | On TB treatment at cART initiation | R | All | 100 | 38 | 34 | 153 | NS | 22 | CD4 |
| 2011 | Dronda[21] | Spain | 1986 | 110 (6) | Yes | Definite or presumptive diagnosis of TB in the 6 months prior to cART initiation | P | All | 100 | 76 | 38 | 196 | 5.0 | 7 | Both |
| 2011 | Hermans[35] | Uganda | 3797 | 570 (15) | Yes | On TB treatment at cART initiation | R | All | 100 | 34 | 37 | 100 | NS | NS | CD4 |
| 2011 | Lartey[25] | Ghana | 74 | 34 (46) | Yes | On TB treatment at cART initiation | P | EFV | 100 | 49 | NS | 83 | 5.4 | 11 | Both |
| 2012 | Bassett[44] | South Africa | 951 | 343 (36) | Yes | Newly diagnosed by sputum culture at cART enrollment or previously diagnosed and currently on treatment | P | All | 100 | 41 | 36 | 90 | NS | 7 | Both |
| 2012 | Bastard[17] | Malawi, Kenya, Uganda, Cambodia | 1580 | 305 (9) | No | On TB treatment at cART initiation | R | All | 97 | 36 | 36 | 119 | NS | NS | HIV RNA |
| 2012 | Hardwick (a)[22]f | Ethiopia | 649 | 365 (56) | No | On TB treatment at cART initiation | P | EFV | 100 | NS | NS | 94 | 5.4 | NS | CD4 |
| 2012 | Hardwick (b)[22]f | Tanzania | 353 | 147 (42) | No | On TB treatment at cART initiation | P | EFV | 100 | NS | NS | 99 | 5.8 | NS | CD4 |
| 2012 | Julg[24] | South Africa | 442 | 187 (42) | No | Concurrent TB co-infection at cART initiation | R | All | 100 | 39 | 35 | 95 | NS | NS | CD4 |
| 2012 | Mugusi[42] | Tanzania | 473 | 220 (47) | No | Newly diagnosed at cART enrollment by smear microscopy, histology or clinical criteria | P | EFV | 100 | 43 | 40 | 92 | 5.7 | 12 | CD4 |
| 2012 | Odo[37] | Nigeria | 5338 | 290 (5) | Yes | On TB treatment at cART initiation | R | All | 100 | 38 | 36 | 159 | NS | NS | CD4 |
| 2013 | Schomaker[43] | South Africa | 15646 | 1052 (7) | Yes | On TB treatment for confirmed or probable TB at cART initiation | P | All | 100 | 32 | 34 | 98 | 4.9 | NS | Both |
Abbreviations: EFV, Efavirenz-based cART; HIV, human immunodeficiency virus; NS, not specified; NVP, Nevirapine-based cART; P, prospective study; R, retrospective study; TB, tuberculosis.
See Table, Supplemental Digital Content 2 for further information on the timing of TB treatment in relation to cART initiation from each study, if available
See Table, Supplemental Digital Content 1 for detailed information on cART regimens from each study, if available
See Table, Supplemental Digital Content 3 for detailed information on methods used by each study to handle mortality and loss-to-follow-up, if available
(a) Nevirapine-based cART; (b) Efavirenz-based cART
(a) patients with baseline CD4 counts of 100-200 cells/μL; (b) patients with baseline CD4 counts <100 cells/μL
(a) a cohort from Ethiopia; (b) a cohort from Tanzania
Seventeen studies examined TB treatment at cART initiation as the main exposure of interest [18, 19, 21, 23, 25-27, 29, 30, 32, 33, 35, 37-39, 43, 44]. The other eight studies examined TB treatment as a secondary exposure; five aimed to describe general cART outcomes [16, 24, 28, 40, 41], and one each examined the primary exposures of timeliness of clinic attendance [17], β-defensin genomic copy number [22], and liver enzyme abnormalities [42]. The type of TB being treated varied across studies. Only one study had a subset of bacteriologically-confirmed TB cases [44], while others included both confirmed and probable TB cases. One study focused solely on pulmonary TB [32], and three excluded patients who developed incident TB from the reference group [18, 30, 35]. Sixteen studies reported detail on the duration of TB treatment at the time of cART initiation (see Table, Supplemental Digital Content 2, which provides study-specific information on the timing of TB treatment, if available).
Overall loss-to-follow-up was reported by 15 studies and ranged from 0% to 64% (median 10%, interquartile range 7% to 12%) (see Table, Supplemental Digital Content 3, which details methods utilized by studies to handle loss-to-follow-up and mortality). 5 studies limited their analysis to those who completed follow-up [16, 30, 35, 37, 44] and 3 studies considered those who discontinued cART for a variety of reasons and/or lacked follow-up laboratory data as treatment failures [21, 26, 27]. While most studies did not describe how they handled patients who switched cART regimens, one excluded patients who stopped or changed cART during follow-up [18], three explicitly retained patients who switched [24, 30, 40], and one did a sensitivity analysis considering those who discontinued stavudine as treatment failures [27].
All studies included both genders, with the proportion male ranging from 21% to 92% (median 45%). All patients were ≥14 years of age; mean patient age ranged from 31 to 41 years (median 36). Median baseline CD4 count ranged from 29 to 196 cells/μL (median 94), and baseline HIV RNA ranged from 4.9 to 5.8 log10 copies/mL (median 5.3). One study reported results stratified by baseline CD4 count [33].
Virologic suppression
There was heterogeneity in how each study quantified virologic response with respect to the reported effect measure, the cut-off used (50 or 400 copies/mL), and the timing of measurement (see Table, Supplemental Digital Content 4 for virologic measures as reported by each study). In total, 17 studies reported virologic suppression, either directly or as a measure that allowed conversion into virologic suppression. Times of reported virologic suppression ranged from 1 to 48 months following cART initiation, with some studies reporting multiple time points. While most studies had overall suppression proportions >75%, several observed relatively low suppression. The study with the shortest follow-up time (1 month) reported the lowest overall proportion suppressed (46%) [23]. Manosuthi et al. (2006, 2008, 2010) also reported low suppression among Thai patients: 69% at 6 months [39], 59% at 33 months [26] and 51% at 48 months [27]. Three other studies reported suppression rates between 64 and 70% [16, 21, 32]. However, three of these studies that reported low suppression rates considered those who discontinued cART or lacked follow-up laboratory data as treatment failures [21, 26, 27].
In total, 15 studies reported RRs for virologic suppression in those receiving vs. not receiving TB treatment at cART initiation (Fig. 2). Overall, the random-effects relative risk (RRRE) for suppression was 0.97 (95% CI 0.92-1.03). When estimates were categorized according to follow-up time, the RRRE for suppression was 1.06 (0.86-1.29) at 1-4 months, 0.91 (0.83-1.00) at 6 months, 0.99 (0.94-1.05) at 11-12 months, and 0.99 (0.77-1.28) at 18-48 months after cART initiation (Table 2). In meta-regression analysis, a lower limit of detection of 50 or 400 copies/mL and type of cART regimen did not substantially influence the summary relative risks (see Table, Supplemental Digital Content 5 for meta-regression results).
Figure 2.
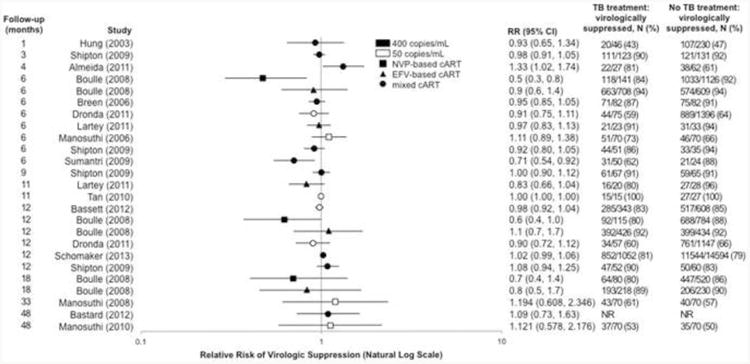
Relative risk of virologic suppression in those receiving vs. not receiving tuberculosis treatment at cART initiation by length of follow-up time, as reported by 15 studies. Estimates were abstracted according to the precision and stratification used by the original authors. Estimates calculated using available data are reported to 2 decimal places. Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; EFV, efavirenz; NR, not reported; NVP, nevirapine; RR, relative risk.
Table 2. Meta-analysis results for the effect of TB treatment on virologic suppression after combination antiretroviral therapy initiation, by length of follow-up time.
| Length of follow-up time | 1-4 months | 6 months | 11-12 months | 18-48 months | 1-48 months |
|---|---|---|---|---|---|
| No. of estimates | 3 | 8 | 7 | 5 | 13 |
| Homogeneity p-value | 0.088 | 0.064 | 0.134 | 0.718 | 0.060 |
| Estimate of between-study variance (τ2) | 0.019 | 0.008 | 0.002 | 0.000 | 0.003 |
| RRRE (95% CI) | 1.06 (0.86, 1.29) | 0.91 (0.83, 1.00) | 0.99 (0.94, 1.05) | 0.99 (0.77, 1.28) | 0.97 (0.92, 1.03) |
| 95% population effects interval | (0.81, 1.38) | (0.76, 1.09) | (0.91, 1.08) | Undefineda | (0.87, 1.08) |
| Opposite effects proportion | 34.9% | 14.9% | 41.1% | Undefineda | 29.6% |
| 95% prediction interval | (0.70, 1.58) | (0.73, 1.14) | (0.88, 1.12) | (0.74, 1.33) | (0.84, 1.12) |
Abbreviations: CI, confidence interval; RRre, random-effects summary relative risk; TB, tuberculosis
Undefined because τ2 = 0.000
Additionally, 6 studies provided data on cART regimen-specific relative risks of virologic suppression (see Figure, Supplemental Digital Content 6 for forest plot). The three lowest RRs for TB treatment exposure all correspond to three follow-up time-points of the nevirapine-based cART arm of Boulle et al. (2008) [18].
The funnel plot of overall suppression relative risks did not appear asymmetrical due to publication bias or other factors, with Begg's and Egger's p-values for small study effects of 0.26 and 0.71, respectively (see Figure, Supplemental Digital Content 7, for the funnel plot).
Virologic failure
Measures of virologic failure were highly heterogeneous (Supplemental Digital Content 4), with studies measuring whether patients reached HIV RNA levels of >5000 copies/mL [17], failed to suppress <400 copies/mL [18], rebounded after being previously undetectable or never became undetectable [23, 25, 26], time to first value ≥400 [18], time to 2 consecutive values ≥5000 copies/mL [18], and time to first value >500 among those who initially suppressed [28]. Six of these studies did not find TB treatment to have a significant effect on virologic failure [17, 18, 23, 25, 26, 40]. Interestingly, Boulle et al. found an association between TB treatment and virologic failure among those on nevirapine-based cART, but not among patients on efavirenz-based cART in their 2008 study [18], but reported the opposite finding in their 2010 study [40]. The substantial heterogeneity among virologic failure outcome measures precluded a formal meta-analysis.
CD4 count response to cART
Methods for measuring and reporting CD4 count response were even more heterogeneous than those used for virologic response, due to measurements at different time points and use of a diversity of outcome measures. Eight studies reported mean or median change in CD4 count from baseline, five measured mean or median absolute CD4 count during follow-up, and three reported the difference in CD4 count gain from baseline in patients receiving vs. not receiving TB treatment at cART initiation (Table 3). In addition, some studies defined a specific measure of immunologic success [21, 24] or immunologic failure [24, 41], and two studies described the CD4 count recovery trajectory [24, 43] (see Table, Supplemental Digital Content 8 for detailed immunologic measures). Two studies limited reporting of CD4 count response to virologically suppressed patients [24, 40].
Table 3. Types of outcome measures used by 21 studies to quantify CD4 count response to cART.
| Measure of CD4 count response | Number of studies reporting this measure | Length of follow-up used in reporting this measure (months) |
|---|---|---|
| Immunologic success | ||
| Change in CD4 count from baselinea | 8 | 1; 3; 6; 9; 11; 12 |
| Absolute CD4 count at a specific follow-up timeb | 5 | 3; 6; 9; 11; 48 |
| Difference in the increase in CD4 count from baselinec | 3 | 6; 18; 22 |
| Increase of ≥50 cells/μL from baseline | 1 | 6 |
| Increase of ≥100 cells/μL from baseline | 1 | 12 |
| Absolute CD4 count >200 cells/μL | 1 | 12 |
| Absolute CD4 count >500 cells/μL | 1 | 30 |
| Rate of CD4 count increase from baseline (cells/month) | 1 | 30 |
| Difference in CD4 count recovery slope (cells/6 months) | 1 | 6; 48 |
| Median on treatment peak CD4 count | 1 | 53 |
| Median change between baseline and on treatment peak CD4 count | 1 | 53 |
| Immunologic failure | ||
| CD4 count decline from baseline, CD4 count <100 cells/μL, or 50% decline from peak CD4 count after ≥6 months of cART | 1 | 36 |
| Rate of immunologic failure | 1 | 36 |
| Absolute CD4 count <500 cells/μL | 1 | 30 |
Abbreviations: cART, combination antiretroviral therapy
An additional 2 studies provided data that enabled the calculation of this measure.
An additional 3 studies provided data that enabled the calculation of this measure.
An additional 10 studies provided data that enabled the calculation of this measure.
Overall, those receiving TB treatment at cART initiation tended to have lower baseline CD4 counts, greater increases in CD4 count from baseline, and lower absolute CD4 counts during follow-up. Median change in CD4 count from baseline after 6 months of cART (reported by 7 studies) ranged from 97 to 200 cells/μL (median 167) among TB treatment-exposed patients and from 89 to 177 cells/μL (median 138) among those not on TB treatment. At 11-12 months, median change in CD4 count from baseline (reported by 5 studies) ranged from 124 to 234 cells/μL (median 155) among TB treatment-exposed patients and from 104 to 205 cells/μL (median 165) among those not on TB treatment. This corresponds to a differential gain in CD4 count between patients receiving vs. not receiving TB treatment at cART initiation ranging from -10 to 60 more CD4 cells/μL (median 27) at 6 months and -10 to 29 more CD4 cells/μL (median 6) at 11-12 months. Heterogeneity among CD4 count response outcomes measures prevented formal meta-analysis.
Discussion
In this systematic review and meta-analysis of the effect of TB treatment on virologic and CD4 count response to cART, the first meta-analysis of this topic to our knowledge, we found that exposure to TB treatment at cART initiation does not impair virologic suppression or CD4 count gain. The effect on the risk of virologic failure could not be assessed. Our findings indicate that despite concerns about drug-drug interactions, toxicity, high pill burden, and IRIS, TB treatment does not appear to reduce the efficacy of cART in regards to virologic suppression and CD4 count response. The reported outcome measures were however highly heterogeneous, impeding sound between-study comparisons or meta-analytic summarization for outcome measures other than virologic suppression. While rigorous meta-analysis methods could not be applied for CD4 response, we did observe similar within-study effects of TB treatment, and the overall impression is that TB treatment exposure does not have a substantial impact on CD4 recovery.
Furthermore, time points reported by individual studies were also heterogeneous. The optimal time point for evaluating the effect of exposure to TB treatment on response to cART is unclear. Follow-up times shorter than 4 months may be too early to accurately describe response to cART, and follow-up times longer than two years may underestimate the impact of TB treatment at cART initiation, especially if patients who switch treatments or take second-line therapy are included in the analysis. For the sake of completeness, all reported outcome measures and follow-up times were retained in this review.
The exposure, TB treatment at cART initiation, captured both exposure to active TB disease and exposure to anti-tuberculosis drugs. This combined exposure is useful from a health systems perspective, particularly in low-resource countries, where active TB cannot always be confirmed, especially in PLWH. This is further highlighted by the fact that the included studies used a variety of methods for determining who had active TB and should receive treatment, and no studies were limited to bacteriologically-confirmed TB cases and only one study described this subset. Consequently, active TB could have been misclassified and some patients included in this meta-analysis may have received TB treatment even though they did not have TB.
Since being treated for active TB cannot be studied in a randomized controlled trial, all studies included in our review were observational. Consequently, there was much heterogeneity in the duration of TB treatment prior to cART initiation, with some patients on TB therapy for up to eight months and others beginning TB treatment and cART concurrently. While the timing of TB treatment in relation to cART initiation is an important factor when evaluating mortality [45-49], it is unclear whether TB treatment timing would influence virologic or CD4 count response. Unfortunately, the included studies did not provide enough information on duration of TB treatment to systematically evaluate its effect on our results. Similarly, a lack of provided data on cART regimen switching during follow-up precluded a systematic evaluation of this factor.
This systematic review and meta-analysis may have been subject to some biases. First, virologic and immunologic response cannot be evaluated in those who have died or were lost-to-follow-up. Loss rates varied widely, ranging from 0% to 64% though most studies lost ≤12%, and studies handled loss-to-follow-up in a variety of ways, which may have influenced their results. Missing patients may systematically differ from those retained in the analysis. If response to cART among lost or deceased patients was differential by TB treatment status, than the results of these studies and our review could have been biased. Second, in 8 of 25 studies, TB treatment was not the primary exposure and covariates included in some multivariable models may differ from ideal confounder adjustment for this research question. Third, some bias may have been introduced by estimation methods used when a study did not directly report an outcome measure but provided the necessary data to calculate the desired effect measures [8, 9].
In conclusion, this recent comprehensive review of studies assessing the effect of TB treatment on response to cART indicates that TB treatment does not affect virologic suppression or CD4 count gain after cART initiation and we were unable to assess the effect on virologic failure. These findings will allow health care workers to be more confident in their clinical decision-making and in their communication to patients about the need to start cART during TB treatment. The heterogeneity in outcome measures posed a challenge to the interpretation and summarization of the virologic and CD4 count response to cART. Between-study comparisons could be greatly facilitated by methodological standardization of outcome measures and their time points in future studies.
Supplementary Material
Supplemental Digital Content 1. Table of combination antiretroviral therapy regimens used in each study.pdf
Supplemental Digital Content 2. Table of timing of TB treatment in relation to cART initiation, by study.pdf
Supplemental Digital Content 3. Table of methods for handling loss-to-follow-up and mortality utilized by each study.pdf
Supplemental Digital Content 4. Table of quantification of virologic response to combination antiretroviral therapy, stratified by TB treatment status, as reported by 17 studies.pdf
Supplemental Digital Content 5. Table of meta-regression results for the effect of TB treatment on virologic suppression after combination antiretroviral therapy initiation.pdf
Supplemental Digital Content 6. Figure of cART-regimen specific relative risks of virologic suppression.pdf
Supplemental Digital Content 7. Figure of funnel plot of overall relative risk of virologic suppression.pdf
Supplemental Digital Content 8. Table of quantification of CD4 count response to combination antiretroviral therapy, stratified by TB treatment status, as reported by 21 studies.pdf
Acknowledgments
H.M.S. and A.V.R. devised and designed the study, developed the search strategy, established inclusion criteria, reviewed abstracts, and interpreted data. H.M.S. did all searches of published work, abstracted the study data, was responsible for the statistical analysis, created tables and figures, and drafted the report. M.R.P. double-abstracted the study data, discussed discrepancies and participated in revising the report. J.J.E. and S.N. helped design the analysis, provided expert clinical opinion, and participated in report revision.
The authors are grateful to Mellanye Lackey for her assistance with developing our search strategy, and to Harry Moultrie and Alan Brookhart for their thoughtful comments and guidance.
M.R.P. was partially supported by NIH training grant 2T32AI070114. J.J.E. and S.N. were partially supported by the University of North Carolina Center for AIDS Research (CFAR), an NIH-funded program (P30 AI50410).
Sources of funding: M.R.P. was partially supported by NIH training grant 2T32AI070114. J.J.E. and S.N. were partially supported by the University of North Carolina Center for AIDS Research (CFAR), an NIH-funded program (P30 AI50410).
Footnotes
Conflicts of interest: H.M.S., S.N., and A.V.R. have no conflicts of interest. M.R.P. was a summer intern and research assistant at GlaxoSmithKline (GSK) in 2010-2011. J.J.E. is a consultant to GSK, Bristol MyersSquibb, Gilead, Merck, and Janssen, and an investigator on clinical research studies at UNC Chapel Hill supported by GSK.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva: WHO; 2012. [Google Scholar]
- 2.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 3.The Global Plan to Stop TB 2011-2015. Geneva: World Health Organization and Stop TB Partnership; 2010. [Google Scholar]
- 4.Volberding P, Sande M, Lange J, Greene W. Global HIV/AIDS Medicine. Philadelphia: Elsevier; 2008. [Google Scholar]
- 5.Curran A, Falco V, Pahissa A, Ribera E. Management of Tuberculosis in HIV-Infected Patients. AIDS Rev. 2012;14:231–246. [PubMed] [Google Scholar]
- 6.Breton G, Bourgarit A, Pavy S, Bonnet D, Martinez V, Duval X, et al. Treatment for tuberculosis-associated immune reconstitution inflammatory syndrome in 34 HIV-infected patients. The International Journal of Tuberculosis and Lung Disease. 2012;16:1365–1370. doi: 10.5588/ijtld.11.0693. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger M, Smith GD, Altman DG. Systematic reviews in health care: Meta-analysis in context. 2nd. London: BMJ Publishing Group; 2001. [Google Scholar]
- 9.Greenland S, O'Rourke K. Meta-analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 652–682. [Google Scholar]
- 10.Borenstein M, Hedges L, Higgins JP, Rothstein H. Introduction to Meta-Analysis. West Sussex: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 11.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society Series A. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 16.Almeida JM, Letang E, Nhampossa T, Ayala E, David C, Menendez C, et al. Rapid suppression of HIV-RNA is associated with improved control of immune activation in Mozambican adults initiating antiretroviral therapy with low CD4 counts. AIDS Res Hum Retroviruses. 2011;27:705–711. doi: 10.1089/AID.2010.0200. [DOI] [PubMed] [Google Scholar]
- 17.Bastard M, Pinoges L, Balkan S, Szumilin E, Ferreyra C, Pujades-Rodriguez M. Timeliness of clinic attendance is a good predictor of virological response and resistance to antiretroviral drugs in HIV-infected patients. PLoS One. 2012;7:e49091. doi: 10.1371/journal.pone.0049091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S, Abrahams M, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 19.Breen RA, Miller RF, Gorsuch T, Smith CJ, Ainsworth J, Ballinger J, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–1440. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]
- 20.Cingolani A, Cozzi Lepri A, Castagna A, Goletti D, De Luca A, Scarpellini P, et al. Impaired CD4 T-Cell count response to combined antiretroviral therapy in antiretroviral-naive HIV-infected patients presenting With tuberculosis as AIDS-defining condition. Clin Infect Dis. 2012;54:853–861. doi: 10.1093/cid/cir900. [DOI] [PubMed] [Google Scholar]
- 21.Dronda F, Sobrino P, Hernandez-Novoa B, Caro-Murillo AM, Montero M, Iribarren JA, et al. Response to HAART in treatment-naive HIV-infected patients with a prior diagnosis of tuberculosis or other opportunistic infections. Curr HIV Res. 2011;9:229–236. doi: 10.2174/157016211796320324. [DOI] [PubMed] [Google Scholar]
- 22.Hardwick RJ, Amogne W, Mugusi S, Yimer G, Ngaimisi E, Habtewold A, et al. beta-defensin genomic copy number is associated with HIV load and immune reconstitution in sub-saharan Africans. J Infect Dis. 2012;206:1012–1019. doi: 10.1093/infdis/jis448. [DOI] [PubMed] [Google Scholar]
- 23.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–2622. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 24.Julg B, Poole D, Ghebremichael M, Castilla C, Altfeld M, Sunpath H, et al. Factors predicting discordant virological and immunological responses to antiretroviral therapy in HIV-1 clade C infected Zulu/Xhosa in South Africa. PLoS One. 2012;7:e31161. doi: 10.1371/journal.pone.0031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lartey M, Sagoe KW, Yang H, Kenu E, Xexemeku F, Oliver-Commey J, et al. Viral decay rates are similar in HIV-infected patients with and without TB coinfection during treatment with an Efavirenz-based regimen. Clin Infect Dis. 2011;52:547–550. doi: 10.1093/cid/ciq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manosuthi W, Tantanathip P, Prasithisirikul W, Likanonsakul S, Sungkanuparph S. Durability of stavudine, lamivudine and nevirapine among advanced HIV-1 infected patients with/without prior co-administration of rifampicin: a 144-week prospective study. BMC Infect Dis. 2008;8:136. doi: 10.1186/1471-2334-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manosuthi W, Tantanathip P, Chimsuntorn S, Eampokarap B, Thongyen S, Nilkamhang S, et al. Treatment outcomes of patients co-infected with HIV and tuberculosis who received a nevirapine-based antiretroviral regimen: a four-year prospective study. Int J Infect Dis. 2010;14:e1013–1017. doi: 10.1016/j.ijid.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Mussini C, Manzardo C, Johnson M, Monforte A, Uberti-Foppa C, Antinori A, et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS. 2008;22:2461–2469. doi: 10.1097/QAD.0b013e328314b5f1. [DOI] [PubMed] [Google Scholar]
- 29.Patel A, Patel K, Patel J, Shah N, Patel B, Rani S. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naive patients in India who are coinfected with tuberculosis and HIV-1. J Acquir Immune Defic Syndr. 2004;37:1166–1169. doi: 10.1097/01.qai.0000135956.96166.f0. [DOI] [PubMed] [Google Scholar]
- 30.Shipton LK, Wester CW, Stock S, Ndwapi N, Gaolathe T, Thior I, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. Int J Tuberc Lung Dis. 2009;13:360–366. [PMC free article] [PubMed] [Google Scholar]
- 31.Siika AM, Yiannoutsos CT, Wools-Kaloustian KK, Musick BS, Mwangi AW, Diero LO, et al. Active tuberculosis is associated with worse clinical outcomes in HIV-infected African patients on antiretroviral therapy. PLoS ONE. 2013;8:e53022. doi: 10.1371/journal.pone.0053022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumantri S, Djoerban Z. Clinical manifestations and antiretroviral management of HIV/AIDS patients with tuberculosis co-infection in Kramat 128 Hospital. Acta Med Indones. 2008;40:117–123. [PubMed] [Google Scholar]
- 33.Wanchu A, Kuttiatt VS, Sharma A, Singh S, Varma S. CD4 cell count recovery in HIV/TB co-infected patients versus TB uninfected HIV patients. Indian J Pathol Microbiol. 2010;53:745–749. doi: 10.4103/0377-4929.72070. [DOI] [PubMed] [Google Scholar]
- 34.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M Loss to follow-up and mortality among those co-infected with TB at ART initiation in Durban, South Africa (TUPDB303). XVIII International AIDS Conference; Vienna, Austria. 2010. [Google Scholar]
- 35.Hermans S, Van Leth F, Kiragga AN, Hoepelman AI, Lange JM, Manabe YC. CD4+ T cell recovery after ART in HIV/TB Co-infected patients is blunted in patients with ART-associated TB compared to patients with TB treated prior to ART. 18th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2011. [Google Scholar]
- 36.Muzoora C, Bangsberg D, Geng EH, Emenyonu N, Andia I, Bwana M Impact of tuberculosis on T cell activation and subsequent mortality in HIV-infected Ugandans initiating antiretroviral therapy. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 37.Odo M, Olareqaju S, Oladele E, Adedokun O, Ogbanufe O, Odafe S Comparison of immune recovery among HIV and TB-HIV co-infected patients on antiretroviral therapy in Nigeria [PC-855-17]. The 43rd World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease; Kuala Lumpur, Malaysia. 2012. [Google Scholar]
- 38.Tan HY, Yong YK, Ong LY, Lee YM, Omar SFS, Sasheela S Impact of active tuberculosis on the immune recovery of HIV-infected individuals receiving ART. XVIII International AIDS Conference; Vienna, Austra. 2010. [Google Scholar]
- 39.Manosuthi W, Sungkanuparph S, Thakkinstian A, Rattanasiri S, Chaovavanich A, Prasithsirikul W, et al. Plasma nevirapine levels and 24-week efficacy in HIV-infected patients receiving nevirapine-based highly active antiretroviral therapy with or without rifampicin. Clin Infect Dis. 2006;43:253–255. doi: 10.1086/505210. [DOI] [PubMed] [Google Scholar]
- 40.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 41.Auld AF, Mbofana F, Shiraishi RW, Sanchez M, Alfredo C, Nelson LJ, et al. Four-year treatment outcomes of adult patients enrolled in Mozambique's rapidly expanding antiretroviral therapy program. PLoS One. 2011;6:e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mugusi S, Ngaimisi E, Janabi M, Minzi O, Bakari M, Riedel KD, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-Based HAART with or without tuberculosis co-infection in Tanzania. PLoS ONE. 2012;7:e40180. doi: 10.1371/journal.pone.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schomaker M, Egger M, Maskew M, Garone D, Prozesky H, Hoffmann C, et al. Immune recovery after starting ART in HIV-infected patients presenting and not presenting with tuberculosis in South Africa. J Acquir Immune Defic Syndr. 2013;63:142–145. doi: 10.1097/QAI.0b013e318288b39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassett IV, Chetty S, Wang B, Mazibuko M, Giddy J, Lu Z, et al. Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr. 2012;59:25–30. doi: 10.1097/QAI.0b013e31823d3aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westreich D, MacPhail P, Van Rie A, Malope-Kgokong B, Ive P, Rubel D, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23:707–715. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table of combination antiretroviral therapy regimens used in each study.pdf
Supplemental Digital Content 2. Table of timing of TB treatment in relation to cART initiation, by study.pdf
Supplemental Digital Content 3. Table of methods for handling loss-to-follow-up and mortality utilized by each study.pdf
Supplemental Digital Content 4. Table of quantification of virologic response to combination antiretroviral therapy, stratified by TB treatment status, as reported by 17 studies.pdf
Supplemental Digital Content 5. Table of meta-regression results for the effect of TB treatment on virologic suppression after combination antiretroviral therapy initiation.pdf
Supplemental Digital Content 6. Figure of cART-regimen specific relative risks of virologic suppression.pdf
Supplemental Digital Content 7. Figure of funnel plot of overall relative risk of virologic suppression.pdf
Supplemental Digital Content 8. Table of quantification of CD4 count response to combination antiretroviral therapy, stratified by TB treatment status, as reported by 21 studies.pdf