Abstract
During critical periods of development early in life, excessive or scarce nutritional environments can disrupt the development of central feeding and metabolic neural circuitry, leading to obesity and metabolic disorders in adulthood. A better understanding of the genetic networks that control the development of feeding and metabolic neural circuits, along with knowledge of how and where dietary signals disrupt this process, can serve as the basis for future therapies aimed at reversing the public health crisis that is now building as a result of the global obesity epidemic. This review of animal and human studies highlights recent insights into the molecular mechanisms that regulate the development of central feeding circuitries, the mechanisms by which gestational and early postnatal nutritional status affects this process, and approaches aimed at counteracting the deleterious effects of early over- and underfeeding.
Keywords: obesity, diabetes, neural circuits, neurogenesis, hypothalamus, diet
INTRODUCTION
Over the past 30 years, global rates of obesity and comorbid health problems like type 2 diabetes (T2D), cardiovascular disease, and cancer have increased dramatically (1). The scale of this problem is staggering. An estimated 43 million children were estimated to be overweight or obese in 2010, and this number is estimated to increase to 60 million by 2020 (2). Increased body weight has moved in lockstep with pediatric prevalence of T2D, which now accounts for almost half of all child and adolescent diabetics (3).
Although economic and lifestyle changes, such as increased availability of high-calorie food and reduced physical activity, undoubtedly underlie these changes, developmental changes resulting from early-life overnutrition compound the problem. It has long been known that both under- and overfeeding during gestation or early childhood substantially increase the risk of obesity and comorbid diseases in adulthood (4, 5), a phenomenon referred to as metabolic imprinting (6). The lag between childhood and the onset of adult obesity suggests that permanent changes in central feeding circuitries, induced by maternal diet and adiposity, play an important part in driving the obesity epidemic (7).
This public health crisis provides the backdrop for this review, which covers two closely linked topics. First, because the central homeostat regulating feeding and metabolism is located in the brain, we review how neuronal feeding and metabolism circuitry is formed during embryonic and early postnatal development. Second, we review the long-term effects of developmental exposure to nutritional excess or scarcity, paying particular attention to how metabolic signals modulate the development of neural circuitry regulating feeding and metabolism.
OVERVIEW OF CENTRAL NEURAL CIRCUITRY REGULATING FEEDING AND METABOLISM
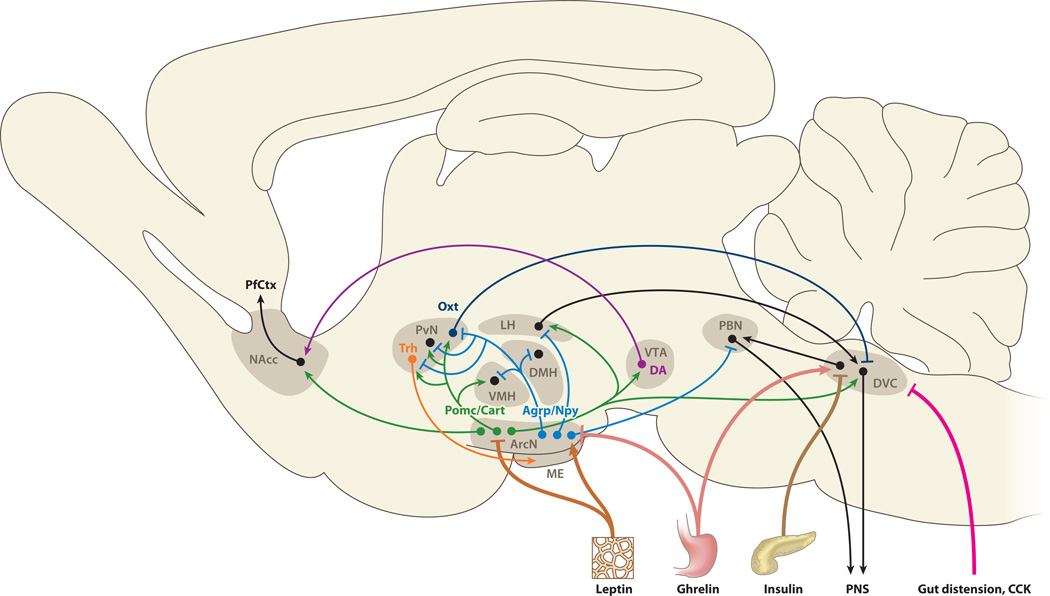
The hypothalamus is the key brain region that detects internal nutrient levels and uses this information to control feeding behavior. Neurons of the hypothalamic arcuate nucleus (ArcN) respond directly to circulating hunger- and satiety-promoting signals. The peptide hormone ghrelin, the most potent hunger-promoting peripheral substance identified, is released into the circulation by P/1D cells of the stomach fundus and pancreatic epsilon cells in response to low nutrient levels. Upon binding to the Gshr1a receptor, ghrelin rapidly activates GABAergic neurons in the ArcN that coexpress neuropeptide Y (Npy) and agouti gene–related peptide (Agrp) to stimulate feeding (8). Direct activation of Agrp-expressing neurons by using optical or chemical genetic tools leads to ravenous eating (9, 10). Signals that strongly repress feeding include insulin, which is released from the pancreas, and the cytokine leptin, which is produced by white adipose tissue; both of these activate arcuate neurons that coexpress pro-opiomelanocortin (Pomc) and cocaine- and amphetamine-regulated transcript (Cart). In addition, both leptin and insulin inhibit the activation of orexigenic Agrp neurons. The opposing actions of Pomc- and Agrp-expressing neurons are further amplified by a reciprocal inhibitory local circuit, with Agrp acting as an inverse agonist of melanocortin receptor 4 (Mcr4), the principal effector of the Pomc cleavage product α-melanocyte-stimulating hormone (α-MSH). In turn,Mcr4 activation inhibits excitation of Agrp neurons (11). The resulting bistable circuit allows for robust and stable internal representation of hunger and satiety and enables rapid transition between these two physiological states in response to internal hormonal signals.
Both Agrp and Pomc neurons directly project to multiple neuronal subtypes in the hypothalamic paraventricular nucleus (PvN). Inhibition of oxytocin (Oxt)-expressing PvN neurons by Agrp neurons is both necessary and sufficient for acute induction of feeding (10). Oxt neurons in turn send descending projections to the dorsal vagal complex (DVC) of the medulla, which in turn controls the action of the peripheral nervous system to produce the somatic manifestations of hunger and satiety. The DVC includes the nucleus of the tractus solitarius (NTS) and the area postrema, a circumventricular organ that, like the hypothalamic median eminence (ME), lies outside the blood-brain barrier. Pomc is expressed in a subset of NTS neurons, which, like ArcN neurons, are leptin responsive (12, 13). Taste and other sensory inputs required for feedback regulation of digestive, respiratory, and cardiovascular organs are conveyed to the central nervous system by visceral sensory ganglia, which converge at the NTS. Descending parallel inhibitory projections to the DVC are also relayed through unidentified neurons in the lateral hypothalamus (LH), where both Pomc-positive cells and Agrp-positive cells send prominent projections. LH lesions that affect these descending efferents typically induce anorexia, whereas disruption of descending PvN efferents results in hyperphagia and obesity (14, 15). Agrp neuron innervation of the pontine parabrachial nucleus (PBN) is necessary but not sufficient for acute feeding (10, 16) and may be required to suppress descending glutamatergic projections from both the PBN and DVC that induce visceral disgust and inhibit feeding (17).
Other intrahypothalamic Agrp and α-MSH projections may also play important roles in modulating food intake. α-MSHergic efferents to the ventromedial hypothalamus (VMH) trigger release of brain-derived neurotrophic factor (Bdnf), which suppresses feeding (18), whereas reduction in α-MSHergic signaling to the dorsomedial hypothalamic nucleus (DMH) can trigger hyperphagia (19). Neurosecretory PvN cells also play a critical role in body weight homeostasis downstream of Pomc and Agrp signaling by regulating thyroid function through the release of thyrotrophin-releasing hormone (Trh).
Finally, the neural networks that control hunger and satiety are connected to central reward circuitries through the ventral tegmental area (VTA) and the nucleus accumbens (NAcc), which are connected by dopaminergic projections of the basal forebrain bundle that pass directly through the ventral hypothalamus. Ghrelin directly targets the VTA to increase motivation to eat (20), whereas both leptin and insulin act in the VTA to suppress hedonic feeding and reward-associated food intake (12, 21). Insulin may elicit these behavioral changes by directly stimulating reuptake of dopamine, thereby potently reducing synaptic dopamine levels (22). Other projections that target the VTA and suppress feeding include α-MSHergic projections from the ArcN (23, 24) and oxytocinergic projections from the PvN (25). α-MSHergic projections also extend to the NAcc and suppress feeding during chronic psychological stress (26). Figure 1 summarizes the central neural circuitry regulating feeding and appetite.
Figure 1.
An overview of neural circuitry controlling both appetitive and hedonic aspects of food intake is shown. Excitatory synaptic inputs are indicated by arrowheads, and inhibitory inputs are indicated by bars. Abbreviations: Agrp, agouti gene–related peptide; ArcN, hypothalamic arcuate nucleus; Cart, cocaine- and amphetamine-regulated peptide; CCK, cholecystokinin; DA, dopamine; DMH, dorsomedial hypothalamic nucleus; DVC, dorsal vagal complex; LH, lateral hypothalamus; ME, median eminence; NAcc, nucleus accumbens; Npy, neuropeptide Y; Oxt, oxytocin; PBN, parabrachial nucleus; PfCtx, prefrontal cortex; PNS, peripheral nervous system; Pomc, pro-opiomelanocortin; PvN, paraventricular nucleus; Trh, thyrotrophin-releasing hormone; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
DEVELOPMENT OF CENTRAL FEEDING CIRCUITRY IN MAMMALS
Patterning and Cell Fate Specification in the Developing Hypothalamus
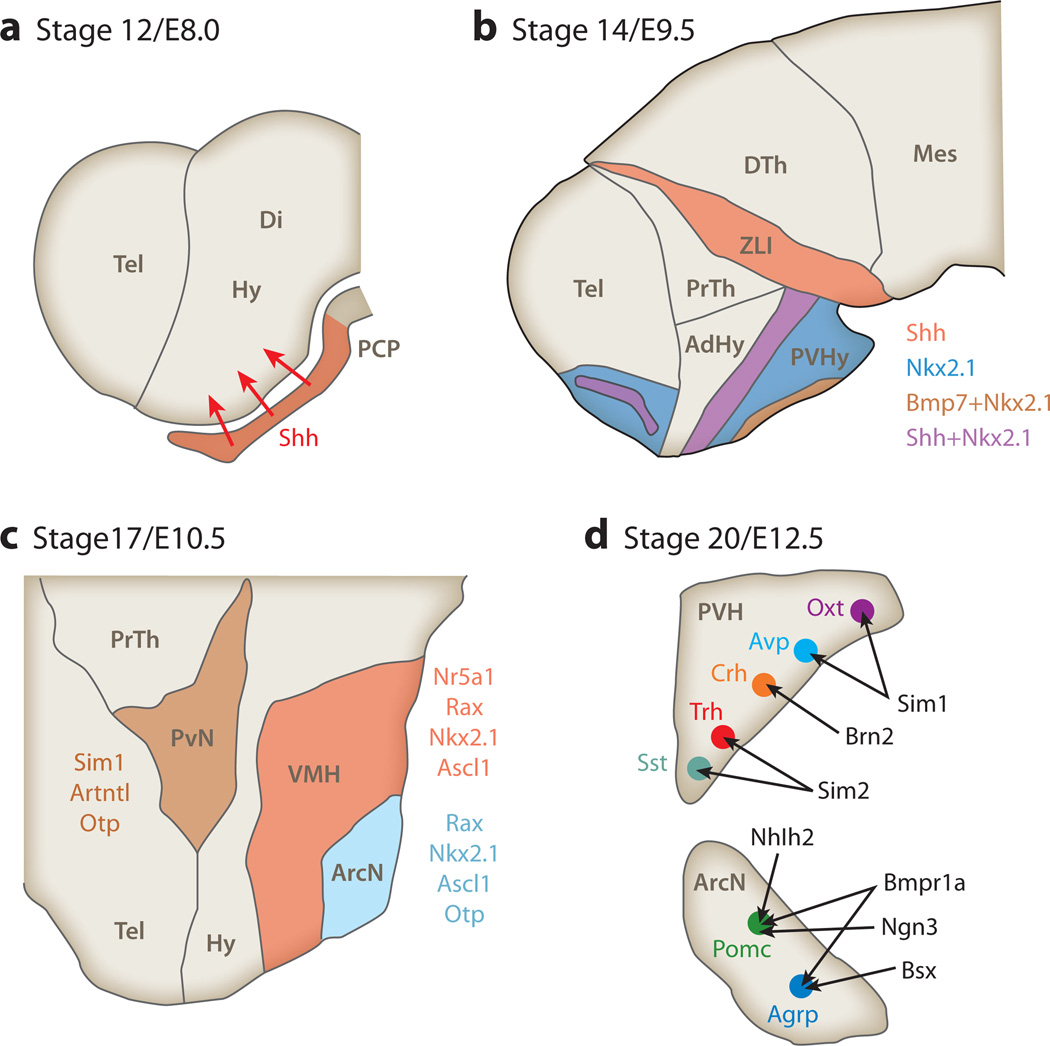
The hypothalamic primordium is initially patterned by sonic hedgehog (Shh) secreted by the prechordal plate (PCP), the anteriormost extension of the notochord (27). PCP-derived Shh induces Shh expression in the hypothalamic floor plate, which in turn induces expression of bone morphogenetic protein 7 (Bmp7) in ventral hypothalamic neuroepithelium (28). Bmp7 then both suppresses ventral Shh expression and induces Shh expression in the more dorsally located basal plate domain (Figure 2a). Basal plate–derived Shh both drives progenitor proliferation and maintains identity of anterior and tuberal hypothalamic nuclear primordia (Figure 2b) (29, 30). Wnt signaling is required for initial specification of hypothalamic primordium (31) and later for specification of posterior hypothalamic structures (32).
Figure 2.
Molecular mechanisms controlling hypothalamic development. (a) Early hypothalamic patterning by prechordal plate–derived sonic hedgehog (Shh) in sagittal sections of mouse hypothalamus. (b) Expression of Shh, bone morphogenetic protein 7 (Bmp7), and NK2 homeodomain 1 (Nkx2.1) in hypothalamic neuroepithelium prior to the onset of neurogenesis. (c) Transcription factors required for nucleogenesis and the early stages of neurogenesis in hypothalamic nuclei that regulate feeding. (d) Control of neuronal subtype specification in the developing PvN and ArcN. Panels a, b, and c are shown in sagittal orientation, whereas panel d is in coronal orientation. Other abbreviations: AdHy, anteriodorsal hypothalamus; Agrp, agouti gene–related peptide; ArcN, hypothalamic arcuate nucleus; Avp, arginine vasopressin; Crh, corticotrophin-releasing hormone; Di, diencephalon; DTh, dorsal thalamus; Hy, hypothalamus; Mes, mesencephalon; Oxt, oxytocin; PCP, prechordal plate; Pomc, pro-opiomelanocortin; PrTh, prethalamus; PVH, paraventricular hypothalamic nucleus; PVHy, posterioventral hypothalamus; PvN, paraventricular nucleus; Sst, somatostatin; Tel, telencephalon; Trh, thyrotrophin-releasing hormone; VMH, ventromedial hypothalamus; ZLI, zona limitans intrathalamica.
Nucleogenesis and Cell Fate Specification in the Developing Hypothalamus
Multiple transcription factors are required for different stages of ArcN development. Shh signaling induces NK2 homeodomain 1 (Nkx2.1) expression in posterioventral hypothalamic progenitors; loss of Nkx2.1 blocks the development of hypothalamic nuclei in this region, including VMH and ArcN (Figure 2c) (33). The paired-type homeodomain Rax is also expressed in early hypothalamic progenitors and is necessary to maintain expression of both Nkx2.1 and Pomc, along with that of the VMH marker gene Nr5a1, which encodes SF-1 (34).
Transcription factors expressed in progenitors that give rise to the VMH and ArcN also control terminal differentiation of both structures. For example, Nr5a1 is selectively required for normal development of the VMH. Although VMH neurons are generated in normal numbers in Nr5a1 mutants, VMH cellular organization is abnormal, as are its afferent and efferent projections. Nr5a1-deficient animals fail to express Bdnf in the VMH, which potently suppresses feeding, and both embryonic deletion and postnatal deletion of Nr5a1 in the VMH lead to leptin resistance and obesity (35). The homeobox factor Otp is also prominently expressed in ArcN progenitors, with loss of function leading to hypocellularity (36).
The onset of neurogenesis, which begins at approximately embryonic day (E)10.5 in the mouse (37), triggers expression of the neurogenic basic helix-loop-helix (bHLH) factors Ascl1 (Mash1), Ngn3, and Nhlh2 in both the ArcN and VMH. These bHLH proteins control the development of specific subsets of neurons in the ArcN and VMH (Figure 2d). Ascl1−/− mice show a reduction of more than 90% in Nr5a1-, Pomc-, and Agrp-expressing neurons (38). Genetic fate mapping reveals that Ngn3-expressing progenitors give rise to subsets of Pomc-, Npy-, and Nr5a1-positive neurons in the ArcN and VMH. Furthermore, deletion of Ngn3 reveals that it normally promotes the development of Pomc- and Nr5a1-positive neurons while inhibiting the development of Npy neurons (39), consistent with the recent finding that selective loss of Ngn3 in the developing hypothalamus of Ngn3 results in early-onset obesity due to hyperphagia (40). Similarly, loss of Nhlh2 results in obesity (41), with Nhlh2−/− mice exhibiting a reduction in MSH processing caused by defective expression of prohormone convertase-1 in Pomc neurons (42). The homeodomain transcription factor encoded by Bsx, which is prominently expressed in the developing ArcN (43), is required for normal Npy and Agrp expression (44). However, although Bsx−/− mice show reduced food intake, overall body weight is unaffected, implying that compensatory changes take place elsewhere in the central feeding circuitry (45).
Relatively little is known about extrinsic factors that influence neuronal subtype identity in the developing ArcN, with the exception of a recent finding that Bmpr1a-dependent activin signaling is critical for the development of Agrp- and Npy-positive neurons; to a lesser extent, this same signaling pathway also affects the development of other ArcN cell types (46). Mice lacking Bmpr1a expression in ArcN neuronal precursors develop severe anorexia (46), although because the Olig1-Cre line used in this study is expressed in many other regions of the developing brain, it is unclear whether anorexia results directly from the observed defects in ArcN development. An intriguing study mapping the Pomc-expressing cell lineage in the ArcN revealed that a subset of Pomc-expressing cells switch to expressing Agrp beginning at approximately E14, with nearly 25% of adult Agrp-expressing neurons deriving from Pomc-positive precursors (47). This switch from anorexigenic to orexigenic identity may be under the control of extrinsic factors and may be one way in which gestational under- or overfeeding regulates cell composition in the ArcN.
The transcriptional network controlling PvN neuroendocrine cell development is much better understood than for other regions of the hypothalamus. The Per-Arnt-Sim family gene Sim1 is expressed in PvN progenitors beginning at approximately E10.5 (Figure 2c). Although Sim1−/− mice lose essentially all neurosecretory cell markers, Sim1 gene dosage differentially affects the development of neuroendocrine cell types, with a selective reduction in Oxt- and Avp-positive cell numbers in Sim1+/− mice (Figure 2d) (48, 49). Sim1 haploinsufficiency causes obesity in both humans (50) and mice (51). In contrast, Sim1 overexpression in PvN can partially reverse weight gain induced by high-fat diet (HFD) or by defective Mcr4 signaling (52). Sim1+/− mice exhibit defective descending oxytocinergic PvN projections to the NTS; these animals are also hypersensitive to Oxt (48, 49). Furthermore, postnatal Sim1 deletion also results in obesity by 12 weeks of age due to hyperphagia (53), thus implying that metabolic defects in Sim1-deficient mice may be attributable in part to Sim1’s role in the maintenance of cell identity and function. Taken together, these findings imply that the loss of descending Oxt projections from the PvN partially mediates the effects of Sim1 haploinsufficiency on body weight and suggest that restoring the full complement of Sim1 activity may offer a possible treatment for morbidly obese individuals harboring one of these rare Sim1 mutations.
Other transcription factors controlling PvN neuroendocrine cell differentiation act both in parallel and downstream of Sim1. Otp is a factor that is expressed in PvN progenitors and acts independently of Sim1 to control terminal differentiation of PvN neuroendocrine cells; this function is in addition to Otp’s role in ArcN development (36, 54). Arnt2 is an obligatory heterodimer of Sim1 for both DNA binding and transcriptional activation, with Arnt2 mutants phenocopying Sim1 loss of function (55, 56). The Sim1 homolog Sim2 acts downstream of Sim1 to promote the development of Trh- and somatostatin (Sst)-expressing neuroendocrine cells (Figure 2d) (57). Brn2 is a critical downstream target of Sim1 and Arnt2, as evidenced by the selective loss of Crh-, Oxt-, and Avp-expressing PvN and supraoptic nucleus neurons. Unfortunately, an assessment of behavioral and metabolic parameters in Brn2-null mice is precluded by their early neonatal lethality (58, 59).
Although the past few years have seen much progress in our understanding of the molecular pathways regulating the specification of neurons in the central feeding circuits, much remains unknown. For example, nothing is known about the extrinsic molecular factors controlling neuroendocrine cell development or about the transcriptional networks guiding the differentiation of neurons in the LH, which also play important roles in regulating feeding. Moreover, with the notable exception of the trophic actions of leptin and insulin, which are discussed below, we are likewise ignorant of the molecular pathways affecting axonal pathfinding and synaptogenesis during later stages of the formation of these neural circuits. However, recent large-scale gene expression screens have identified large numbers of both transcription factors and growth and differentiation factors dynamically expressed in all major hypothalamic nuclei, providing a rich source of candidate genes for future studies (30).
Postnatal and Adult Hypothalamic Neurogenesis
The induction of neurogenesis is a potential mechanism by which central feeding circuitry can enduringly adapt to nutritional availability. Although evidence for neural progenitor cells in the juvenile hypothalamus and adult hypothalamus is rapidly mounting (60–62), the identity, location, and precise functions of neural progenitor cells in the postnatal hypothalamus are controversial.
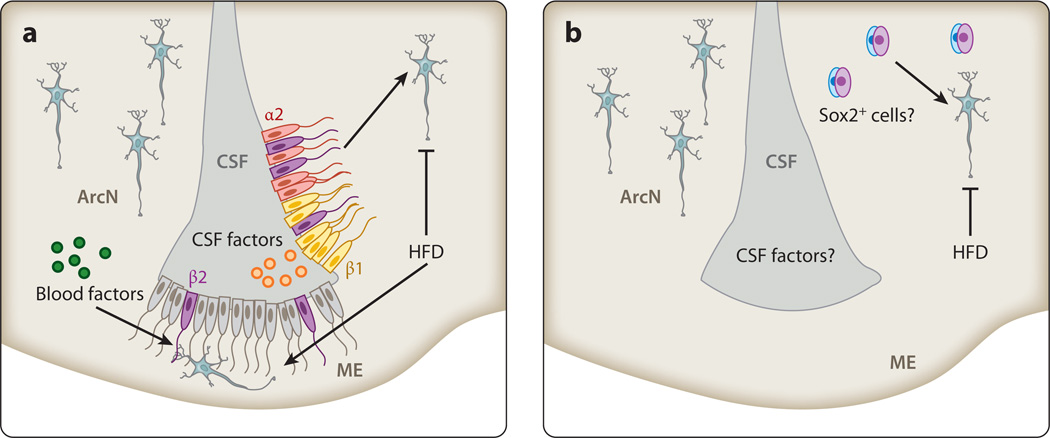
Recent studies suggested that tanycytes, a radial glial-like ependymal cell that lines the ventral portion of the third ventricle, are a potential source of adult-born neurons (Figure 3) (63). Tanycytes express many genes selectively enriched in embryonic hypothalamic progenitors and adult neural stem cells of the subventricular zone of the lateral ventricles and the subgranular zone of hippocampal formation (30, 60, 64). This tanycyte cell population is divided into four subtypes, α1, α2, β1, and β2, which can be distinguished by functional differences, position, the expression of differentially important functional molecules and receptors, and ultrastructure (64). Circumstantial evidence implicating tanycytes as potential neural progenitors first arose from in vitro neurosphere or stemness assays using tissue obtained from the lining of the hypothalamic third ventricle (65, 66). Subsequent prospective genetic-fate-mapping studies demonstrated that one tanycyte subtype, the β2 tanycyte, generates neurons in the ME of juvenile mice, providing direct in vivo evidence that tanycytes are neurogenic (60). Tanycyte neurogenesis is regulated by nutrient availability, as HFD selectively stimulated neurogenesis in both juvenile and young adult murine ME (Figure 3a), whereas focal irradiation of the ME attenuated weight gain when young adults were fed HFD (60). Although this study reported little evidence that other tanycyte subtypes were substantially neurogenic, lineage analysis using Fgf10-CreER knock-in mice showed that a substantial number of hypothalamic neurons were derived from α2 and β1 tanycytes of the ArcN (67). These differences may arise from the use of different ages and inducible Cre lines, with the Fgf10-CreER mouse line used in this study showing modest but potentially confounding ectopic recombination in hypothalamic parenchymal neurons and glia (67). Another recent study, in which prospective lineage analysis was conducted by using a Glast-CreER transgene, also demonstrated that α tanycytes can give rise to both neurons and glia in adult hypothalamus, although the fact that this line does not express Cre in β tanycytes precluded a direct analysis of the contribution of this cell type to adult hypothalamic neurogenesis (68).
Figure 3.
Potential sources of postnatal hypothalamic neurogenesis. (a) α2 (red) and β1 (yellow) tanycytes of the hypothalamic arcuate nucleus (ArcN) and β2 tanycytes (purple) of the median eminence (ME) function as neural progenitors, with newborn neurons migrating along tanycyte foot processes into the hypothalamic parenchyma. High-fat diet (HFD) stimulates neurogenesis from β2 tanycytes of the ME but inhibits neurogenesis in more dorsal tanycytic neural progenitors. Factors present in the cerebrospinal fluid (CSF) directly contact the apical surface of tanycytes, whereas blood-borne factors contact β2 tanycytes. (b) HFD inhibits an as-yet-uncharacterized, constitutively proliferating parenchymal progenitor population.
Evidence exists for a slowly proliferating neural progenitor population among neurons and glial parenchymal cells of the adult mouse hypothalamus. The suggestion that constitutive neurogenesis occurred throughout adult forebrain parenchyma was initially supported by widespread BrdU incorporation into neurons in the striatum, thalamus, and hypothalamus following continuous intracerebroventricular (icv) delivery of Bdnf and bromodeoxyuridine (BrdU), a nucleotide analog incorporated into replicating DNA (69). Later, increased hypothalamic neurogenesis was reported in studies of icv delivery of ciliary neurotrophic factor (Cntf) to the lateral ventricles. Moreover, simultaneous icv delivery of AraC, which inhibits cell proliferation, blocked the effects of Cntf (70). Other studies found that both long-term HFD (71) and activation of the nuclear factor κB (NFκB) cytokine signaling pathway (72) inhibit BrdU incorporation into hypothalamic Pomc neurons. Furthermore, enhancing NFκB signaling impairs survival and differentiation of a Sox2-positive candidate neural progenitor cell population in ArcN parenchyma and results in increased food intake and body weight at 10 months post–gene delivery. Taken together, these findings suggest that parenchymal hypothalamic neurogenesis may primarily generate anorexigenic neuronal subtypes and attenuate diet-induced increases in body weight.
Although studies concerning hypothalamic parenchymal neurogenesis triggered considerable excitement about the potential modulation of hypothalamic neural circuitry by de novo neurogenesis, some caution is necessary in interpreting these findings. Importantly, the extensive number of labeled BrdU+ neurons is seen only when BrdU is continuously delivered by icv, but not with intraperitoneal or oral delivery pulses of BrdU. The sparse number of BrdU-labeled neurons observed with these more conventional delivery routes indicates that the overwhelming majority of BrdU+ cells in forebrain parenchyma are NG2+ oligodendrocyte precursors rather than neurons (73). These discrepant results using either pulses or continuous BrdU delivery may suggest that BrdU+ neurons in hypothalamic parenchyma derive from exceptionally slowly proliferating progenitors or, alternatively, may reflect BrdU incorporation independent of cell division. Indeed, DNA synthesis can be initiated independently of mitosis during gene duplication, repair, or apoptosis (74, 75), implying that all dividing cells incorporate BrdU but that not all cells labeled with BrdU necessarily divide. Furthermore, continuous BrdU long-term infusion may pose a toxicity risk to hypothalamic neurons and increase BrdU incorporation due to increased DNA repair (76).
Recent evidence suggests that Sox2+ cells may be a parenchymal source of adult-born hypothalamic neurons. Viral tracing studies that marked Sox2+ cells by using a lentiviral construct expressing Cre recombinase under the control of the Sox2 promoter concluded that newborn parenchymal hypothalamic neurons derive from a Sox2+ progenitor pool (72). However, the fidelity of this particular virally delivered Sox2-Cre reagent has not been clearly demonstrated in vivo. Revisiting this question using Sox2-CreERT2 knock-in mice, which fully recapitulates Cre-mediated recombination from endogenous Sox2 (77), may resolve this question. Thus, unlike tanycytic neurogenesis, the identity of a putative parenchymal neural progenitor cell(s) remains to be definitively determined.
Studies reporting tanycyte neurogenesis rest on firmer ground, having used mutant mice in which inducible Cre recombinase is selectively expressed in tanycytes for prospective lineage mapping (60, 67). However, focal irradiation of HFD-fed mice performed in these studies (60) blocked not only neurogenesis, but also generation of all cell types in the ME. Intersectional genetic approaches to selectively disrupt tanycyte-derived neurogenesis will be needed to definitively prove the importance of this process in regulating body weight and metabolism (78).
Development of Brain Stem Components of Feeding Circuitry
The molecular mechanisms regulating the development of brain stem circuitry involved in feeding are even less well understood than those controlling hypothalamic development. The homeodomain transcription factor encoded by Phox2b controls the development of both the NTS and its visceral sensory ganglia afferent regions (79). Genetic fate mapping reveals that Phox2b acts in peripheral sensory neurons to promote visceral identity while repressing somatosensory identity (80). The neurogenic bHLH factor encoded by Olig3 is required for NTS neuronal development (81). Neurogenesis has also been reported in area postrema of adult mice, a circumventricular organ like the hypothalamic ME, although its regulation by dietary signals and its role in regulating feeding are unclear (82, 83).
DISORDERS THAT DISRUPT DEVELOPMENT OF CENTRAL FEEDING CIRCUITRY
Genetic variants that regulate body weight and metabolism can also disrupt the development of central feeding circuitry. These variants include not only the rare mutations mentioned in the previous section, which play central roles in hypothalamic development, but also a range of syndromic and single-gene disorders known or suspected to disrupt hypothalamic development. One of the best-characterized examples of syndromic developmental obesity is Prader-Willi syndrome (PWS), the prevalence of which ranges from 1:15,000 to 1:30,000 (84). Among multiple other neurological and behavioral disturbances, the disorder presents with uncontrolled hyperphagia and an obsession with food beginning at approximately 2–8 years of age (84). If food intake is not controlled externally, body mass increases and progresses to obesity and, eventually, to diabetes (85). Serum ghrelin is already elevated in PWS (86) by the first year of life, preceding the onset of hyperphagia (87).
Co-occurrence of hyperphagia with central hypogonadism and small stature suggests that disrupted hypothalamic development is a central etiology of PWS (88). Most cases result from a 5–7-Mb deletion in paternally inherited chromosome 15q11.2-q13, which contains multiple genes subject to parental imprinting. Loss-of-function mouse models of some of these imprinted genes have given insight into the genetic underpinnings of PWS. Mice deficient in Magel2 or Necdin (Ndn) demonstrate features reminiscent of PWS and strongly support the hypothesis that PWS results from aberrant hypothalamic development. Ndn−/− mice have profound deficits in some hypothalamic neuronal subtypes (89); similarly, Magel2−/− mice have fewer Agrp-positive neurons, excessive weight gain after weaning, and altered adult metabolism (90, 91). Despite these genetic insights, considerable controversy due to the variable penetrance of PWS remains, with patient studies indicating that deletions, including those of MAGEL2 and NDN, may not be sufficient to cause disease (92). These conflicting results may be explained by interactions of PWS genes with maternally derived genetic modifiers (93), although the relevant genetic variants remain unidentified.
Other individual genes that control hypothalamic development have also been proposed to underlie obesity and related metabolic disorders. One such gene is delta-like 1 homolog (Dlk1), a transmembrane protein that is homologous to the Delta family of Notch ligands and that acts as a noncanonical Notch signaling inhibitor in vitro (94). Like Magel2 and Ndn, Dlk1 undergoes parental imprinting and is prominently and selectively expressed in both the developing hypothalamus and the adult hypothalamus (95). Loss of function of paternally inherited human DLK1 leads to extreme child and adolescent obesity (96). Likewise, increased adiposity and obesity are seen in mice heterozygous for a paternally, but not maternally, inherited mutant allele of Dlk1 (97).
Bdnf and its receptor TrkB regulate neurogenesis, synaptogenesis, and neuronal survival in multiple brain regions. Bdnf is robustly expressed in several hypothalamic regions, with haploin-sufficiency leading to early-onset obesity in mice (98). Both BDNF haploinsufficiency and TRKB haploinsufficiency similarly lead to early-onset obesity in humans (99, 100). The human Val66Met polymorphism decreases BDNF secretion (101) and is associated with increased risk of obesity (102, 103). Despite extensive data demonstrating an anorexigenic function for Bdnf, its role in hypothalamic development is unclear. However, because intrahypothalamic infusion of recombinant Bdnf acutely inhibits feeding (104) and restoring Bdnf to the hypothalami of Bdnf-deficient adult mice normalizes body weight, any effects of Bdnf deficiency during development are apparently reversible later in life. It is unclear whether restoring the function of other obesity-associated genes in adulthood can normalize weight, a critical consideration for any targeted gene therapy approaches aimed at correcting these defects.
Other genes linked to control of body weight and adiposity in human association studies includeFTO(fat mass and obesity–associated gene), which encodes an RNA demethylase expressed prominently in the developing hypothalamus and the adult hypothalamus (105). FTO couples mT or activity to intracellular amino acid levels (106). Common FTO variants are associated with a substantial increase in the risk of early-onset obesity in humans (107), and maternal obesity induces Fto mRNA expression in neonatal, but not adult, offspring (108). Other genes associated with early-onset obesity in human populations include those encoding the protein kinase PRKCH and the cell adhesion molecule NEGR1 and the long non coding RNA RMST (109, 110), which are all prominently expressed in the developing hypothalamus (30). Negr1-deficient mice show substantially reduced food intake and physical activity (111).
Understanding the molecular pathways that mediate hypothalamic development will inform our understanding of how inappropriate development of feeding and metabolic circuits contributes to the manifestation of metabolic disorders. Such knowledge will allow for the use of genetic biomarkers in formulating treatment plans for metabolic disorders tailored to underlying individual disease mechanisms.
EARLY-LIFE ENVIRONMENTAL REGULATION OF ADULT METABOLISM
Although this review thus far focuses on the molecular and genetic mechanisms guiding the development of central feeding and metabolic circuitry, dietary factors and hormonal factors regulate this process at all stages. Animal and epidemiological studies show that maternal nutrition during early critical periods (e.g., gestation and lactation) can produce lasting changes in metabolism and physiology of offspring. These early environmental effects on adult body weight have been appreciated for at least half a century (4, 112) and have matured conceptually into metabolic imprinting theory, initially proposed by Hales & Barker (113). These studies systematically demonstrated an elevated risk of diabetes, obesity, and cardiovascular disease in offspring of malnourished mothers (114). Such effects may reflect adaptive changes by the developing fetus that are aimed at maximizing energy utilization in the face of continued poor nutrition following birth, the so-called thrifty phenotype hypothesis (113). More recently, it has become clear that overfeeding in early life can produce deleterious effects much like those of early malnutrition and that long-term effects of early-life nutritional excess or scarcity depend on the timing, duration, and severity of the treatment and on the sex of affected offspring (115). This section summarizes current knowledge on how nutrient levels during critical developmental periods inform long-term metabolic changes.
In general, gestational obesity and early postnatal obesity are often sufficient to rewire central homeostatic and hedonic regulatory circuitry, leading to increased food intake and lower activity rates later in life. Paradoxically, similar effects are also observed with early caloric deprivation. When undernutrition during early critical periods is accompanied by improved nutrition later, many plants and animals (including humans) display compensatory or accelerated catch-up growth. Studies conducted on survivors of the Dutch winter famine of 1944–1945 revealed that undernutrition during the first half of pregnancy induced adult obesity and T2D in humans (116, 117), despite lower final trimester and early postnatal body weight (5). Gestation and birth during postwar famine in Nigeria (118), China (119), and Austria (120) likewise led to a dramatic increase in rates of adult T2D.
It has been hypothesized that metabolic changes from early malnutrition arise from accelerated catch-up growth, which can bring rapid benefits to the organism at a substantial cost later in life (121). This same mechanism may account for the deleterious metabolic effects of early overnutrition. Timing of catch-up growth appears to be critical, with a much higher incidence of obesity and diabetes noted when catch-up occurs close to weaning age (122). In humans, low birth weight coupled with rapid weight gain between ages 3 and 11 greatly increases the risk of developing T2D (123). Long-term food deprivation not followed by abundance, such as during the 3-year-long siege of Leningrad during World War II, does not lead to rapid compensatory catch-up growth (124) and is not associated with increased obesity or T2D rates in adulthood (125). Furthermore, a longitudinal study of 13,517 men and women born at Helsinki University Hospital from 1924 to 1944 suggests that between 25 and 63% of the incidence risk of adult diabetes, hypertension, and coronary heart disease may be attributed to low birth weight and/or to accelerated newborn-to-adolescent weight gain (123).
Gestational and Postnatal Undernutrition
Although maternal undernutrition can elevate obesity andT2Drisk in offspring, this effect depends on the timing and duration of caloric restriction. Undernutrition during the first half of human pregnancy increases obesity risk in offspring, whereas undernutrition during the final trimester or first few postnatal months is associated with lower adult body weight (5). Fetuses exposed to nutritional deprivation have impaired glucose tolerance (116) and higher food intake and adiposity (126, 127). Nutritional deprivation in animal models reduces sympathetic innervation of white adipose tissue in male, but not female, mice (128). Gestational caloric restriction increases cell proliferation in the PvN, VMH, ArcN, and ME in the early postnatal period, most likely reflecting increased gliogenesis or ongoing reactive gliosis (129). However, a 20% caloric restriction during early gestation (E1–12) in rats reduces both Npy and Pomc neuron numbers in the ArcN, along with decreases in both LepR and InsR mRNA(130). Caloric restriction likewise blunts the postnatal surge in leptin and inhibits the formation of ArcN-PvN melanocortinergic projections (131). Maternal malnutrition during lactation can likewise have detrimental effects on offspring and is associated with higher risks of obesity and leptin resistance (132).
Gestational and Early Postnatal Overnutrition
Recent reports of an association between excessive weight in human pregnancy and the BMI of adolescent children support the developmental overnutrition hypothesis, suggesting that maternal obesity may predispose offspring to increased obesity risk (133, 134). Animal studies confirmed maternal obesity as causal for elevated adult hyperphagia and obesity in progeny (135). Neonatal offspring of obese dams display amplified and prolonged leptin surges, which may remodel postnatal central feeding circuitry (136). Hypothalamic Fto mRNA expression is also upregulated by maternal obesity, and the level of such expression is correlated with higher visceral fat in adulthood (108). Early neonatal overfeeding is likewise a risk factor for adult obesity and metabolic disorders, leading to leptin resistance (137), consistent with higher hypothalamic SOCS3 expression and lower STAT3 activity levels in adult rats; both of these transcription factors participate in leptin signaling (138). Moreover, in rodents, the metabolic risks of maternal obesity and postweaning HFD consumption are additive and cumulative (139).
Gestational Diabetes
In a similar vein, offspring of diabetic mothers are predisposed to obesity and T2D later in life. As in humans, murine gestational diabetes (GD) elevates body weight of offspring (140) and reduces mean neuronal areas in both the PvN and the VMH (141). More Npy-positive neurons were observed in the ArcN of both neonatal and adult offspring of GD mothers (141). Neural projections from the ArcN to the PvN are also markedly reduced in progeny of insulin-deficient dams (140). These data show that GD leads to structural changes in central feeding circuitry.
Dietary Signals that Control Development of Central Feeding Circuitry
Epidemiological studies indicate that low maternal intake of dairy and meat protein in late pregnancy is associated with low birth weight (142). In animal models, a gestational low-protein diet (LPD) also results in offspring with lower body weight, as well as in hypoglycemia and hypoinsulinemia (143). Significant structural changes in the hypothalami of LPD dams’ progeny include increased VMH volume and increased VMH and PvN neuron density; both the PvN and the LH also showed increased Npy and Agrp immunoreactivity (129, 143, 144). Although a minimal amount of maternal protein intake is necessary for proper central-feeding-circuitry development, excessive dietary protein in early life likewise has long-term deleterious effects on metabolism. In animals, a gestational high-protein diet (HPD) leads to increased adiposity and to decreased energy expenditure in adult offspring (145), and epidemiological studies suggest that similar effects may occur in humans (146).
A high-carbohydrate diet (HCD) during gestation induces leptin and insulin resistance and increases Npy and Agrp mRNA expression in the ArcN. However, in contrast to HPD and HFD, HCD does not increase offspring weight gain (147). Supplementation of maternal diets with 20% fructose have yielded inconsistent metabolic effects in offspring; one study reported elevated blood leptin and glucose in P10 female rat pups, but not in male rat pups, although whether this effect persisted into adulthood was unclear (148). Another study reported high body weights in adult offspring of mothers fed 20% fructose during gestation (149). It is uncertain whether any observed long-term effects of carbohydrate supplementation resulted from carbohydrates per se or simply from excess caloric intake, as control diets tested in these studies were not isocaloric. The pressing need for additional studies on this topic is highlighted by multiple epidemiological studies that link increased consumption of high-fructose corn syrup with elevated childhood obesity rates, suggesting a possible effect of this supplement on metabolic imprinting (150, 151).
In contrast, it is well established that maternal HFD is a major trigger for long-term deleterious effects on progeny body weight and metabolism (152). HFD increases protein, cholesterol, and triglyceride content in breast milk, elevating total caloric and fat intake in nursing pups (153). In rats, maternal HFD during gestation or nursing increased body weight, adiposity, food consumption, and plasma leptin concentration in pups and led to impaired glucose tolerance and leptin resistance (154). Some studies reported these effects to be sexually dimorphic, with increased adult adiposity and hyperphagia observed only in male progeny, although impaired glucose tolerance occurred in offspring of both sexes (153, 154). In contrast, other studies reported no sex differences in these effects (155).
High-Fat Diet and Regulation of Embryonic and Postnatal Neurogenesis
HFD appears to mediate long-term effects on feeding through controlling the generation and differentiation of hypothalamic neurons. The offspring of pregnant rats fed HFD between E6 and P15 showed elevated hypothalamic cell proliferation at E14, as measured by BrdU incorporation. At later time points, offspring exposed to HFD showed selective increases in neuronal number in the PvN and the LH, but not in the ArcN. Selective increases in the number of galanin- and dynorphin-expressing cells were observed in PvN, along with increases in orexin in the LH and increases in body weight and adiposity (155). Because these neuropeptides are sometimes orexigenic, these findings suggest that embryonic HFD may stimulate the production of neurons that promote food intake.
Several recent studies reported that HFD also controls neurogenesis in the postnatal hypothalamus, although other studies report opposing effects of HFD on neurogenesis and body weight. One possible explanation for this apparent contradiction is differences in the ages and sexes of the animals tested, as Li et al. (60) examined 3–6-month-old males, whereas Lee et al. (72) examined 6–11-week-old females. Furthermore, the two studies targeted different hypothalamic regions, suggesting that hypothalamic parenchymal neural progenitors may predominantly generate anorexigenic neurons, whereas ME tanycytic neural progenitors may predominantly generate orexigenic neurons. In addition, Li et al. (60) examined mice exposed to HFD over several months, while Lee et al. (72) administered HFD for only one month. The recent finding that HFD induces a rapid and transient stimulation of neurogenesis in ventrobasal hypothalami of adult female mice, as measured by BrdU incorporation (156), may underlie the increase in neurogenesis in ME observed by Lee et al. (72). Finally, different hypothalamic neural progenitor pools may produce different sets of anorexigenic or orexigenic neurons in response to changes in nutritional availability, which would also explain the different physiological and inflammatory responses to acute versus chronic HFD (157). These newly adult-born neurons may respond to short-term changes in nutrient availability and may ultimately affect long-term energy homeostasis (158).
Diet-Regulated Hormonal Signals
Changes in gestational or early postnatal diet may trigger long-term changes in feeding regulatory circuitries by affecting known hormone signaling pathways involved in energy homeostasis. Among these, the most heavily studied hormones are leptin and insulin, which are tightly regulated by fatty acid and carbohydrate levels, respectively. Leptin readily crosses the placenta (159), relaying maternal dietary status to the fetus. In contrast, insulin crosses the placenta poorly (160), but because insulin is produced in fetal pancreatic β cells before most hypothalamic neurogenesis begins, it can potentially regulate the earliest stages of central-feeding-circuitry development. Both leptin and insulin promote proliferation in neurosphere cultures of fetal rat hypothalamus, whereas low birth weight leads to depletion of hypothalamic neural progenitor cells in neurosphere assays (161, 162). Furthermore, intrahypothalamic infusion of insulin in neonatal rats led to a decrease in neuronal cell size in the VMH, with a corresponding increase in neuronal size in the DMH, indicating that hypothalamic nuclei important for regulating feeding exhibit a differential susceptibility to the developmental effects of insulin signaling (163).
Leptin plays a critical neurotrophic role for ArcN projection neurons. In rodents, ArcN projections are immature at birth and innervate, in succession, the DMH, PvN, and LH within distinct temporal windows (164). Axonal outgrowth and pathfinding from the ArcN to the PvN are leptin dependent (136), with innervation coinciding with a dramatic increase in circulating leptin levels during the second postnatal week in mice (165). Absence of leptin during this stage disrupts the formation of axonal projections from the ArcN to the PvN (136), whereas a premature surge in leptin levels increases the density of these projections (166). Leptin exerts different effects on the targeting of Agrp and Pomc axonal projections: Leptin-deficient mice exhibit reduced Agrp and Pomc innervation of PvN neuroendocrine cells, whereas only Agrp innervation of preautonomic NTS neurons is affected in these animals. Leptin also induces rapid changes in the strength and number of excitatory and inhibitory synapses contacting ArcN neurons that express either Npy or Pomc; the opposite effects are observed with ghrelin (167).
Leptin regulates hypothalamic circuitry development by different signaling mechanisms downstream of the leptin receptor (LepR), activating both Stat3 phosphorylation and PI3K/Akt/Erk signaling. The formation of Agrp projections to the PvN requires LepR-dependent activation of both Stat3 and Erk1/2, whereas leptin-dependent formation of Pomc projections requires only Stat3 phosphorylation (168). LepR-dependent phosphorylation of Stat3 triggers the nuclear entry of Stat3, allowing it to activate transcription. Erk and Akt signaling in turn triggers phosphorylation of the transcription factor FoxO1 and inhibits nuclear entry of Stat3. Erk and Akt act in a mutually antagonistic manner in appetite-regulating neurons of the ArcN. FoxO1 directly activates transcription of Agrp and inhibits Stat3-dependent activation of Pomc transcription, whereas Stat3 in turn inhibits Agrp transcription (169).
Hypothalamic Inflammation and Cellular Stress
Recent studies observed elevated levels of circulating cytokines and adipokines prior to the induction of overnutrition-related diseases such as obesity andT2D(170, 171), suggesting that metabolic inflammation may be a cause, rather than just an effect, of metabolic diseases (172). This hypothesis is supported by animal studies showing increased proinflammatory cytokine (IL1β, IL6, TNFα) and Nfκb pathway gene expression in the hypothalamus, along with an induction of reactive hypothalamic gliosis, shortly after introduction of HFD (3–7days) (157, 173). Central activation and inactivation of Nfκb signaling in the mediobasal hypothalamus show that this pathway plays a central role in regulating body weight (174). Selective activation of Nfκb signaling in putative neural progenitors in ArcN parenchyma inhibits adult neurogenesis, pointing to one potential pathway by which hypothalamic inflammation can trigger long-term changes in body weight (72).
Although a proinflammatory environment precedes the incidence of overnutrition-related diseases in adolescence and adulthood, it remains unclear how Nfκb and cytokines might modulate central-feeding-circuitry development during maternal overnutrition. In adult mice, Nfκb signaling inhibits the expression of Socs3, a critical inhibitor of both insulin signaling and leptin signaling in the hypothalamus (174). HFD likewise downregulates hypothalamic Socs3 expression, a process that occurs at least in part through activation of the endoplasmic reticulum (ER) stress response (174). Interestingly, direct pharmacological activation of the ER stress response through icv administration of thapsigargin blocks the anorexigenic effects of both leptin and insulin; such a mechanism involving a change in the ER stress response may underlie leptin resistance associated with overnutrition (175). As in adults, exposure to maternal circulating cytokines may help program metabolic neural circuits in the developing fetal hypothalamus. Maternal overnutrition may also activate hypothalamic Nfκb signaling, leading to insulin and leptin resistance, which may in turn trigger long-lasting epigenetic changes in hypothalamic neurons regulating feeding and metabolism (176, 177). Thus far, however, the precise mechanism by which a dietary increase in fatty acids leads to systemic hypothalamic inflammation remains to be determined.
Psychosocial Stress and the Hypothalamic-Pituitary-Adrenal Axis
Accumulating evidence suggests that gestational stress or early postnatal stress increases the susceptibility of offspring to diet-induced obesity and metabolic disorders (178, 179). Elevated stress—which may be dietary, physical, or psychological—increases energy storage through multiple mechanisms. Stressed pregnant dams give birth to offspring that gain more weight and have greater adiposity than do control littermates. Consistent with their body composition, offspring of stressed dams are hyperleptinemic and hyperinsulinemic at weaning. However, these effects are reversible: Weaning onto a standard chow diet reverses early signs of obesity. Metabolic effects of perinatal stress are likely mediated by increases in the hypothalamic releasing hormone Crh and the subsequent elevation of glucocorticoids, which acutely influence food intake levels (180) and can chronically induce the symptoms of metabolic syndrome, including glucose intolerance, hyperlipidemia, and hyperinsulinemia (181).
Epigenetic Regulation of Metabolic Imprinting
Developmental epigenetic modification of genes important for the function of central feeding circuitry may be a critical mechanistic component of metabolic imprinting. In maternal undernutrition studies of pregnant sheep, offspring had reduced DNA methylation of the Pomc and glucocorticoid receptor (Gr) promoters. Alterations in Gr methylation were restricted to the hypothalamus, with no changes observed in the hippocampus (182). In another study, mice exposed to HFD during the embryonic and preweaning periods had hypomethylation of the vesicular dopamine transporter (Dat), the µ-opioid receptor (Mor), and preproenkephalin (Penk) promoters in the brain (183). These effects were associated with elevated Dat expression in the VTA, the NAcc, and the prefrontal cortex, but with reduced expression in the hypothalamus. There was increased expression of Mor and Penk in all these areas, suggesting that hedonic aspects of feeding may be altered in these animals. As with most examples of epigenetic changes induced by environmental signals, the mechanism by which these modifications are selectively targeted to the regulatory regions of these specific genes is unclear.
Reversing the Deleterious Effects of Metabolic Imprinting
Given that early-life exposure to challenging nutritional environments can produce deleterious metabolic effects in adulthood, one might ask whether there are any available interventional strategies that would mitigate the preclinical risks for metabolic disorders. Some studies suggest that low-cost and essentially risk-free environmental changes may blunt the developmental effects of metabolic imprinting. For example, a 3-week period of wheel running in rats fed HFD in early postnatal life leads to sustained increases in leptin sensitivity in adulthood (184). Enriched environmental conditions (EEC) are even more potent in reversing prenatal HFD-induced leptin resistance, triggering ArcN synaptic changes that increase Pomc neuron excitability and decrease Agrp neuron excitability (185). The impact of EEC is age dependent, with the greatest impact on the leptin-hypothalamic axis when environmental stimulation is applied during postnatal development. In corroboration, previous studies on the cerebral cortex found that early EEC accelerates neuronal maturation through an IGF1-dependent mechanism (186).
EEC may also alter central feeding circuitries by regulating Bdnf expression. Hypothalamic Bdnf mRNA is selectively upregulated by EEC, with a 2-week exposure inducing a twofold increase in Bdnf expression in the ArcN and VMH of young adult mice (187). Hypothalamic Bdnf potently inhibits both feeding and fat storage (18, 188, 189), and hypothalamic knockdown of Bdnf blocks EEC effects on circulating leptin levels (187). Pharmacological approaches may mimic the effects of early EEC at later stages of development. In the visual cortex, fluoxetine administration induces Bdnf expression, restoring ocular dominance plasticity in adulthood (190, 191). The prominent role of Bdnf in regulating the development and function of central hypothalamic feeding circuitry suggests that a similar, targeted approach may prove useful here.
CONCLUSIONS AND FUTURE DIRECTIONS
Our understanding of the neurodevelopmental regulation of metabolism and the central mechanisms regulating feeding has exploded in the past decade. We now know the key peripheral signals communicating hunger and satiety to the brain, along with at least a subset of the first-order hypothalamic neurons that relay these signals. Our understanding of the genetics of body weight and hypothalamic development has advanced to the point that clear links between defective hypothalamic differentiation and early-onset obesity can be made. Finally and most importantly, a plethora of both animal and human studies have convincingly established the link between (a) both over- and undernutrition early in life and (b) metabolic disturbances in adulthood.
Despite this, much work remains to be done, and we remain ignorant of many fundamental aspects of this system and its development. We still know only the barest outlines of central-feeding-circuitry organization. We are only just beginning to identify postsynaptic targets of the Pomc and Agrp neurons of the ArcN and to study their role in regulating feeding and satiety. Ghrelin, leptin, and insulin modulate the activity of multiple different neuronal subtypes in the hypothalamus and brain stem, and the precise identity of these neurons and their role in regulating feeding remain to be determined. Other hypothalamic neuronal subtypes are in turn connected to neurons responsive to dietary hormones; ultimately, these connections signal hunger, satiety, and the hedonistic aspects of feeding. Finally, virtually all studies of central feeding circuitry thus far have focused on identifying cells that acutely regulate food intake. However, early-life metabolic imprinting often leads to only modest increases in adult food intake that act gradually to increase adult body weight and T2D risk. Thus, neuronal subtypes that remain entirely unidentified may control long-term changes in food intake. Even with the advent of powerful new tools of optical and chemical genetics that allow for reversible and selective activation of individual neuronal subtypes, the tremendous molecular heterogeneity of the hypothalamus makes this problem particularly challenging. The availability of public repositories of molecular markers and regional connectivity through the Allen Brain Atlas (192), coupled with new technologies for comprehensive identification of neuronal subtypes activated by specific physiological stimuli (193), will contribute importantly to expanding our understanding of central feeding circuitry.
Knowledge of the genetic networks controlling regional specification, cell identity, and neuronal connectivity in the hypothalamus and in the brain stem is even less well developed than our understanding of central feeding circuitry. The anatomy of these structures is highly complex, particularly at the early stages of development. Unraveling the complexity of the hypothalamus has historically been hampered by the lack of robust regional and cell subtype–specific molecular markers. However, the recent publication of an annotated molecular atlas of the developing hypothalamus, along with the release of in situ hybridization data for several thousand genes from mouse embryos of multiple different developmental stages (30, 194), has gone a long way toward bypassing this roadblock. When combined with the availability of conditional alleles for hundreds of genes, intersectional genetic approaches can be designed to selectively study how candidate genes guide the development of specific cell types that compose the neural feeding circuitry (195, 196). This intersectional approach can also directly determine the contribution of individual neuronal cell types to feeding regulation, provided that mutant animals survive gestation. In addition, new, robust assays for feeding and metabolism will allow investigators in the field to use powerful genetic tools available in zebra fish (197–199). The next few years will likely see much progress both in our understanding of how central neural circuitry controlling feeding is assembled during development and in the extent to which genetic variants influencing body weight and metabolism disrupt this process.
This work will complement and provide firm mechanistic insight into the flood of human genetic data now being generated. Dozens of both rare and common genetic variants that predispose humans to early-onset differences in body weight and metabolism will be identified in the next few years, although in most cases how these variants produce these changes will be unclear. The same genetic approaches and model organisms used for defining the development of central feeding circuitries can also be employed to ask whether and how these genetic variations account for juvenile obesity and diabetes.
Finally, despite compelling evidence that gestational and early postnatal dietary signals induce long-lasting changes in body weight and metabolism, precisely how the developing brain senses nutrient status is still unclear. What are the relative contributions of peripheral hormonal signals like insulin and leptin compared with local sensing of metabolites by the mTor pathway or, in the case of HFD, by inflammation? When and where these signals act are likewise unclear. What are the critical periods in which over- and underfeeding exert long-term effects on central feeding circuitry? Do these signals trigger shifts in cell identity, neural connectivity, and/or synapse formation?
Knowing precisely where to look for such dietary-induced changes in neuronal differentiation is challenging. However, it should become possible to more precisely correlate changes in diet and hormonal levels with long-term alterations in feeding circuitry as our understanding of central feeding circuitry expands; as global changes in gene expression triggered by dietary signals emerge; and as new technologies develop for the rapid visualization of developmental changes in neuronal subtype, connectivity, and morphology (200). As these new data converge with a basic understanding of normal hypothalamic development and the dietary signaling pathways, the molecular and cellular mechanisms mediating metabolic imprinting should ultimately be resolved. This knowledge can then serve as the basis for therapies aimed at halting or reversing the public health crisis now building as a result of the obesity epidemic.
SUMMARY POINTS.
Global rates of obesity and comorbid health problems, such as diabetes, cardiovascular disease, and cancer, have increased dramatically during the past 30 years.
Both human studies and animal studies have firmly established that developmental exposure to either nutritional excess or scarcity results in an increased probability of obesity and associated disorders in adulthood, a process known as metabolic imprinting. The effects of metabolic imprinting are likely to exacerbate and prolong the public health problems resulting from the obesity epidemic.
The past decade has seen a substantial increase in our understanding of the organization of central feeding circuitry and its development. A number of transcription factors have been found to be essential for the specification and function of the hypothalamic neuronal subtypes that regulate feeding.
Postnatal and adult hypothalamic neurogenesis appears to play a critical role in controlling feeding.
Investigators have identified a range of syndromic and single-gene disorders that result in the dysregulation of body weight and metabolism, in part by disrupting the development of central feeding circuitry.
Nutritional signals affect multiple aspects of hypothalamic development from embryonic neurogenesis and cell fate specification to neural circuit formation. These signals appear to mediate their effects by diet-regulated hormones such as leptin and insulin, as well as by the induction of hypothalamic inflammation.
Challenging nutritional environments in early life can produce deleterious metabolic effects in adulthood. The use of low-cost and risk-free changes may mitigate the preclinical risk for metabolic disorders.
Although much progress has been made in recent years, our current understanding of all aspects of metabolic imprinting—from the organization and development of central feeding circuitry to the mechanisms by which dietary signals disrupt neuronal development—is still poor.
ACKNOWLEDGMENTS
We thank W. Yap, T. Moran, and J. Bedont for helpful feedback on the manuscript.
Glossary
- T2D
type 2 diabetes
- ArcN
hypothalamic arcuate nucleus
- Npy
neuropeptide Y
- Agrp
agouti gene–related peptide
- Pomc
pro-opiomelanocortin
- α-MSH
α-melanocyte-stimulating hormone
- PvN
paraventricular hypothalamic nucleus
- DVC
dorsal vagal complex
- NTS
nucleus of the tractus solitarius
- ME
median eminence
- LH
lateral hypothalamus
- VMH
ventromedial hypothalamus
- Bdnf
brain-derived neurotrophic factor
- DMH
dorsomedial hypothalamic nucleus
- Trh
thyrotrophin-releasing hormone
- VTA
ventral tegmental area
- NAcc
nucleus accumbens
- Shh
sonic hedgehog
- HFD
high-fat diet
- BrdU
bromodeoxyuridine
- Cntf
ciliary neurotrophic factor
- LPD
low-protein diet
- HPD
high-protein diet
- HCD
high-carbohydrate diet
- EEC
enriched environmental conditions
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc. R. Soc. B. 1963;158:329–342. doi: 10.1098/rspb.1963.0051. [DOI] [PubMed] [Google Scholar]
- 5.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 6.Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos. Trans. R. Soc. B. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popkin BM, Conde W, Hou N, Monteiro C. Is there a lag globally in overweight trends for children compared with adults? Obesity. 2006;14:1846–1853. doi: 10.1038/oby.2006.213. [DOI] [PubMed] [Google Scholar]
- 8.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 9.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 13.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 14.Kirchgessner AL, Sclafani A. PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol. Behav. 1988;42:517–528. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- 15.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol. Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 16.Wn Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Sugiyama Y, Yates BJ. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R965–R975. doi: 10.1152/ajpregu.00680.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Williams SM, Grove KL, Smith MS. Melanocortin 4 receptor–mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. J. Neurosci. 2004;24:5091–5100. doi: 10.1523/JNEUROSCI.0588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 2012;36:2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS ONE. 2011;6:e25864. doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roseberry AG. Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats. Psychopharmacology. 2013;226:25–34. doi: 10.1007/s00213-012-2879-6. [DOI] [PubMed] [Google Scholar]
- 25.Wn Z, Xu Y, Zhu Y, Sutton AK, Zhao R, et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7:e45167. doi: 10.1371/journal.pone.0045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, et al. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- 28.Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, et al. Regional morphogenesis in the hypothalamus: A BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell. 2006;11:873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Szabo NE, Zhao T, Cankaya M, Theil T, Zhou X, Alvarez-Bolado G. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J. Neurosci. 2009;29:6989–7002. doi: 10.1523/JNEUROSCI.1089-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapsimali M, Caneparo L, Houart C, Wilson SW. Inhibition of Wnt/Axin/β-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development. 2004;131:5923–5933. doi: 10.1242/dev.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- 33.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, et al. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 34.Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, et al. Rax is a selector gene for mediobasal hypothalamic cell types. J. Neurosci. 2013;33:259–272. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc. Natl. Acad. Sci. USA. 2011;108:10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, et al. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 1973;41:163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- 38.McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol. Endocrinol. 2006;20:1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 39.Pelling M, Anthawal N, McNay D, Gradwohl G, Leiter AB, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 2011;349:406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Anthwal N, Pelling M, Claxton S, Mellitzer G, Collin C, et al. Conditional deletion of neurogenin-3 using Nkx2. 1 iCre results in a mouse model for the central control of activity and obesity. Disease Model. Mech. 2013;6:1133–1145. doi: 10.1242/dmm.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat. Genet. 1997;15:397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 42.Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides α melanocyte–stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology. 2004;145:1503–1513. doi: 10.1210/en.2003-0834. [DOI] [PubMed] [Google Scholar]
- 43.Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expr. Patterns. 2004;4:47–51. doi: 10.1016/s1567-133x(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 44.Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, et al. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–463. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Nogueiras R, Lopez M, Lage R, Perez-Tilve D, Pfluger P, et al. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology. 2008;149:3009–3015. doi: 10.1210/en.2007-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng CY, Mukhopadhyay A, Jarrett JC, Yoshikawa K, Kessler JA. BMP receptor 1A regulates development of hypothalamic circuits critical for feeding behavior. J. Neurosci. 2012;32:17211–17224. doi: 10.1523/JNEUROSCI.2484-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duplan SM, Boucher F, Alexandrov L, Michaud JL. Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur. J. Neurosci. 2009;30:2239–2249. doi: 10.1111/j.1460-9568.2009.07028.x. [DOI] [PubMed] [Google Scholar]
- 49.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 51.Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 52.Kublaoui BM, Holder JL, Jr, Tolson KP, Gemelli T, Zinn AR. SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology. 2006;147:4542–4549. doi: 10.1210/en.2006-0453. [DOI] [PubMed] [Google Scholar]
- 53.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and Oxytocin expression. J. Neurosci. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- 55.Hosoya T, Oda Y, Takahashi S, Morita M, Kawauchi S, et al. Defective development of secretory neurones in the hypothalamus of Arnt2-knockout mice. Genes Cells. 2001;6:361–374. doi: 10.1046/j.1365-2443.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 56.Keith B, Adelman DM, Simon MC. Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc. Natl. Acad. Sci. USA. 2001;98:6692–6697. doi: 10.1073/pnas.121494298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goshu E, Jin H, Lovejoy J, Marion JF, Michaud JL, Fan CM. Sim2 contributes to neuroendocrine hormone gene expression in the anterior hypothalamus. Mol. Endocrinol. 2004;18:1251–1262. doi: 10.1210/me.2003-0372. [DOI] [PubMed] [Google Scholar]
- 58.Schonemann MD, Ryan AK, McEvilly RJ, O’Connell SM, Arias CA, et al. Development and survival of the endocrine hypothalamus and posterior pituitary gland requires the neuronal POU domain factor Brn-2. Genes Dev. 1995;9:3122–3135. doi: 10.1101/gad.9.24.3122. [DOI] [PubMed] [Google Scholar]
- 59.Nakai S, Kawano H, Yudate T, Nishi M, Kuno J, et al. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 1995;9:3109–3121. doi: 10.1101/gad.9.24.3109. [DOI] [PubMed] [Google Scholar]
- 60.Lee DA, Bedont JL, Pak T, Wang H, Song J, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee DA, Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int. J. Dev. Neurosci. 2012;30:615–621. doi: 10.1016/j.ijdevneu.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- 63.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 66.McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, et al. α-Tanycytes of the adult hypothalamic third ventricle induce distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 69.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 71.McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Tang Y, Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, et al. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J. Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duque A, Rakic P. Different effects of bromodeoxyuridine and [3H] thymidine incorporation into DNA on cell proliferation, position, and fate. J. Neurosci. 2011;31:15205–15217. doi: 10.1523/JNEUROSCI.3092-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, et al. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 79.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 80.D’Autreaux F, Coppola E, Hirsch MR, Birchmeier C, Brunet JF. Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc. Natl. Acad. Sci. USA. 2011;108:20018–20023. doi: 10.1073/pnas.1110416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Storm R, Cholewa-Waclaw J, Reuter K, Brohl D, Sieber M, et al. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136:295–305. doi: 10.1242/dev.027193. [DOI] [PubMed] [Google Scholar]
- 82.Hourai A, Miyata S. Neurogenesis in the circumventricular organs of adult mouse brains. J. Neurosci. Res. 2013;91:757–770. doi: 10.1002/jnr.23206. [DOI] [PubMed] [Google Scholar]
- 83.Bauer S, Hay M, Amilhon B, Jean A, Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130:75–90. doi: 10.1016/j.neuroscience.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 84.Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur. J. Hum. Genet. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader-Labhart-Willi syndrome. J. Pediatr. 1999;134:222–225. doi: 10.1016/s0022-3476(99)70419-1. [DOI] [PubMed] [Google Scholar]
- 86.DelParigi A, Tschop M, Heiman ML, Salbe AD, Vozarova B, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 87.Feigerlova E, Diene G, Conte-Auriol F, Molinas C, Gennero I, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008;93:2800–2805. doi: 10.1210/jc.2007-2138. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton CR, Jr, Scully RE, Kliman B. Hypogonadotropinism in Prader-Willi syndrome. Induction of puberty and sperm altogenesis by clomiphene citrate. Am. J. Med. 1972;52:322–329. doi: 10.1016/0002-9343(72)90019-8. [DOI] [PubMed] [Google Scholar]
- 89.Muscatelli F, Abrous DN, Massacrier A, Boccaccio I, Le Moal M, et al. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum. Mol. Genet. 2000;9:3101–3110. doi: 10.1093/hmg/9.20.3101. [DOI] [PubMed] [Google Scholar]
- 90.Bischof JM, Stewart CL, Wevrick R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum. Mol. Genet. 2007;16:2713–2719. doi: 10.1093/hmg/ddm225. [DOI] [PubMed] [Google Scholar]
- 91.Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, et al. The imprinted gene Magel2 regulates normal circadian output. Nat. Genet. 2007;39:1266–1272. doi: 10.1038/ng2114. [DOI] [PubMed] [Google Scholar]
- 92.Kanber D, Giltay J, Wieczorek D, Zogel C, Hochstenbach R, et al. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader-Willi syndrome. Eur. J. Hum. Genet. 2009;17:582–590. doi: 10.1038/ejhg.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chamberlain SJ, Johnstone KA, DuBose AJ, Simon TA, Bartolomei MS, et al. Evidence for genetic modifiers of postnatal lethality in PWS-IC deletion mice. Hum. Mol. Genet. 2004;13:2971–2977. doi: 10.1093/hmg/ddh314. [DOI] [PubMed] [Google Scholar]
- 94.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim. Biophys. Acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Villanueva C, Jacquier S, de Roux N. DLK1 is a somato-dendritic protein expressed in hypothalamic arginine-vasopressin and oxytocin neurons. PLoS ONE. 2012;7:e36134. doi: 10.1371/journal.pone.0036134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wermter AK, Scherag A, Meyre D, Reichwald K, Durand E, et al. Preferential reciprocal transfer of paternal/maternal DLK1 alleles to obese children: first evidence of polar overdominance in humans. Eur. J. Hum. Genet. 2008;16:1126–1134. doi: 10.1038/ejhg.2008.64. [DOI] [PubMed] [Google Scholar]
- 97.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 100.Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 102.Hotta K, Nakamura M, Nakamura T, Matsuo T, Nakata Y, et al. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J. Hum. Genet. 2009;54:727–731. doi: 10.1038/jhg.2009.106. [DOI] [PubMed] [Google Scholar]
- 103.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 104.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1037–R1045. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 105.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gulati P, Cheung MK, Antrobus R, Church CD, Harding HP, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl. Acad. Sci. USA. 2013;110:2557–2562. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caruso V, Chen H, Morris MJ. Early hypothalamic FTO overexpression in response to maternal obesity—potential contribution to postweaning hyperphagia. PLoS ONE. 2011;6:e25261. doi: 10.1371/journal.pone.0025261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 111.Lee AW, Hengstler H, Schwald K, Berriel-Diaz M, Loreth D, et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE. 2012;7:e41537. doi: 10.1371/journal.pone.0041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubos R, Savage D, Schaedler R. Biological Freudianism. Lasting effects of early environmental influences. Pediatrics. 1966;38:789–800. [PubMed] [Google Scholar]
- 113.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 114.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pico C, Palou M, Priego T, Sanchez J, Palou A. Metabolic programming of obesity by energy restriction during the perinatal period: different outcomes depending on gender and period, type and severity of restriction. Front. Physiol. 2012;3:436. doi: 10.3389/fphys.2012.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 117.de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, et al. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 118.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy AK, et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS ONE. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Hoek M, Langendonk JG, de Rooij SR, Sijbrands EJ, Roseboom TJ. Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes. 2009;58:1440–1444. doi: 10.2337/db08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thurner S, Klimek P, Szell M, Duftschmid G, Endel G, et al. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc. Natl. Acad. Sci. USA. 2013;110:4703–4707. doi: 10.1073/pnas.1215626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 122.Coupe B, Grit I, Darmaun D, Parnet P. The timing of “catch-up growth” affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R813–R824. doi: 10.1152/ajpregu.00201.2009. [DOI] [PubMed] [Google Scholar]
- 123.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 124.Gerrard DE, Grant AL. Principles of Animal Growth and Development. Dubuque, IA: Kendall Hunt; 2002. [Google Scholar]
- 125.Stanner SA, Yudkin JS. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4:287–292. doi: 10.1375/1369052012498. [DOI] [PubMed] [Google Scholar]
- 126.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 127.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am. J. Obstet. Gynecol. 2007;196:555.e1–555.e7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garcia AP, Palou M, Sanchez J, Priego T, Palou A, Pico C. Moderate caloric restriction during gestation in rats alters adipose tissue sympathetic innervation and later adiposity in offspring. PLoS ONE. 2011;6:e17313. doi: 10.1371/journal.pone.0017313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coupe B, Dutriez-Casteloot I, Breton C, Lefevre F, Mairesse J, et al. Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. J. Neuroendocrinol. 2009;21:40–48. doi: 10.1111/j.1365-2826.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 130.Garcia AP, Palou M, Priego T, Sanchez J, Palou A, Pico C. Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and αMSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes. Metab. 2010;12:403–413. doi: 10.1111/j.1463-1326.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 131.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 132.de Silva Lima N, de Moura EG, Passos MC, Nogueira Neto FJ, Reis AM, et al. Early weaning causes undernutrition for a short period and programmes some metabolic syndrome components and leptin resistance in adult rat offspring. Br. J. Nutr. 2011;105:1405–1413. doi: 10.1017/S0007114510005064. [DOI] [PubMed] [Google Scholar]
- 133.Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, et al. Parental determinants of neonatal body composition. J. Clin. Endocrinol. Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lawlor DA, Smith GD, O’Callaghan M, Alati R, Mamun AA, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am. J. Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 135.Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE. 2009;4:e5870. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 137.Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, et al. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology. 2010;151:1598–1610. doi: 10.1210/en.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodrigues AL, de Moura EG, Passos MC, Trevenzoli IH, da Conceição EP, et al. Postnatal early overfeeding induces hypothalamic higher SOCS3 expression and lower STAT3 activity in adult rats. J. Nutr. Biochem. 2011;22:109–117. doi: 10.1016/j.jnutbio.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 139.Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS ONE. 2009;4:e6259. doi: 10.1371/journal.pone.0006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152:4171–4179. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Plagemann A, Harder T, Janert U, Rake A, Rittel F, et al. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev. Neurosci. 1999;21:58–67. doi: 10.1159/000017367. [DOI] [PubMed] [Google Scholar]
- 142.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dorner G. Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J. Nutr. 2000;130:2582–2589. doi: 10.1093/jn/130.10.2582. [DOI] [PubMed] [Google Scholar]
- 144.Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides. 2000;34:1–6. doi: 10.1054/npep.1999.0778. [DOI] [PubMed] [Google Scholar]
- 145.Daenzer M, Ortmann S, Klaus S, Metges CC. Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. J. Nutr. 2002;132:142–144. doi: 10.1093/jn/132.2.142. [DOI] [PubMed] [Google Scholar]
- 146.Ota E, Tobe-Gai R, Mori R, Farrar D. Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database Syst. Rev. 2012;9 doi: 10.1002/14651858.CD000032.pub2. CD000032. [DOI] [PubMed] [Google Scholar]
- 147.Beck B, Richy S, Archer ZA, Mercer JG. Ingestion of carbohydrate-rich supplements during gestation programs insulin and leptin resistance but not body weight gain in adult rat offspring. Front. Physiol. 2012;3:224. doi: 10.3389/fphys.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vickers MH, Clayton ZE, Yap C, Sloboda DM. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology. 2011;152:1378–1387. doi: 10.1210/en.2010-1093. [DOI] [PubMed] [Google Scholar]
- 149.Borcarsly ME, Barson JR, Haucau JM, Hoebel BG, Leibowitz SF, et al. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol. Behav. 2012;107:568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 151.Lakhan SE, Kirchgessner A. The emerging role of dietary fructose in obesity and cognitive decline. Nutr. J. 2013;12:114. doi: 10.1186/1475-2891-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carmody JS, Wan P, Accili D, Zeltser LM, Leibel RL. Respective contributions of maternal insulin resistance and diet to metabolic and hypothalamic phenotypes of progeny. Obesity. 2011;19:492–499. doi: 10.1038/oby.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franco JG, Fernandes TP, Rocha CP, Calvino C, Pazos-Moura CC, et al. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J. Physiol. 2012;590:5503–5518. doi: 10.1113/jphysiol.2012.240655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes. 2012;61:2833–2841. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goaze A, Brenachot X, Rigault C, Krezymon A, Rauch C, et al. Cerebral cell renewal in adult mice controls the onset of obesity. PLoS ONE. 2013;8:e72029. doi: 10.1371/journal.pone.0072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zeltser LM, Seeley RJ, Tschop MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat. Neurosci. 2012;15:1336–1342. doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- 159.Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: Transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024–3030. doi: 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- 160.Boskovic R, Feig DS, Derewlany L, Knie B, Portnoi G, Koren G. Transfer of insulin lispro across the human placenta: in vitro perfusion studies. Diabetes Care. 2003;26:1390–1394. doi: 10.2337/diacare.26.5.1390. [DOI] [PubMed] [Google Scholar]
- 161.Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Desai M, Li T, Ross MG. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: neurotrophic effects of leptin and insulin. Brain Res. 2011;1378:29–42. doi: 10.1016/j.brainres.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Plagemann A, Harder T, Rake A, Janert U, Melchior K, et al. Morphological alterations of hypothalamic nuclei due to intrahypothalamic hyperinsulinism in newborn rats. Int. J. Dev. Neurosci. 1999;17:37–44. doi: 10.1016/s0736-5748(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 164.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Investig. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 167.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 168.Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 2012;32:1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Coppari R, Ramadori G, Elmquist JK. The role of transcriptional regulators in central control of appetite and body weight. Nat. Clin. Pract. Endocrinol. Metab. 2009;5:160–166. doi: 10.1038/ncpendmet1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 171.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 172.Cai D. NFκB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542–2548. doi: 10.4161/cc.8.16.9386. [DOI] [PubMed] [Google Scholar]
- 173.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 174.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Won JC, Jang PG, Namkoong C, Koh EH, Kim SK, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity. 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 176.Martinez JA, Cordero P, Campion J, Milagro FI. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc. 2012;71:276–283. doi: 10.1017/S0029665112000055. [DOI] [PubMed] [Google Scholar]
- 177.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 178.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 180.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008;94:169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 181.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 182.Begum G, Stevens A, Smith EB, Connor K, Challis JR, et al. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 2012;26:1694–1703. doi: 10.1096/fj.11-198762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R537–R548. doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mainardi M, Scabia G, Vottari T, Santini F, Pinchera A, et al. A sensitive period for environmental regulation of eating behavior and leptin sensitivity. Proc. Natl. Acad. Sci. USA. 2010;107:16673–16678. doi: 10.1073/pnas.0911832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guzzetta A, Baldini S, Bancale A, Baroncelli L, Ciucci F, et al. Massage accelerates brain development and the maturation of visual function. J. Neurosci. 2009;29:6042–6051. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cao L, Liu X, Lin EJ, Wang C, Choi EY, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang C, Godar RJ, Billington CJ, Kotz CM. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1320–R1332. doi: 10.1152/ajpregu.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 191.Maya-Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- 192.Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, et al. Allen Brain Atlas: an integrated spatiotemporal portal for exploring the central nervous system. Nucleic Acids Res. 2013;41:D996–D1008. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151:1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dymecki SM, Kim JC. Molecular neuroanatomy’s “three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shimada Y, Hirano M, Nishimura Y, Tanaka T. A high-throughput fluorescence-based assay system for appetite-regulating gene and drug screening. PLoS ONE. 2012;7:e52549. doi: 10.1371/journal.pone.0052549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gut P, Baeza-Raja B, Andersson O, Hasenkamp L, Hsiao J, et al. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nat. Chem. Biol. 2013;9:97–104. doi: 10.1038/nchembio.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen S, Oikonomou G, Chiu CN, Niles BJ, Liu J, et al. A large-scale in vivo analysis reveals that TALENS are significantly more mutagenic than ZFNs generated using context-dependent assembly. Nucleic Acids Res. 2013;41:2769–2778. doi: 10.1093/nar/gks1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]