Abstract
Nephrotic syndrome is a renal disease accompanied by abnormal body fluid balance. The present experiments investigated the role of behavioral mechanisms in contributing to disordered fluid homeostasis in rats with experimentally-induced nephrotic syndrome. The studies examined water and sodium ingestion under ad libitum conditions and in response to dehydration-related challenges in rats made nephrotic by treatment with the antibiotic, Adriamycin. Rats with nephrotic syndrome had greater ad libitum water intakes beginning 3 weeks after treatment, but daily sodium (0.3 M NaCl) intakes were not affected. Nephrotic rats showed attenuated water and sodium intakes after combined treatment with furosemide (10 mg/kg) and captopril (2 mg/kg), reduced water intakes after 20 hr of water deprivation, and diminished water intakes, plasma renin activity and aldosterone secretion after subcutaneous isoproterenol (30 μg/kg). However, the Adriamycin-treated animals had normal water intakes in response to subcutaneous hypertonic saline (4% at 0.75 ml/100 g) and central injections of angiotensin II (10, 20, and 50 ng). The results suggest that water and sodium ingestion in response to hypovolemic/hypotensive stimuli are disturbed in nephrotic rats, and provide evidence that the disordered behaviors reflect disturbances of the peripheral renin-angiotensin-aldosterone system.
INDEX TERMS: Furosemide, Captopril, Isoproterenol, Angiotensin II, Plasma renin activity, Vasopressin, Aldosterone, Adriamycin
1. Introduction
Nephrotic syndrome is a clinical disorder of body fluid regulation characterized by edema, hypoalbuminemia and proteinuria (4). Much of the pathology of nephrotic syndrome can be modeled in animals by inducing renal damage with systemic injections of Adriamycin (doxorubicin), an anthracycline antibiotic. In the rat, a single intravenous (iv) injection of this drug induces severe proteinuria that appears within 2 wks, plateaus around 4 wks and remains elevated for several months (2). Rats made nephrotic by Adriamycin treatment also exhibit sodium retention, polyuria, and increased plasma urea nitrogen and plasma creatinine concentrations (2, 7, 8, 16). Histological studies show that Adriamycin-injected rats have severe generalized renal lesions characterized by tubular dilation and atrophy, interstitial fibrosis and focal, global glomerulosclerosis (7).
Renal damage associated with nephrotic syndrome alters fluid balance by affecting not only excretory mechanisms, but also other renal functions important for maintaining body fluid homeostasis. There are data indicating alterations in renin-angiotensin mechanisms in nephrotic syndrome and that such changes may contribute to various aspects of the pathology of the nephrotic syndrome. For example, infusions of renin or angiotensin increase the urinary excretion of protein in nephrotic animals (3) and proteinuria can be reduced in nephrotic humans and some experimental animal models by administering angiotensin converting enzyme inhibitors, such as captopril (9).
There is evidence that drinking mechanisms are altered in nephrotic syndrome. Hall et al. (7) reported elevated water intake and urine output in Adriamycin-treated rats suggesting that drinking is altered in nephrotic syndrome. However, it is not clear whether the change in drinking is primary or secondary to increased urine output. Additionally, a complete assessment of changes in stimulated drinking has not yet been done. Therefore, the goal of the present studies was to investigate the effects of Adriamycin treatment on 1) ad libitum water and sodium ingestion; 2) water and sodium ingestion in response to acute intracellular and extracellular thirst challenges; 3) short-term water and sodium ingestion mediated by peripheral and/or brain renin-angiotensin systems; and 4) blood pressure and hormonal responses to activation of the peripheral renin-angiotensin system.
2. General Methods
2.1. Animals
Male Sprague-Dawley rats weighing 370–430 g at the beginning of the experiment were purchased from Harlan Laboratories (Indianapolis, IN). They were housed individually in hanging wire-mesh cages for at least 5 days before being introduced into experimental protocols. Standard diet (Purina Rat Chow 5012), tap water and 0.3 M NaCl solution were available ad libitum except as indicated. Water and 0.3 M NaCl were provided from 100 ml graduated cylinders with 1 ml divisions that were fitted with metal drinking spouts. A 12-hr light-dark cycle (0600–1800, light) was maintained in the colony. Room temperature was controlled at approximately 22°C. All work was conducted according to procedures approved by the University of Iowa Institutional Animal Care and Use Committee and conformed to the guidelines of the American Physiological Society.
For all surgeries, rats were anesthetized with an Equithesin-like anesthetic cocktail (composed of 0.97 g of sodium pentobarbital and 4.25 g of chloral hydrate/100 ml distilled water and prepared by the University of Iowa Hospitals and Clinics Pharmacy; 0.33 ml/100 g body-weight) (6).
2.2 Materials and Procedures
2.2.1. Adriamycin Treatment
Rats were randomly assigned to experimental and control groups and anesthetized. Adriamycin (3.5 mg/kg) or an equal volume of isotonic saline vehicle (1 ml/kg) was injected through the sublingual vein, and the rats were returned to their home cages.
2.2.2. Catheter Surgery
Rats received carotid catheters under anesthesia. The catheters were made from 25 cm pieces of polyethylene tubing (PE-50) that were heat-welded to 4-cm pieces of PE-10. The PE-10 end was inserted into the carotid artery and the other end was tunneled under the skin to exit at the base of the neck. Catheters were filled with heparinized saline (50 U/ml) and plugged with 23-gauge obturators, and the rats were allowed to recover from surgery for at least 2 days before testing.
2.2.3. Cannula Surgery
Anesthetized rats were placed in a Kopf stereotaxic instrument. The scalp was incised and the periosteum reflected. The skull was then leveled between bregma and lambda. A 23-gauge stainless steel cannula was implanted to terminate in the lateral ventricle using the coordinates 1.2 mm caudal to bregma, 1.5 mm left of the midline and 4.0 mm below the dura mater. The cannula was fixed to the cranium using dental acrylic resin and jeweler’s screws and a 30-gauge metal obturator was placed in the cannula between tests.
2.2.4. Blood Pressure Measurements
Arterial catheters were connected to transducers and recorders by l m of PE-50 tubing. Arterial blood pressure was recorded on a polygraph (Dynograph Recorder, Model R611, Sensormedic, Anaheim, CA) using Cobe transducers. Mean arterial blood pressure (MAP) was obtained by reducing the gain on the electronic arterial signal.
2.2.5 Drugs
Adriamycin (Sigma, St. Louis, MO) was dissolved in sterile isotonic saline and administered at a dose of 3.5 mg/kg in 1 ml/kg volume through the sublingual vein. Furosemide (Furo; 10 mg/kg body-weight; Elkins-Sinn Inc., Cherry Hill, NJ) was administered subcutaneously. Captopril (Cap; 2 mg/kg body-weight; 1 mg/ml volume; SQ-14, 225; Bristol-Myers-Squibb (Princeton, NJ), was dissolved in sterile isotonic saline immediately before each experiment and was also administered subcutaneously. Isoproterenol (HCl, Elkins-Sinn Inc., Cherry Hill, NJ) was diluted with isotonic saline to 30 μg/ml before each test and administered (30 μg/kg body-weight) subcutaneously. Angiotensin II (ANG II; Sigma, St. Louis, MO) was dissolved in isotonic saline and stored frozen in aliquots. Aliquots were thawed, and diluted to the appropriate concentration with isotonic saline, immediately before each test.
2.2.6. Statistical Analysis
The results are reported as means ± SEM. The data were analyzed using one-way or two-way ANOVA. Urinary protein and acute (i.e., 90–120 min) water and 0.3 M NaCl intakes were analyzed with treatment (control vs Adriamycin) as the between-subjects factor. Daily water and 0.3 M NaCl intakes were analyzed with treatment as the between-subjects factor and time (i.e., days, weeks) as the within-subjects repeated factor. Mean arterial pressure and plasma hormones were analyzed with treatment and drug condition (vehicle vs isoproterenol) as between-subjects factors. Water drinking to icv ANG II was analyzed with treatment and dose as factors. Differences were considered significant at p < 0.05.
3. Experiment 1: The Effects of Adriamycin Treatment on Ad Libitum and Experimentally-Induced Thirst and Sodium Appetite
3.1. Experimental groups
Experiment 1 determined the effects of nephrotic syndrome on daily, ad libitum intakes of water and saline, and on water and saline drinking responses to acute dipsogenic and natriorexigenic challenges. Twenty-two rats weighing 370–430 g were randomly assigned to receive Adriamycin (n = 12) or vehicle (n = 10) treatment. Ad libitum water and 0.3 M NaCl intakes were recorded for the next 23 days. Then rats received a series of drinking tests with a few days between testing. Urine was also collected on one day to evaluate effectiveness of Adriamycin treatment by measuring urinary protein excretion.
3.2. Experimental protocols
3.2.1. Quantitative Total Urine Protein Test
Twenty four-hr urine samples were collected on day 28 after Adriamycin treatment to verify the presence of proteinuria. Urine samples were centrifuged to remove suspended matter. Total urinary protein was measured with a turbidimetric test kit (Stanbio Laboratory, Inc., San Antonio, TX). Absorbance reflecting the turbidity of the solution was determined spectrophotometrically at 420 nm (Spectronic 601, Milton Roy, Riviera Beach, FL) and compared to protein standards.
3.2.2. Furo/Cap Test
The combined administration of Furo and Cap (5, 13) was used to induce a rapid-onset sodium appetite and thirst. The Furo/Cap test was conducted on day 23 after Adriamycin treatment. Rats were placed in metabolic cages with stainless steel funnels suspended underneath. Urine was collected in polypropylene tubes secured beneath the funnels. Food was not available during testing. The animals were injected with Furo (10 mg/kg) followed 5 to 10 min later by Cap (2 mg/kg). One hr later, the rats were given access to water and 0.3 M NaCl from 0.1 ml calibrated glass burettes with attached metal drinking spouts. Intakes were measured for 2 hrs.
3.2.3. Hypertonic Saline Test
The water intake induced by administering hypertonic saline was tested in the animals’ home cages on day 32 post-Adriamycin treatment. Food access was blocked beginning 30 min before the test. The rats were weighed, and 4% hypertonic saline was administered subcutaneously at 0.75 ml/100 g body-weight. Water was provided from glass burettes immediately after injection and intakes were measured for 90 min.
3.2.4. Water Deprivation Test
Water deprivation testing was conducted on day 36 post-Adriamycin treatment. Rats remained in their home cages with food available but without access to water and 0.3 M NaCl overnight (1500 to 1100). Food access was blocked beginning 30 min before the return of water and remained unavailable during the test period. Water was provided from glass burettes and intakes were measured for 2 hrs.
3.2.5. Isoproterenol Test
Isoproterenol testing occurred on day 44 post-Adriamycin treatment. Rats remained in their home cages during the test. Food access was blocked beginning 30 min before the test. Isoproterenol was administered subcutaneously at 30 μg/kg body-weight in a 1 ml/kg volume of saline. Water burettes were attached to the cages immediately after injection and intakes were recorded for 2 hrs.
3.3. Results
3.3.1. Daily Water and Saline Intakes
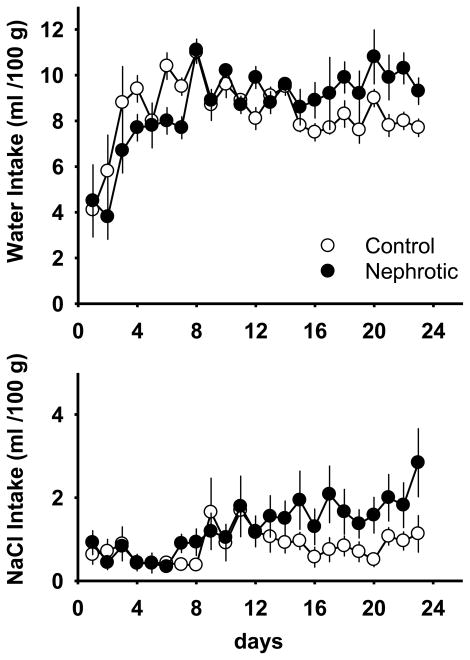
There were significant differences in body-weight between Adriamycin-treated and control rats starting 2 wk after the Adriamycin treatment, 376 ± 4 and 406 ± 4 g, respectively [F(1,20) = 4.35; p < 0.01]. This difference persisted throughout the duration of the experiment. Therefore, water drinking and saline intakes were normalized for body-weight. There were significant differences in daily water intake between the treatments [interaction effect; F (22,440) = 3.45; p < 0.01] (Figure 1). In a follow-up analysis, data were averaged across the first, middle and third weeks after treatment. Daily water intakes of Adriamycin-treated rats were significantly less than those of control rats in the first week after treatment, were equivalent with those of control rats during the second week after treatment, and were significantly greater than those of control rats during the third week after treatment. There were no significant treatment effects on daily 0.3 M saline intakes (Figure 1). However, a significant effect of days [F (22,440) = 3.28; p < 0.01] revealed that saline intakes for both groups increased with time.
Figure 1.
Mean daily water and 0.3 M NaCl intake from day 1 to day 23 post-treatment in control and Adriamycin-treated (nephrotic) rats. Intakes are normalized for body-weight. Values are means ± SE. Note change in axis between measures.
3.3.2. Urinary Protein Test
Twenty-eight days after treatment, the animals in the Adriamycin group had significantly higher 24-hr total urinary protein excretion compared to control treatment, 334.7 ± 16.5 vs. 40.1 ± 5.3 mg, respectively [F(1,19) = 224.5; p < 0.01]. One Adriamycin-treated rat died before the assessment of urinary protein.
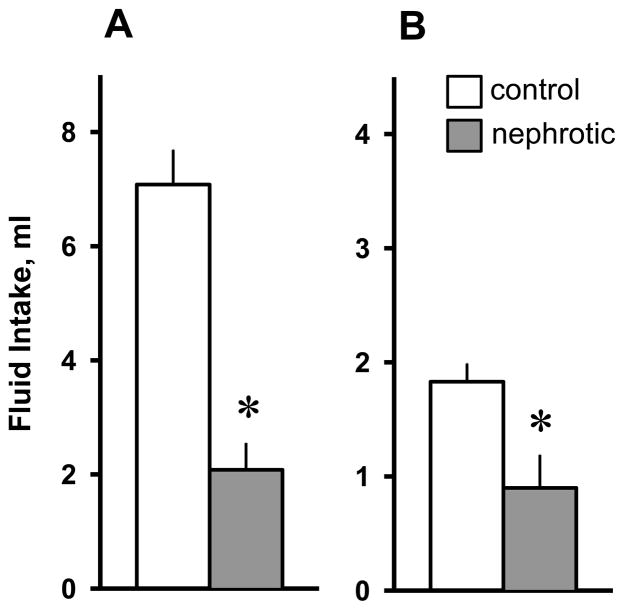
3.3.3. Furo/Cap Test
Rats treated with Adriamycin had significantly reduced water [F(1,19) = 6.50; p < 0.01] and 0.3M NaCl intakes [F(1,19) = 6.59; p < 0.05] compared to control rats in response to Furo/Cap treatment (Figure 2). Total urine volume excreted by Adriamycin-treated rats during the Furo/Cap test was not significantly different from those of control-treated rats, 13.2 ± 1.1 ml and 15.4 ± 0.8 ml, respectively, [F(1,19) = 1.94; p > 0.05]. (Results in the Furo/Cap test were not adjusted for body-weights because the doses of drugs given to induce the ingestive responses were determined for each animal on the basis of body-weight. For the same reason, the fluid intakes in the hypertonic saline test and the isoproterenol test presented below were not adjusted.)
Figure 2.
Water intake (A) and 0.3 M NaCl intake (B) in control and Adriamycin-treated (nephrotic) rats following the Furosemide/Captopril treatment. Both water and 0.3 M NaCl intakes were significantly reduced in nephrotic rats. Values are means ± SE. * = main effect of nephrotic treatment, p < 0.05.
3.3.4. Hypertonic Saline Test
There were no significant differences in water intake between the groups [F(1,19) = 0.14; p > 0.05; Figure 3A].
Figure 3.
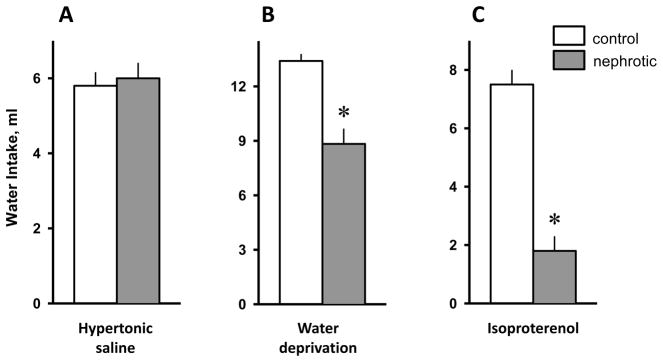
Water intake in control and Adriamycin-treated (nephrotic) rats following 4% hypertonic saline injected subcutaneously (A), overnight water deprivation (B) and isoproterenol injected subcutaneously (30 μg/kg)(C). Water intake was significantly reduced in nephrotic rats following hypertonic saline and isoproterenol. Values are means ± SE. Note change in axis between measures. * = main effect of nephrotic treatment, p < 0.01.
3.3.5. Water Deprivation Test
Adriamycin-treated rats drank significantly less water than control rats after 24-hr water deprivation [F(1,19) = 17.71; p < 0.01; Figure 3B]. The difference remained significant after adjusting for body-weight [F(1,19)= 5.19; p < 0.05].
3.3.6. Isoproterenol Test
Water intake induced by 30 μg/kg isoproterenol subcutaneously was significantly reduced in the Adriamycin-treated group [F(1,16) = 18.37; p < 0.01; Figure 3C]. Three Adriamycin-treated rats died before the isoproterenol test.
4. Experiment 2: The Effects of Adriamycin Treatment on Blood Pressure and Plasma Hormone Levels Following Subcutaneous Isoproterenol
4.1. Experimental groups
Experiment 2 determined the MAP and hormonal profiles of Adriamycin-treated (n = 18) and control (n = 14) rats following subcutaneous injections of isoproterenol. Six Adriamycin-treated rats died during or shortly after catheter implantation.
4.2. Experimental protocol
4.2.1. MAP and Hormonal Profile after Subcutaneous Isoproterenol
On day 25 after Adriamycin treatment, urine protein concentration was measured using the turbidimetric method as described. Rats were instrumented with carotid catheters the next day and MAP was measured beginning four days later (days 30 to 37). Two additional Adriamycin-treated rats died before the beginning of testing. The remaining 24 rats were assigned to 4 groups: 1) Adriamycin-treated receiving isoproterenol treatment (n = 6), 2) Adriamycin-treated receiving isotonic saline (n = 4), 3) controls receiving isoproterenol (n = 7), and 4) controls receiving isotonic saline (n = 7). For determination of MAP, rats were brought to a separate room, connected to the polygraph, and allowed at least 30 min to adapt. Baseline MAP was measured for 5 min immediately before the injection of isoproterenol (30 μg/kg) or subcutaneous isotonic saline (1 ml/kg). Blood pressure was recorded for 45 min after the injections. The maximum fall in pressure after injection was calculated as change MAP from baseline values. At the end of blood pressure measurements, the rats were decapitated for collection of trunk blood and determination of plasma renin activity (PRA) and plasma levels of vasopressin and aldosterone. Trunk blood was collected in centrifuge tubes containing heparin (20 IU, USP) and EDTA-Na2 (1.5 mg/ml blood). Plasma was separated by centrifugation (1,200 g, 10 min at 4°C), transferred to polypropylene tubes, and frozen (−20° C) until extraction. PRA was determined by measuring the formation of angiotensin I (ANG I) using a radioimmunoassay kit from New England Nuclear (Du Pont, Wilmington, DE) (21). Plasma aldosterone was measured according to previously published methods (18) following extraction by dichloromethane and isolation by LH-20 column chromatography. The radioimmunoassay for aldosterone was performed with a sheep antiserum to the mineralocorticoid. Plasma levels of vasopressin were measured as described in previous studies (15, 21). The plasma samples (0.5–1.0 ml) were extracted before radioimmunoassay with acetone-petroleum ether. The plasma extracts were dried with a Speed Vac Concentrator (Savant Instruments, Farmingdale, NY). Dried extracts were stored at −20°C until the time of assay when they were redissolved in 1 ml solution of 0.03% acetic acid in saline. Plasma levels of vasopressin were then determined by radioimmunoassay, using a non-equilibrium technique described elsewhere (14).
4.3. Results
4.3.1. Urinary Protein Concentration
Urinary protein concentrations were significantly higher in Adriamycin-treated rats compared to controls, 7.01± 0.55 vs. 0.84 ± 0.09 mg/ml, respectively, [F(1,19) = 108.75; p < 0.01].
4.3.2. MAP Measurements
Statistical analysis was conducted on baseline MAP and the maximum reduction in MAP following drug injection. Baseline MAP, averaged over 5 min prior to drug injection, was not different between the Adriamycin-treated and control groups (126 ± 4 vs. 134 ± 3 mmHg, respectively). The maximum decrease in MAP was significantly greater after isoproterenol compared to isotonic saline (23 ± 2 vs. 3 ± 0.2 mmHg, respectively) [F(1,20) = 74.92; p < 0.001]. However, there was no significant difference in the isoproterenol-induced decrease in MAP between the Adriamycin-treated and control groups.
4.3.3. Endocrine Measurements
Levels of PRA were significantly higher in isoproterenol-treated than in isotonic saline-treated rats [F(1,20) = 9.91; p < 0.01] and significantly reduced in Adriamycin-treated rats compared to control rats [F(1,20) = 10.11; p < 0.01](Figure 4A). There were no effects of isoproterenol or Adriamycin treatments on values of plasma vasopressin (Figure 4B). Plasma aldosterone levels were significantly higher after isoproterenol treatment compared to isotonic saline treatment [F(1,20) = 8.86; p < 0.01] and significantly reduced in Adriamycin-treated rats compared to controls [F(1,20) = 10.40; p < 0.01](Figure 4C).
Figure 4.
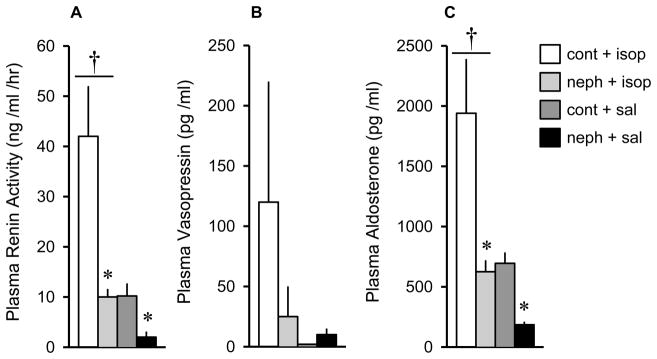
Levels of plasma renin activity (A), vasopressin (B) and aldosterone (C) in control and Adriamycin-treated (nephrotic) rats following isoproterenol (30 μg/kg, sc). Measures were obtained 45 min after injection. Isoproterenol caused significantly higher levels of plasma renin activity and aldosterone compared to control injections. Nephrotic animals had significantly reduced levels of plasma renin activity and aldosterone compared to control animals. There were no effects on plasma vasopressin levels. Values are means ± SE. cont = control; isop = isoproterenol; neph = nephrotic; sal = saline. * = main effect of nephrotic treatment, p < 0.01. †= main effect of isoproterenol, p < 0.01.
5. Experiment 3: The Effects of Adriamycin Treatment on Drinking Following Central Angiotensin II (ANG II)
5.1. Experimental groups
Experiment 3 determined the effects of nephrotic syndrome on water drinking responses to centrally administered ANG II. Twenty-eight Sprague-Dawley male rats weighing 330–390 g at the beginning of the experiment were used. Each rat was implanted with a 23-gauge cannula into the lateral ventricle.
5.2. Experimental protocol
5.2.1. Central ANG II Injection
The rats received three intracerebroventricular (icv) ANG II screening tests administered starting day 7 post-surgery. Injections into the lateral ventricle were made using 10 μl Hamilton syringes connected with polyethylene tubing (PE 30; 1 m) to 30-gauge injection cannulas. On the day of testing, water bottles were replaced with glass burettes. Rats were weighed and food access was blocked for the duration of the test period. Rats were removed from the cage, the obturator was withdrawn and the injection cannula was introduced into the chronically implanted guide cannula. Rats were held by hand during injection of ANG II (50 ng in 1μl volume). After injection, the obturators were replaced and the rats were placed back into the home cage. Water intake was measured for l hr. All rats drank greater than 3 ml of water on average. After the screening tests, rats were randomly divided into Adriamycin-treated and control groups with 14 rats in each group.
Rats received 3.5 mg/kg Adriamycin in 1 ml/kg volume or the same volume of isotonic saline as described in Experiment I. Two Adriamycin-treated rats died during the surgery. Beginning 25 days after Adriamycin treatment, each rat received three tests with ANG II administered icv at doses of 10, 25 and 50 ng/1 μl in random order according to procedures outlined above with at least one day intervening between tests. Cumulative water intakes were measured at the end of 1 hr.
5.3. Results
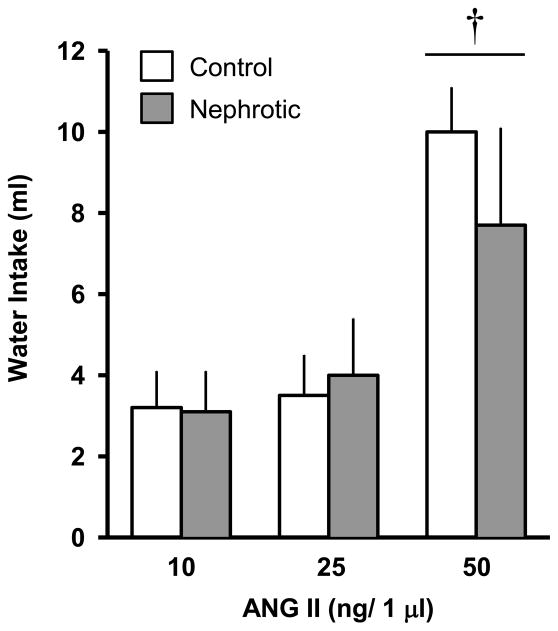
There were no significant differences in the water drinking responses to the icv ANG II screening tests between the groups of rats designated to receive either Adriamycin or vehicle treatment. On subsequent tests with icv ANG II, the rats drank more water in response to the 50 ng dose than the other doses [F(2,48) = 92.28; p < 0.001], but there was no effect of Adriamycin treatment (Figure 5). Water intakes were adjusted for body-weight, but no significant differences were found.
Figure 5.
One hr water intake in control and Adriamycin-treated (nephrotic) rats following icv angiotensin II (ANG II). All rats received all doses. Water intake was not different between groups. The 50 ng dose caused more drinking than the other doses. Values are means ± SE. † = main effect of dose, p < 0.001.
6. General Discussion
The main finding of the present experiments is that rats with Adriamycin-induced experimental renal nephrosis have impaired water and salt intake in response to fluid challenges associated with activation of the peripheral renin-angiotensin system. This includes attenuated drinking responses to overnight water deprivation and isoproterenol treatment and impaired water and 0.3 M NaCl intakes in response to treatment with Furo/Cap. These behavioral impairments may be attributable to relative inability of Adriamycin-treated rats to secrete renin, e.g., after isoproterenol, in comparison to controls, and not to reductions in central responding to ANG II as central administration of ANG II elicits drinking in Adriamycin-treated rats as it does in controls.
Our observations on daily water intakes in Adriamycin-treated rats are consistent with those of Hall and colleagues (7). The increase in daily water intakes developed during the third week post-Adriamycin injection and may reflect increased sodium retention (8) or increased urinary volume (16) after treatment. However, neither the work of Hall and colleagues (7) nor our additional observations on ad libitum intake provide an explanation of the mechanisms which may account for altered daily fluid intake in rats with nephrotic syndrome.
Drinking responses to various thirst-inducing paradigms are usually caused by dehydrating a body fluid compartment or by stimulating changes in components that are associated with the fluid deficits of one of the compartments. For example, subcutaneous injection of 4% hypertonic saline dehydrates only the intracellular compartment. Isoproterenol treatment elevates circulating levels of ANG II (10) and reduces arterial blood pressure (17), two results typically associated with fluid loss from the extracellular compartment. Water deprivation results in body fluid loss from both the intracellular and extracellular compartments. The observation that drinking differences between Adriamycin and control groups occurred in response to isoproterenol treatment and to 20 hr of water deprivation, but not to subcutaneous hypertonic saline suggests that some aspects of the control of extracellular, but not cellular, thirst are disturbed in Adriamycin-treated rats.
Data from the present studies also suggest that the mechanisms controlling sodium appetite are disturbed in nephrotic rats since a depressed sodium appetite was observed in response to the Furo/Cap treatment. Because the rapid onset of sodium appetite induced by the Furo/Cap test depends on the renin-angiotensin system (20, 21), the present results suggest that impaired renin-angiotensin mechanisms may account for the attenuated response to Furo/Cap treatment.
In order to evaluate the systemically generated humoral and neural controls of thirst and sodium appetite, we examined hydromineral-related endocrine and blood pressure changes in response to isoproterenol treatment. The elevation of PRA and aldosterone levels observed after isoproterenol treatment (19) were attenuated in Adriamycin-treated rats providing evidence that the renin-angiotensin system is less responsive to blood pressure/volume-related challenges. This result may account for the decreased water intake after isoproterenol treatment and water deprivation and possibly explains the attenuated water and hypertonic saline intakes after Furo/Cap treatment. However, the differences in drinking to isoproterenol are unlikely to be caused by a greater reduction in MAP because the fall in MAP was comparable between the groups. We note that the large amount of variability between the groups for plasma vasopressin levels suggests that potential differences in vasopressin secretion may not be detected with the approaches employed here.
There are two distinct renin-angiotensin systems that potentially contribute to the altered fluid intake responses observed here, namely, the “classic” renal renin-angiotensin system and the brain renin-angiotensin system. Both systems have been implicated in the control of thirst and sodium appetite (see 11 for review). In order to assess the potential involvement of the central renin-angiotensin system in water intake responses of nephrotic rats, ANG II was injected into the lateral ventricle. If central ANG II mechanisms are impaired in Adriamycin-treated animals, a depressed drinking response to icv ANG II would be expected in these animals. Our data did not support this hypothesis as there was no significant difference in drinking in response to icv ANG II between the groups. Given that rats with nephrotic syndrome had no impairment in the response to centrally administered ANG II on water intake, but did have impaired increases in plasma renin activity and aldosterone levels in response to isoproterenol, this suggests that impairments in mobilization of the systemic renin-angiotensin system may in large part account for the attenuated ingestive responses observed in rats with nephrotic syndrome.
Several nephrotic rats were lost during the course of testing. Therefore, there is a question if the decreased water and sodium intakes of Adriamycin-treated rats are a function of general illness or debilitation (e.g., motor impairments) rather than specific decreases of renin-dependent water and sodium ingestion. We do not have additional measures of behavioral competence of the animals. However, as the intakes in response to systemic hypertonic saline and centrally-administered angiotensin were indistinguishable between Adriamycin-treated and control rats, it is unlikely that the decreased responses of Adriamycin-treated rats are due to non-specific, general debilitation. In addition, Adriamycin (doxorubicin) has been known to cause cardiomyopathy and heart failure. In humans, heart failure is often associated with profound thirst (1). Animal models of heart failure produce alterations in thirst and sodium ingestion attributable to the associated changes in cardiovascular function and hormonal profile (12). Therefore, while Adriamycin treatment clearly caused renal damage in the present studies, the potential contributions of heart damage to the present results cannot be ruled out.
7. Conclusions
The present experiments represent an initial step towards understanding the effects on fluid balance-related ingestive behaviors that accompany nephrotic syndrome. The current results suggest that disturbed drinking patterns in Adriamycin-treated rats may be the result of alterations in the dynamics of the peripheral renin-angiotensin system. However, the precise mechanisms and extent of their involvement in contributing to the disturbed drinking behavior and sodium appetite in Adriamycin-treated rats will require further investigation.
Highlights.
Injections of Adriamycin were used to create a model of nephrosis in rats.
Adriamycin treatment attenuated water drinking to renin-dependent challenges.
Adriamycin treatment reduced sodium intake after acute hypovolemic challenge.
Adriamycin treatment attenuated release of renin and aldosterone to isoproterenol.
Water drinking to osmotic challenge and angiotensin II were not affected.
Acknowledgments
This research was supported by MH59239 and AG25465 to RLT and HL098207, MH80241 and HL14388 to AKJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allida SM, Inglis SC, Davidson PM, Lal S, Hayward CS, Newton PJ. Thirst in chronic heart failure: a review. J Clin Nurs. 2015;24:916–26. doi: 10.1111/jocn.12732. [DOI] [PubMed] [Google Scholar]
- 2.Bertani T, Rocchi G, Sacchi G, Mecca G, Remuzzi G. Adriamycin-induced glomerulosclerosis in the rat. Am J Kidney Dis. 1986;7:12–19. doi: 10.1016/s0272-6386(86)80051-8. [DOI] [PubMed] [Google Scholar]
- 3.Bohrer MP, Deen WM, Robertson CR, Brenner BM. Mechanism of angiotensin II-induced proteinuria in the rat. Am J Physiol. 1977;233:F13–F21. doi: 10.1152/ajprenal.1977.233.1.F13. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JS. The nephrotic syndrome and its complications. Am J Kidney Dis. 1987;10:157–171. doi: 10.1016/s0272-6386(87)80170-1. [DOI] [PubMed] [Google Scholar]
- 5.Fitts DA, Masson DB. Forebrain sites of action for drinking and salt appetite to angiotensin or captopril. Behav Neurosci. 1989;103:865–872. doi: 10.1037/h0092457. [DOI] [PubMed] [Google Scholar]
- 6.Gandal CP. Avian anesthesia. Fed Proc. 1969;28:1533–1534. [PubMed] [Google Scholar]
- 7.Hall RL, Wilke WL, Fettman MJ. The progression of adriamycin-induced nephrotic syndrome in rats and the effect of captopril. Toxicol Appl Pharmacol. 1986;82:164–174. doi: 10.1016/0041-008x(86)90448-5. [DOI] [PubMed] [Google Scholar]
- 8.Herman PJ, Sawin LL, DiBona GF. Role of renal nerves in renal sodium retention of nephrotic syndrome. Am J Physiol. 1989;256:F823–F829. doi: 10.1152/ajprenal.1989.256.5.F823. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison FN. Hormonal modulation of proteinuria in the nephrotic syndrome. Am J Nephrol. 1993;13:337–346. doi: 10.1159/000168648. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AK, Mann JFE, Rascher W, Johnson JK, Ganten D. Plasma angiotensin II concentrations and experimentally induced thirst. Am J Physiol. 1981;240:R229–R234. doi: 10.1152/ajpregu.1981.240.3.R229. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 12.Lane JR, Purhar KK, Fitts DA. Drinking in sodium-depleted rats with bile duct, vena cava or portal vein obstruction. Physiol Behav. 1997;62:1145–54. doi: 10.1016/s0031-9384(97)00309-0. [DOI] [PubMed] [Google Scholar]
- 13.Masson DB, Fitts DA. Subfornical organ connectivity and drinking to captopril or carbachol in rats. Behav Neurosci. 1989;103:873–880. doi: 10.1037/h0092456. [DOI] [PubMed] [Google Scholar]
- 14.Matsuguchi H, Schmid PG, Van Orden D, Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension. 1981;3:174–181. doi: 10.1161/01.hyp.3.2.174. [DOI] [PubMed] [Google Scholar]
- 15.Montes R, Johnson AK. Efferent mechanisms mediating renal sodium and water excretion induced by centrally administered serotonin. Am J Physiol. 1990;259:R1267–R1273. doi: 10.1152/ajpregu.1990.259.6.R1267. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell MP, Michels L, Kasiske B, Raij L, Keane WF. Adriamycin-induced chronic proteinuria: a structural and functional study. J Lab Clin Med. 1985;106:62–67. [PubMed] [Google Scholar]
- 17.Rettig R, Ganten D, Johnson AK. Isoproterenol-induced thirst: renal and extrarenal mechanisms. Am J Physiol. 1981;241:R152–R157. doi: 10.1152/ajpregu.1981.241.3.R152. [DOI] [PubMed] [Google Scholar]
- 18.Robillard JE, Ramberg E, Sessions C, Consamus B, Van Orden D, Weismann D, Smith FG., Jr Role of aldosterone on renal sodium and potassium excretion during fetal life and newborn period. Dev Pharmacol Ther. 1980;1:201–216. [PubMed] [Google Scholar]
- 19.Thunhorst RL, Grobe CL, Beltz TG, Johnson AK. Effects of {beta}-adrenergic receptor agonists on drinking and arterial blood pressure in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2001;300:R1001–8. doi: 10.1152/ajpregu.00737.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thunhorst RL, Johnson AK. Renin-angiotensin, arterial blood pressure, and salt appetite in rats. Am J Physiol. 1994;266:R458–R465. doi: 10.1152/ajpregu.1994.266.2.R458. [DOI] [PubMed] [Google Scholar]
- 21.Thunhorst RL, Morris M, Johnson AK. Endocrine changes associated with a rapidly developing sodium appetite in rats. Am J Physiol. 1994;267:R1168–R1173. doi: 10.1152/ajpregu.1994.267.5.R1168. [DOI] [PubMed] [Google Scholar]