TO THE EDITOR
Ibrutinib, an inhibitor that binds covalently to C481 of Bruton’s tyrosine kinase (BTK), has produced remarkable responses in patients with relapsed and refractory chronic lymphocytic leukemia (CLL).1–4 However, 5.3% of patients have disease progression, and the mechanism of resistance is largely unknown. Herein we describe the mechanism of resistance in such a case.
A 49-year-old woman had a diagnosis of CLL established in 2000. After the failure of multiple treatments, she began receiving ibrutinib at a dose of 560 mg daily in 2010 as part of a phase 1, dose-escalation study of ibrutinib in B-cell cancers.1 By month 11, a partial response was achieved with an absolute lymphocyte count of 4530 cells per cubic millimeter. Computed tomography at month 18 showed a marked but incomplete reduction of lymphadenopathy. At month 21, a rapidly rising lymphocyte count and progressive lymphadenopathy were noted. Despite a dose escalation to 840 mg daily, CLL progressed during the next 4 weeks (for details, see the Supplementary Appendix, available with the full text of this letter at NEJM.org). Peripheral-blood samples were collected before ibrutinib administration (day –52), while the patient was having a response to the drug (day 472), when progressive disease was first noted (day 589), and before dose escalation (day 616). Figure S1 in the Supplementary Appendix shows the dates of sample collection in relation to the patient’s absolute lymphocyte count over the treatment course.
RNA sequencing revealed a thymidine-to-adenine mutation at nucleotide 1634 of the BTK complementary DNA (cDNA) (GenBank accession number, NM_000061.2), leading to a substitution of serine for cysteine at residue 481 (C481S). The mutation was detected in the samples collected when progressive disease was first noted (88% of reads) and before dose escalation (92% of reads) but not in those collected before ibrutinib administration or while the patient was having a response (Fig. S2A in the Supplementary Appendix). No other genetic changes were identified that correlated with the patient’s clinical course in the same manner as the BTK mutation. Sanger sequencing of cDNA verified that the mutation was detected only in the samples collected during relapse (Fig. S2B in the Supplementary Appendix). A more sensitive, allele-specific polymerase-chain-reaction assay (1% analytic sensitivity) detected the mutation in the genomic DNA of samples collected during relapse but not in those collected before ibrutinib administration or while the patient was having a response (Fig. S3 in the Supplementary Appendix).
Ibrutinib binds covalently to the sulfhydryl group of C481 of BTK in the active site, resulting in irreversible inhibition of its kinase activity.5 Structural modeling suggested that the C481S mutation would disrupt this covalent binding, changing irreversible binding to reversible binding (Fig. 1A). Fluorescently tagged ibrutinib labeled the nonmutant BTK, and the covalent binding that was formed withstood electrophoresis, whereas reversible binding to the C481S or C481A mutant of BTK did not. This showed biochemically the critical role of cysteine in covalent-bond formation (Fig. S4 in the Supplementary Appendix).
Figure 1. Effect of C481S Mutation of Bruton’s Tyrosine Kinase (BTK) on Ibrutinib Binding and the Ability of Ibrutinib to Inhibit BTK Phosphorylation.
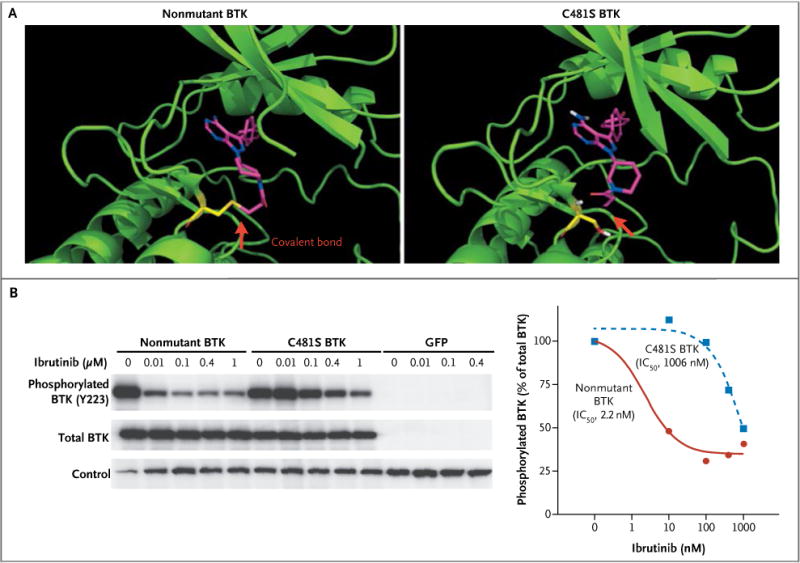
Panel A shows structural modeling of nonmutant and mutant BTK with ibrutinib. The red arrows indicate the covalent bond between ibrutinib (purple and blue) and BTK (green and yellow) before and after the mutation. Panel B shows the inhibition of nonmutant BTK or C481S BTK phosphorylation by ibrutinib in HEK 293 cells. The half-maximal inhibitory concentration (IC50) of ibrutinib for inhibition of BTK phosphorylation was analyzed and plotted with GraphPad Prism. GFP denotes green fluorescent protein.
Phosphorylation of BTK (pY223) reflects BTK kinase activity. Introduction of the recombinant nonmutant and C481S BTK constructs into HEK 293 cells showed that phosphorylation of C481S BTK at Y223 became significantly less sensitive to ibrutinib inhibition than the nonmutant BTK did (half-maximal inhibitory concentration, 1006 nM vs. 2.2 nM) (Fig. 1B).
Taken together, our data indicate that the C481S mutation disrupts the covalent binding between BTK and ibrutinib. The impaired binding leads to a loss of inhibition of BTK enzymatic activity that ultimately results in ibrutinib resistance in the patient. Consistent with the findings reported in the Journal by Woyach et al.,6 our studies confirm that BTK is a relevant pharmacologic target of ibrutinib from a genetic perspective.
Acknowledgments
Supported by grants from the Leukemia and Lymphoma Society and the Prince Family Foundation (both to Dr. Wang).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Richard R. Furman, Weill Cornell Medical College, New York, NY
Shuhua Cheng, Weill Cornell Medical College, New York, NY
Pin Lu, University of Chicago, Chicago, IL
Menu Setty, Memorial Sloan-Kettering Cancer Center, New York, NY
Alexendar R. Perez, Memorial Sloan-Kettering Cancer Center, New York, NY
Ailin Guo, University of Chicago, Chicago, IL
Joelle Racchumi, Weill Cornell Medical College, New York, NY
Guozhou Xu, Boston Children’s Hospital, Boston, MA
Hao Wu, Boston Children’s Hospital, Boston, MA
Jiao Ma, Weill Cornell Medical College, New York, NY
Susanne M. Steggerda, Pharmacyclics, Sunnyvale, CA
Morton Coleman, Weill Cornell Medical College, New York, NY
Christina Leslie, Memorial Sloan-Kettering Cancer Center, New York, NY
Y. Lynn Wang, University of Chicago, Chicago, IL
References
- 1.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [Erratum, N Engl J Med 2014;370:786.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–57. doi: 10.1038/leu.2013.358. [DOI] [PubMed] [Google Scholar]
- 4.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Furman RR, Liu T-M, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]


