Abstract
Over the past several decades, studies have documented the significance of nonsteroidal anti-inflammatory drugs (NSAIDs) on cancer chemoprevention by lowering incidence and slowing down progression of malignant disease, which consequently lead to decline of cancer-related mortality and improvement of disease progression free survival (PFS). Inhibition of cyclooxygenase (COX) has been primarily believed to be the key mechanism responsible for anticancer activity of NSAIDs, while the serious toxicity caused by COX inhibitory effect reduces the enthusiasm to use NSAIDs as chemoprevention agents in the clinic. Recently, more and more studies demonstrate that non-COX inhibitory mechanisms may account for anticancer properties of NSAIDs, at least partially, which potentially support the indication of NSAIDs on cancer chemoprevention. MicroRNAs (miRNAs) are a set of non-coding and small RNA molecules with master regulatory effect on over 30% human genes through the post-transcriptional and translational modulation. Although miRNAs have been reported to be involved in many normal and pathological processes including cell proliferation, apoptosis, differentiation, as well as tumorigenesis, their roles in NSAIDs' properties of cancer chemoprevention have not yet been studied exclusively. Here, we will review the prior studies reporting interactions between miRNAs and COX/non-COX pathways with intent to provide insights into better understanding molecular mechanisms of cancer chemoprevention by NSAIDs.
Keywords: MiRNAs, NSAIDs, Cancer, cyclooxygenase (COX), COX-2, chemoprevention
Introduction
According to the latest world cancer report, the global burden of cancer rose to an estimate of 14 million new cases per year, which is predicted to reach 22 million annually within the next twenty years[1]. Although the cancer burden grows at an alarming pace, fortunately, cancer prevention plays a crucial role in combating the tidal wave of cancer that we have seen coming across the world over the past a few decades. In particular, cancer chemoprevention is referred to reversion, suppression, or prevention of either the initial phase of carcinogenesis or the development of neoplastic cells to cancer by the use of natural, synthetic or biologic substances[2,3]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are generally a chemically diverse group of agents with anti-inflammatory, antipyretic, and/or analgesic properties. Intriguingly, numerous reports support the anti-neoplastic role of NSAIDs in various human tumors, such as colon, breast, lung, prostate, gastric, and bladder cancer [4-6], although the molecular mechanisms by which NSAIDs prevent tumorigenesis and progression have not been understood completely.
MiRNAs are a class of noncoding small (averaging 20 nucleotides) RNA molecules that can negatively regulate gene expression through repressing translation or affecting mRNA stability [7]. It is estimated that over 0ne third of human genes are targeted and regulated by miRNAs[8]. Because of their key role in regulation of gene expression, miRNAs have been recognized as “master” regulators of human gene expression, which are responsible for a multitude of normal biological and pathological events, such as differentiation, proliferation, cell growth, apoptosis, and tumorigenesis. In human cancer, miRNAs have been widely reported to be involved in tumor pathogenesis, progression, metastasis, prognosis, and responses of patients to chemotherapy. However, its role in cancer chemoprevention has not been studied systematically. Given that miRNAs are recognized as the master regulators of gene expression and alternation of their putative targets are almost involved in all cellular events, miRNAs could be assumed as a set of mechanistic mediators accounting for the mechanistic basis of NSAIDs' pleiotropic antineoplastic activities. To support this assumption, we will summarize the published literatures by focusing on the studies that can potentially provide insights into the mechanistic role of miRNAs in cancer chemoprevention by NSAIDs.
1. Cancer chemoprevention of NSAIDs
NSAIDs are referred to a chemically diverse family of drugs that are usually used for treatment of a variety of inflammatory conditions and/or relief of pain caused by diseases, such as arthritis. They are emerging as a particularly valuable class of chemopreventative agents based on their documented anti-tumor properties. Previous studies reported that the use of NSAIDs was associated with a reduced colorectal cancer risk and incidence [9,10], and long-term use of low-dose selective NSAIDs, such as aspirin, could reduce risk for colorectal and other solid tumors [6,11,12]. Thereby, it is obvious that NSAIDs play a role in cancer chemoprevention. Over the past decades, numerous studies have explored the mechanisms by which NSAIDs prevent tumorigenesis and progression, while demonstration of cyclooxygenase (COX) inhibition is one of the most significant achievements in the field of study.
Cyclooxygenase has two informs referring to COX-1 and COX-2, which are named as principle targets of NSAIDs but with distinct expression patterns and bioactivities [13]. COX-1 is a “housekeeping” gene that is commonly found in the kidney, stomach and platelets. It is responsible for the process of producing physiological prostaglandins (PGs), which are of importance for COX-1's homeostatic functions, such as to maintain the renal blood flow, mediate normal platelet function, and regulate the microcirculation of the gastric mucosa. COX-2 is mainly expressed in macrophages, leukocytes and fibroblasts [14], but its expression levels are restricted in normal tissues. Intriguingly, COX-2 can be elevated by the inflammatory reaction. Pro-inflammatory cytokines, such as interleukin 1(IL-1) and tumor necrosis factor-alpha(TNF-α), have been shown to induce the expression of COX-2 [15].
Aside from the phenotypes in inflammation, COX increases in some types of human cancers, such as colon cancer [16]. The resistance to apoptosis, or programmed cell death may be involved in the mechanisms underlying the association between COX-2 overexpression and tumorigenic potential[15]. Given evidence in support of its signature in tumorigenesis and cancer progression, COX-2 has been named as the one of the key targets accounting for anticancer activity of NSAIDs [17,18]. This conclusion is also supported by the following studies showing that COX-1/COX-2 specific inhibitors and non-selective COX inhibitors could not only inhibit tumor cell growth, but also reduces tumor metastasis [19]. A recent preclinical study reported that NSAIDs with properties inhibiting COX-2 can effectively prevent tumor metastasis and disease progression through antiangiogenic mechanisms in vivo [20], which further supports that use of NSAIDs should be a viable option for cancer patients with advanced diseases.
Regardless of anticancer efficacy, NSAIDs are actually not recommended to be used for cancer chemoprevention in the clinic because of COX inhibition is often associated with potentially fatal gastrointestinal, renal and cardiovascular toxicity[21]. However, numerous studies have reported that COX inhibition does not fully account for its antineoplastic activity [22-28], which implies that alternative mechanisms can be targeted to develop safer and more efficacious derivatives. For example, Piazza's group has made a significant contribution in studies of the non-COX inhibitory mechanism by characterizing cyclic guanosine monophosphate (cGMP) phosphodiesterase (PDE) as a novel target of sulindac for breast and colon cancer prevention [29-33]. They found that sulindac sulfide (SS) can inhibit PDE5 and certain other cGMP degrading isozymes, which increase intracellular cGMP levels that activate protein kinase G (PKG) in breast cancer cells and that this activity is COX-independent[30,31]. These studies provide strong evidence in support of the COX-independent properties of sulindac leading to safer and more efficacious derivatives for breast cancer. Moreover, Wnt/β-catenin, AMPK, NF-kb, and PPARγ signaling pathways are reported to be involved in non-COX anticancer activities of selective NSAIDs[34-38]. Based on these previous accomplishments, we think that better understanding of mechanistic basis of NSAIDs' anticancer activity should be a key step to develop novel, safe, and effective agents to prevent tumorigenesis and cancer progression, while the master regulators of gene expression, miRNAs, may be involved in these underlying mechanisms.
2. MiRNAs and Cancer
MiRNAs are of single-stranded, non-coding sequences and naturally occurring small RNA molecules that bind to 3′-UTR of target genes to repress their expression at the post-transcriptional and translational levels[39,40]. Almost 30% of all human genes are regulated by miRNAs in which each is capable of mediating the expression of several hundreds of cognate messenger RNA targets simultaneously, and over one third of human genes appear to be conserved MiRNA targets[8]. Approximately a couple of thousands of human MiRNAs have been identified that are involved in many biological processes including apoptosis, proliferation, differentiation, cell death, immune reactions, tumorigenesis, and metastasis [41,42].
2.1 MiRNA biogenesis
The biogenesis of miRNAs is similar to other RNA molecules starting from DNA transcription. MiRNAs are primarily transcribed by RNA polymerase II (pol II) as long primary transcripts known as primary-miRNAs (pri-miRNAs). These transcripts are often spliced and located within their intronic segments. Additionally, pri-miRNAs contains multiple miRNA sequences and within itself can fold back into stem-loop precursors of approximately 60-80 nucleotides, known as the miRNAs precursor (pre-miRNAs). During the process, the hairpins are recognized and excised from pri-miRNAs in the nucleus by the microprocessor complex formed by the RNase III enzyme Drosha and its binding partner DGCR8[43]. Then pre-miRNAs are rapidly exported to the cytoplasmic by the nuclear export factor - exportin 5[44,45]. And the cytoplasmic processing of pre-miRNAs into double- stranded miRNA duplexes by Dicer, a member of the RNase III superfamily of bidentate nucleases. Further processing to mature miRNAs is through a large protein complex, the RNA-induced silencing complex (RISC), which includes the Argonaute proteins as core components. Thereafter, only one strand binds with the RISC stably to become the mature miRNAs to regulate the expression of target genes. The other strand, also known as the passenger strand or miRNA*, is disposed by two alternative mechanisms: mRNA cleavage or translational repression. When it is loaded into the RISC, the only human Ago protein capable of cleaving target mRNAs, the passenger strand may be cleaved; RISC containing any Ago protein may remove miRNA* that does not require[42,45-47]. The process of miRNA biogenesis and their central function in regulation of gene expression are shown as Fig. 1.
Figure 1.
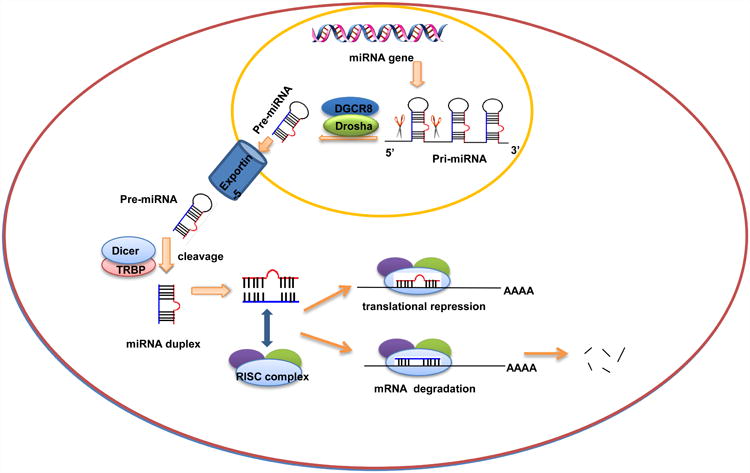
MiRNA biogenesis and mechanism of action in regulation of gene expression.
2.2 Anti-neoplastic activity of MiRNAs
MiRNAs play a key regulatory role in the pathological processes in addition to their curial functions in normal cellular events, such as development, cell proliferation, cell differentiation, and apoptosis[41,48,49]. To date, more than 11,000 literatures have been recorded in PubMed under the key words of “miRNA and cancer.” The first direct study reporting the significance of miRNAs in cancer was published by Calin et al a decade ago [50]. They found that a 30kb deletion within the chromosome 13q14 where miR15 and miR16 are located is correlated to the incidence of B-cell chronic lymphocytic leukemia (B-CLL) [50].
Following this pilot study, scientists and physicians have made significant progress on studies of miRNA and human diseases including cancer in basic, translational, and clinical researches. For example, Song et al reported that miR-200b could inhibit tumor cell growth and migration by suppressing ZEB1 and sequentially augment E-cadherin in vivo [51]. Lu et al used bead-based flow cytometric miRNA expression profiling method to implement a systematic expression analysis of 217 mammalian miRNAs in 334 human samples. Their results showed the aberrant expression patterns of oncogenic and tumor suppressor miRNAs in human tumor tissues when compared to normal tissues, which suggested the potential of miRNA as biomarkers for disease diagnosis[52]. The prognostic values of miRNAs in cancer have also been identified in variety of human tumors. For example, in addition to miR-200c that was reported to be a novel prognostic marker in colorectal cancer by our lab[53], miRNAs are also found to target the tumor suppressor genes to promote hematopoietic stem cell self-renewal and transformation[54]. In this study, overexpression of miR-22 inhibiting the tumor suppressor TET2 was demonstrated to be responsible for the poor clinical outcomes in myelodysplastic syndrome (MDS) and leukemia [54]. A recent study reported that the assays featured by multiple miRNA signatures can not only discriminate hepatocellular carcinoma (HCC) from normal tissues, but also can predict HCC patients' survival[55]. Development of bioinformatics and technology provides new insights into study of miRNA and human diseases. For example, the cancer miRNA regulatory network (http://cmrn.systemsbiology.net/) is built to show the interaction of miRNAs with 2,240 genes based on 46 cancer transcriptome profiling studies. Searching this database is able to quickly identify candidate miRNAs that correlate to selective diseases.
3. MiRNAs, a new player in cancer chemoprevention by NSAIDs
NSAIDs have been proposed as chemopreventive agents for variable human tumors, although the mechanisms of cancer chemoprevention by NSAIDs have not been understood completely [56,57]. As discussed above, COX and non-COX inhibitory pathways have been documented as two of the most important mechanisms accounting for the basis of action [56]; however, it has not been concluded that these known pathways can fully address the pleiotropic activities of NSAIDs in cancer prevention. Given the properties of targeting multiple genes by which miRNAs influence variety of signaling pathways, impaired miRNA regulation could contribute to the development of cancer and other diseases.
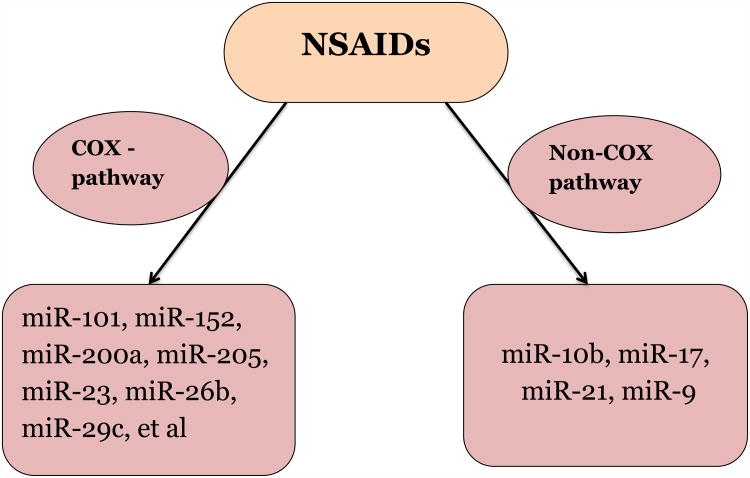
COX-2 has been reported to play an important role in tumorigenesis[58], and overexpression of COX-2 in varieties of cancers has been demonstrated, such as breast cancer[59], colon cancer[58], lung cancer[60], prostate cancer[61,62] and other tumors[63,64]. As discussed above, COX-2 is named as one of the most important targets responsible for the anticancer activity of NSAIDs. Thereby, any miRNAs that are able to trigger COX-2 may be involved in the COX dependent mechanism of NSAIDs anticancer activity. There are only several studies reporting the inhibition of COX-2 signaling by miRNAs[65-67], but few of them are related to human cancer[67]. A latest study reported that miR-101 was downregulated in the cisplatin-resistant human bladder cancer cells T24/CDDP, while overexpression of miR-101 rescued the anticancer activity of cisplatin. The luciferase reporter assay demonstrated that miR-101 could directly target 3′-UTR of COX-2 gene and silence of COX-2 by siRNAs could simulate the phenotype led by overexpression of miR-101[67]. In addition, a study demonstrated that celecoxib, a COX-2 selective inhibitor, could regulate the expression of miRNA-29c in human gastric cancer cells [68]. In this study, miR-29c showed the tumor suppressor signature; whereas COX-2 inhibition of miR-29c might be responsible for progression of gastric cancer [68].
The NSAID sulindac has been shown to display strong efficacy for the treatment of precancerous lesions in patients with familial adenomatous polyposis (FAP) by reducing adenoma size and number by as much as 60-70%[69]. These observations are consistent with a large number of preclinical studies that have shown the ability of sulindac and other NSAIDs to inhibit tumorigenesis in various experimental animal models involving either early or late stage disease[70-73]. In one of our recent publications, we reported that sulindac sulfide (SS) can potently inhibit the invasion of human breast and colon tumor cells at concentrations less than those required to inhibit tumor cell growth in vitro[74]. This inhibitory activity by SS was found to be associated with significant changes in miRNA expression, implying that miRNAs may be involved in the pleiotropic anticancer activity of sulindac against tumor progression and metastasis [74]. Using microarray analysis, we found that SS treatment could alter the expression of 132 miRNAs (17 up and 115 down) in human HCT116 colon tumor cells, in which several have been previously reported to promote tumor metastasis and invasion, such as miR-10b, miR-17, miR-21, and miR-9. We further demonstrated that the mechanism of NSAIDs in control of these oncogenic miRNAs is through the NF-κB signaling. When SS inhibits the entrance of NF-κB to the nucleus, its transcription activity upregulating these oncogenic miRNAs is thereby decreased [74]. Thus, our results support a non-COX-inhibitory mechanism involving miRNAs that is responsible for anti-invasive activity of sulindac. The mechanistic basis of miRNA accounting for anticancer activity of NSAIDs is summarized in Fig. 2.
Figure 2. Mechanistic basis of miRNA accounting for anticancer activity of NSAIDs.
4. Summary
The characteristic of miRNAs has been implicated in the pathogenesis of variable human cancer types and their potential as as diagnostic markers, therapeutic targets and prognostic indicator have been intensively studied [75]; however, it mechanistic role in cancer chemoprevention by NSAIDs has not been well studied. Given the signature targeting over 30% human genes that are involved in most of cellular events, miRNAs have potentials to address the pleiotropic antineoplastic activities of NSAIDs. In support of this assumption, our recent study reported that downregulation of selective oncogenic miRNAs by sulidac through NF-κB signaling is responsible for anti-invasive activity of this NSAID, which is a novel non-COX inhibitory mechanism. In addition, a recent review article by Yiannakopoulou proposes that targeting epigenetic processes and miRNA expression by aspirin and other NSAIDs may be a novel strategy with potential efficacy for cancer therapy and prevention[76]. Altogether, to better understand mechanistic roles of miRNAs in cancer chemoprevention by NSAIDs can provide novel insights into development of novel agents with better efficacy and safety.
Acknowledgments
This study was supported by an American Cancer Society Research Scholar Grant (RSG-13-265-01-RMC, to Yaguang Xi) and NIH/NCI R21 Grants (5R21CA160280 and 1R21CA182754 to Yaguang Xi). We sincerely apologize to those whose work was not cited due to time and space constraints.
Footnotes
Conflict of Interest: Ruixia Ma, Bin Yi, Gary A. Piazza, and Yaguang Xi declare that they have no conflict of interest.
Compliance with Ethics Guidelines: Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Stewart B, Wild CP. World Cancer Report 2014. 2014;16 [Google Scholar]
- 2.Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464(7291):1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35(6):1332–1338. [PubMed] [Google Scholar]
- 4.Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Arch Intern Med. 1999;159(2):161–166. doi: 10.1001/archinte.159.2.161. [DOI] [PubMed] [Google Scholar]
- 5.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson F, Yin L, Lundholm C, Smedby KE, Czene K, Pawitan Y. Low-dose aspirin use and cancer characteristics: a population-based cohort study. Br J Cancer. 2013;109(7):1921–1925. doi: 10.1038/bjc.2013.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106(7):1340–1350. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res. 2014;20(5):1104–1113. doi: 10.1158/1078-0432.CCR-13-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106(2):djt431. doi: 10.1093/jnci/djt431. This clinical study demontrated that daily use of low-dose aspirin can postentially reduce the risk of ovarian cancer, which supports the new indication of this “old” FDA approved generic drug in cancer prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol. 1999;155(3):995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res. 2002;62(23):6846–6849. [PubMed] [Google Scholar]
- 15.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- 16.Ramsay RG, Friend A, Vizantios Y, Freeman R, Sicurella C, Hammett F, et al. Cyclooxygenase-2, a colorectal cancer nonsteroidal anti-inflammatory drug target, is regulated by c-MYB. Cancer Res. 2000;60(7):1805–1809. [PubMed] [Google Scholar]
- 17.Skopinska-Rozewska E, Piazza GA, Sommer E, Pamukcu R, Barcz E, Filewska M, et al. Inhibition of angiogenesis by sulindac and its sulfone metabolite (FGN-1): a potential mechanism for their antineoplastic properties. Int J Tissue React. 1998;20(3):85–89. [PubMed] [Google Scholar]
- 18.Gurpinar E, Grizzle WE, Piazza GA. COX-Independent Mechanisms of Cancer Chemoprevention by Anti-Inflammatory Drugs. Front Oncol. 2013;3:181. doi: 10.3389/fonc.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62(8):2343–2346. [PubMed] [Google Scholar]
- 20.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2 Suppl 7):2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Mazhar D, Gillmore R, Waxman J. COX and cancer. QJM. 2005;98(10):711–718. doi: 10.1093/qjmed/hci119. [DOI] [PubMed] [Google Scholar]
- 22.Alberts DS, Hixson L, Ahnen D, Bogert C, Einspahr J, Paranka N, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biochem Suppl. 1995;22:18–23. doi: 10.1002/jcb.240590804. [DOI] [PubMed] [Google Scholar]
- 23.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3(10):1679–1683. [PubMed] [Google Scholar]
- 24.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52(2):237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 25.Kashfi K, Rigas B. Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol. 2005;70(7):969–986. doi: 10.1016/j.bcp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57(12):2452–2459. [PubMed] [Google Scholar]
- 27.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55(14):3110–3116. [PubMed] [Google Scholar]
- 28.Rigas B, Shiff SJ. Is inhibition of cyclooxygenase required for the chemopreventive effect of NSAIDs in colon cancer? A model reconciling the current contradiction. Med Hypotheses. 2000;54(2):210–215. doi: 10.1054/mehy.1999.0023. [DOI] [PubMed] [Google Scholar]
- 29.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2(6):572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 2011;4(8):1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8(12):3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, et al. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3(10):1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, et al. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5(6):822–833. doi: 10.1158/1940-6207.CAPR-11-0559. This research article presented a novel deteritive of sulindac, SBA that lacked COX-2 inhibitory properties but showed improved suppressive effect on human colorectal tumor cells by targeting cGMP phosphodiesterases, which supports that non-COX inhibitory mechanisms are also of importance to address the anticancer activities of NSAIDs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi Y, Li K, Yan Q, Koizumi S, Shi L, Takahashi S, et al. Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase. J Pharmacol Exp Ther. 2011;339(1):257–266. doi: 10.1124/jpet.111.183020. [DOI] [PubMed] [Google Scholar]
- 36.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 37.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 38.Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/beta-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem Res. 2013;38(11):2313–2322. doi: 10.1007/s11064-013-1142-9. [DOI] [PubMed] [Google Scholar]
- 39.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 40.Xi Y. MicroRNA: A New Player for Cancer Chemoprevention. J Integr Oncol. 2013;2(1) doi: 10.4172/2329-6771.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27(45):5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 42.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94(6):776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 44.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123(4):607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 47.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123(4):621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Lu X, Liu Y, Lv Z, Chen J, Yu W, et al. Expression analysis of miRNAs in BmN cells. Gene. 2012;505(2):240–245. doi: 10.1016/j.gene.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Bukhari SI, Vasquez-Rifo A, Gagne D, Paquet ER, Zetka M, Robert C, et al. The microRNA pathway controls germ cell proliferation and differentiation in C. elegans. Cell Res. 2012;22(6):1034–1045. doi: 10.1038/cr.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song F, Yang D, Liu B, Guo Y, Zheng H, Li L, et al. Integrated microRNA network analyses identify a poor-prognosis subtype of gastric cancer characterized by the miR-200 family. Clin Cancer Res. 2014;20(4):878–889. doi: 10.1158/1078-0432.CCR-13-1844. [DOI] [PubMed] [Google Scholar]
- 52.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 53.Xi Y, Formentini A, Chien M, Weir DB, Russo JJ, Ju J, et al. Prognostic Values of microRNAs in Colorectal Cancer. Biomark Insights. 2006;2:113–121. [PMC free article] [PubMed] [Google Scholar]
- 54.Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13(1):87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei R, Huang GL, Zhang MY, Li BK, Zhang HZ, Shi M, et al. Clinical significance and prognostic value of microRNA expression signatures in hepatocellular carcinoma. Clin Cancer Res. 2013;19(17):4780–4791. doi: 10.1158/1078-0432.CCR-12-2728. [DOI] [PubMed] [Google Scholar]
- 56.Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3(3):166–174. doi: 10.1016/s1470-2045(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 57.Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Drug Targets. 2004;4(1):29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]
- 58.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 59.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26(26):3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 60.Kim GY, Lim SJ, Kim YW. Expression of HuR, COX-2, and survivin in lung cancers; cytoplasmic HuR stabilizes cyclooxygenase-2 in squamous cell carcinomas. Mod Pathol. 2011;24(10):1336–1347. doi: 10.1038/modpathol.2011.90. [DOI] [PubMed] [Google Scholar]
- 61.Khor LY, Bae K, Pollack A, Hammond ME, Grignon DJ, Venkatesan VM, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92-02 trial. Lancet Oncol. 2007;8(10):912–920. doi: 10.1016/S1470-2045(07)70280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. COX-2 is overexpressed in primary prostate cancer with metastatic potential and may predict survival. A comparison study between COX-2, TGF-beta, IL-10 and Ki67. Cancer Epidemiol. 2010;34(3):316–322. doi: 10.1016/j.canep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Ishikawa TO, Jain NK, Herschman HR. Cox-2 gene expression in chemically induced skin papillomas cannot predict subsequent tumor fate. Mol Oncol. 2010;4(4):347–356. doi: 10.1016/j.molonc.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7(2):429–434. [PubMed] [Google Scholar]
- 65.Akhtar N, Haqqi TM. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann Rheum Dis. 2012;71(6):1073–1080. doi: 10.1136/annrheumdis-2011-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104(38):15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bu Q, Fang Y, Cao Y, Chen Q, Liu Y. Enforced expression of miR101 enhances cisplatin sensitivity in human bladder cancer cells by modulating the cyclooxygenase2 pathway. Mol Med Rep. 2014;10(4):2203–2209. doi: 10.3892/mmr.2014.2455. [DOI] [PubMed] [Google Scholar]
- 68.Saito Y, Suzuki H, Imaeda H, Matsuzaki J, Hirata K, Tsugawa H, et al. The tumor suppressor microRNA-29c is downregulated and restored by celecoxib in human gastric cancer cells. Int J Cancer. 2013;132(8):1751–1760. doi: 10.1002/ijc.27862. [DOI] [PubMed] [Google Scholar]
- 69.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 70.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17(8):1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 71.Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, et al. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis. 1998;19(1):87–91. doi: 10.1093/carcin/19.1.87. [DOI] [PubMed] [Google Scholar]
- 72.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57(14):2909–2915. [PubMed] [Google Scholar]
- 73.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, et al. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57(2):267–271. [PubMed] [Google Scholar]
- 74••.Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, et al. Sulindac inhibits tumor cell invasion by suppressing NF-kappaB-mediated transcription of microRNAs. Oncogene. 2012;31(48):4979–4986. doi: 10.1038/onc.2011.655. This research article is for the first time to demonstrate that microRNAs were involved in anti-invasive activity of the NSAID, sulindac in human cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yiannakopoulou E. Targeting epigenetic mechanisms and microRNAs by aspirin and other non steroidal anti-inflammatory agents--implications for cancer treatment and chemoprevention. Cell Oncol (Dordr) 2014;37(3):167–178. doi: 10.1007/s13402-014-0175-7. [DOI] [PubMed] [Google Scholar]