Abstract
Background and Aims Evolution of autonomous selfing may be advantageous because it allows for reproductive assurance. In co-flowering plants competing for pollinators, the least common and/or attractive could suffer pollen limitations. Silene niceensis and S. ramosissima are taxonomically related species sharing the same habitat, although S. ramosissima is less abundant and has a more restricted distribution. They also have the same a priori nocturnal pollinator syndrome, and show an overlapping flowering phenology. The aim of this study was to investigate whether a selfing strategy in S. ramosissima allows it to avoid pollinator competition and/or interspecific pollen transfer with S. niceensis, which would thus enable both species to reach high levels of fruit and seed set.
Methods The breeding system, petal colour, flower life span and degree of overlap between male and female phases, floral visitor abundance and visitation rates were analysed in two sympatric populations of S. niceensis and S. ramosissima in southern Spain.
Key Results Autonomous selfing in S. ramosissima produced very high fruit and seed set, which was also similar to open-pollinated plants. Silene niceensis showed minimum levels of autonomous selfing, and pollen/ovule ratios were within the range expected for the breeding system. In contrast to S. niceensis, flower life span was much shorter in S. ramosissima, and male and female organs completely overlapped in space and time. Upper surface petals of both species showed differing brightness, chroma and hue. Flowers of S. niceensis were actively visited by moths, hawkmoths and syrphids, whereas those of S. ramosissima were almost never visited.
Conclusions The findings show that different breeding strategies exist between the sympatric co-flowering S. niceensis and S. ramosissima, the former specializing in crepuscular–nocturnal pollination and the latter mainly based on autonomous selfing. These two strategies allow both species to share the restricted dune habitat in which they exist, with a high female reproductive success due to the absence of pollinator competition and/or interspecific pollen flow.
Keywords: Caryophyllaceae, floral longevity, Noctuidae, plant mating system, pollen/ovule ratio, Silene niceensis, S. ramosissima, spectral reflectance, Syrphidae, Sphingidae, spontaneous autogamy, supplementary pollination, sympatry
INTRODUCTION
The evolution of selfing is often associated with limitations in pollination, allowing reproductive assurance (Fishman and Wyatt, 1999; Eckert et al., 2006). Insufficient pollination may originate from pollinator-mediated competition among co-flowering plants sharing pollinators (Feinsinger and Tiebout, 1991; Sargent and Ackerly, 2008; Briscoe Runquist and Stanton, 2013). It has been suggested that competition for pollinator services among co-flowering plants is more likely in closely related species with a high phylogenetic signal in floral traits (Sargent and Ackerly, 2008). The causes of pollinator limitation are not only competition for pollinators, but also low mate availability because of reduced population size or plant density (Pannell and Barrett, 1998; Busch and Delph, 2012). Both autonomous and facilitated selfing may provide reproductive assurance; only autonomous selfing may involve reproductive assurance when pollinators are missing, whereas facilitated selfing only provides reproductive assurance when mates are scarce (Brys and Jacquemyn, 2011). Of the three modes of autonomous selfing (prior, competing and delayed; Lloyd, 1979), prior selfing is the most restrictive, because early self-fertilized ovules usually preclude later outcrossing (Davis and Delph, 2005; Eckert et al., 2006). However, prior selfing may be advantageous under conditions of chronic or high pollinator limitation since the plant reduces investment in pollinator attraction, thereby reducing the costs of pollination (Brys and Jacquemyn, 2011; Lepers et al., 2014).
In addition to the direct effects on competition for pollinators, co-flowering plants sharing pollinators may also have negative fitness consequences through interspecific pollen transfer (Waser, 1983). This transfer has two components: conspecific pollen loss and heterospecific pollen deposition (Morales and Traveset, 2008; Schiestl and Schlüter, 2009). Although both mechanisms may have an impact on plant reproduction, some evidence suggests that conspecific pollen loss is more detrimental (Morales and Traveset, 2008). Therefore, as a consequence of interspecific pollen transfer, co-flowering species may evolve a divergence in characters (character displacement), such as the degree of exsertion of reproductive parts (Muchhala and Potts, 2007) or selfing (Fishman and Wyatt, 1999) The consequences of interspecific pollen transfer should be stronger for those species that are relatively rare within the community (Palmer et al., 2003). Character displacement has been demonstrated in different genera. For example, in Ipomoea, I. hederacea shows reduced anther separation when grown with I. purpurea, consequently revealing reduced probability that pollen from I. purpurea reaches their stigmas (Smith and Rausher 2007, 2008). Armbruster et al. (1994) also proposed character displacement in sympatric Stylidium congeners in Australia.
On the other hand, when shifts in mating system and adaptation to different pollinators or groups of pollinators occur in closely related taxa in sympatry, it could be an indicator of divergence through reproductive isolation as part of the speciation process (Grossenbacher and Whittall, 2011; Hopkins, 2013). The reproductive barriers may be pre-zygotic (e.g. ecological habitat differences or pollinator-mediated reproductive isolation) or post-zygotic (e.g. genetic incompatibility or decreased hybrid viability) (Waelti et al., 2008). Pollinator differences between related species can be an important pre-zygotic isolation barrier (Ramsey et al., 2003; Santamaría and Rodríguez-Gironés, 2007; Schiestl and Johnson, 2013). Although less studied, selfing can be an important pre-zygotic reproductive barrier that effectively reduces the gene exchange between congeneric species or subspecies (Levin, 1971; Martin and Willis, 2007; Levin, 2010; Gibson et al., 2012; Briscoe Runquist and Moeller, 2014).
In the present study, we examine a system composed of two closely related species which overlap in space (growing in the same specialized habitat) and time (flowering period) and show the same pollination syndrome. Silene niceensis and S. ramosissima are spring-flowering species that grow in sympatry on coastal dunes in southern Spain, with plants growing intermingled (Fig. 1A). As is common in other species of Silene (Jürgens, 2006; Giménez-Benavides et al., 2007), flowers of both species showed a night-pollination syndrome: petals are extended at dusk, night and dawn, but folded during the day. Interestingly, these two species are the only ones blooming in this period in the populations studied with night-pollination syndrome.
Fig. 1.

(A) Silene ramosissima (pink arrow) and S. niceensis (black arrow) growing together in the PUMB population, (B) S. ramosissima floral stem, (C) S. ramosissima flower with folding petal limbs, (D) S. niceensis flower in male phase with petal limbs spread out, (E) S. niceensis flower in female phase with folded petal limbs, (F) S. ramosissima styles showing own pollen (pink arrow) and pollen added from S. niceensis (black arrow), (G) S. ramosissima (left) and S. niceensis (right) seeds.
Under these circumstances, S. niceensis and S. ramosissima could compete for pollinators, with the subsequent pollen limitation and/or interspecific pollen transfer (Morales and Traveset, 2008). In Silene, self-compatibility is a general pattern, and autonomous selfing occurs in different species of this genus (Jürgens, 2006; Jürgens et al., 2012). Thus, we predict that autonomous selfing could be a way of reducing competition for pollination and/or to avoid interspecific pollen transfer in the less abundant S. ramosissima, with respect to S. niceensis. We also predict that the selfing species will be less visited by pollinators. To test these predictions, we studied breeding systems and pollinator assemblage and activity in depth in two sympatric populations of S. niceensis and S. ramosissima. Specifically, our study had three main objectives: (1) to characterize the degree of autonomous selfing, pollen limitation and the pollen/ovule (P/O) ratio; (2) to analyse flower characteristics related to breeding systems, i.e. floral colour, longevity and overlapping sexual phases; and (3) to determine the spectrum of flower-visiting insects and their visitation rate.
MATERIALS AND METHODS
Study system
Silene niceensis All. and S. ramosissima Desf. inhabit coastal sands; they are particularly specialized to grow on consolidated foredune surfaces (García-Mora et al., 1999). Silene niceensis is a biennial or short-life perennial (rarely annual) distributed around the western Mediterranean basin (from the Iberian Peninsula to Greece). Silene ramosissima is an annual, and distribution is limited to the Iberian Peninsula, Algeria and Morocco (Talavera, 1990). In the Iberian Peninsula, they grow together in some locations on coastal sands of the south and south-east, with S. ramosissima blooming from April to June, whereas S. niceensis blooms from February to July (Talavera, 1990). Both species are hermaphroditic and the petal limbs of the flowers are extended from the evening to the morning and curl up when temperatures increase during the day (Fig. 1). Flowers of S. ramosissima are smaller, with a mean (± s.e., n) petal limb length of 4·10 mm (± 0·117, 31), compared with 7·21 mm (± 0·094, 48) in S. niceensis. They also have shorter calyces, with a mean of 12·07 mm (± 0·145, 31), compared with 13·42 mm (± 0·155, 48) in S. niceensis (M. L. Buide, unpubl. data). Pollen of both species is very similar in shape and size (Fig. 1E), and their seeds are also nearly identical (Fig. 1F). The flowers have different petal colour, whitish in S. niceensis and pink in S. ramosissima, when viewed by the human eye. These two species are taxonomically related, being the only representatives of the section Nicaeenses (Rohrb.) Talavera growing in the Iberian Peninsula and have the same chromosome number (2 n = 24) (Talavera, 1990; Chater and Walters, 1993). However, their phylogenetic relationships are not resolved. In spite of efforts to resolve the phylogeny of Silene and close genera (e.g. Petri and Oxelman, 2011), the relationships among many of the species in this huge genus remain undefined, partly because of the complex reticulate patterns (Frajman and Oxelman, 2007; Erixon and Oxelman 2008), which are mainly due to hybridization processes (Oxelman, 1996; Popp and Oxelman, 2001; Popp et al., 2005; Frajman and Oxelman, 2007). This study was carried out in southern Spain, in two of the populations where both species grow together: La Barrosa in Cádiz (36°20'28'', 6°10'53'W, hereafter BARR) and Punta Umbría-El Portil in Huelva (37°10'16''N, 6°57'5''W, hereafter PUMB).
Levels of autonomous selfing, pollen deficit and P/O ratio
The experiment was carried out in BARR and PUMB in April–May 2013. Fifteen randomly chosen plants per species were marked in PUMB and 20 in BARR. We selected one inflorescence and marked the unopened flowers with red wire, bagging the inflorescences with veil mesh (autonomous selfing). The same number of plants and inflorescences were marked with blue wire for controls (open-pollinated flowers). We followed the marked flowers until senescence and recorded them as either fruit produced or fruit aborted, for fruit set levels. Fruits were collected and their seeds and aborted ovules were counted under the dissecting microscope to calculate seed set. Additionally, ten flowers in PUMB and 15 in BARR were supplementarily pollinated in S. niceensis, although damage to marked plants caused the final data to be taken from six and 11 flowers, which were compared with controls. Supplementary pollination in S. ramosissima is a complicated task, due to the morphology of the flower (see the Results). We did not consider for statistical analysis supplementarily pollinated flowers of S. ramosissima because of the reduced number of data, only two in each population.
Pollen/ovule ratios in S. ramosissima were analysed from ten plants from each population. For S. niceensis, we analysed ten plants from PUMB and nine from BARR. We collected unopened flower buds and preserved them in FAA (95 % ethanol, dH2O, 37–40 % formaldehyde, acetic glacial acid, 10:7:2:1). The total number of ovules was counted under a dissecting microscope. One unopened anther from each flower was selected, and the total number of pollen grains was counted after preparation with Avetissian micro-acetolysis (in Fægri and Iversen, 1975). The total number of pollen grains was multiplied for the number of anthers.
Reflectance spectra measurement and segment classification
In order to know if the petal colour of both species may be distinguished by floral visitors, we haphazardly sampled 14–16 plants of S. niceensis and 9–16 plants of S. ramosissima in the PUMB and BARR population, respectively. For each plant, we selected a flower with no signs of damage and measured the reflectance spectra of the upper and lower surface of one petal limb. UV-VIS spectral reflectance was measured with a Jaz portable spectrophotometer (Ocean Optics, Dunedin, FL, USA) equipped with a deuterium–tungsten halogen light source (200–800 nm). Light was provided at 45° to each sample, and reflectance was measured for a surface of 8 mm diameter. Reflectance data were analysed with SpectraSuite v.10·7·1 software (Ocean Optics), relative to a white standard (WS-1-SL, Ocean Optics). For the analysis of spectral data, we used the colour segment classification proposed by Endler (1990), and modified by Smith (2014). Data were analysed from 400 to 700 nm, binned at 5 nm intervals, because no reflectance was found below 400 nm. This method has been proved to be accurate for quantifying the components of colour, especially brightness and chroma (Grill and Rush, 2000; E. Narbona unpublished results). We did not use the colour hexagon proposed by Chittka (1992) because it is based on the visual system of bees, and most pollinators in the species studied were hawkmoths and moths (see below).
Comparative floral phenology
Unopened flowers of each species were marked on April 2013 in the BARR population. Four hours later, at dusk, we made the first observation, and thereafter observations were made every 12 h, at dawn and dusk, until 112 h from the moment we marked the unopened flower. In S. niceensis, we recorded whether the flower was in male phase or female phase, to determine the period of overlap between both phases. We must note that male–female overlap could be overestimated, as we did not measure stigma receptivity or pollen viability. Some of the marked flowers were still in female phase at the end of the study; therefore, we analysed them separately. Finally, 17 flowers were used for sexual phase overlapping, and ten for flower longevity. In S. ramosissima, stamens and styles do not protrude beyond the petals, making it impossible to determine female and male phases without destroying the flowers. Therefore, we analysed floral longevity in the field in 19 plants and collected five flowers at different developmental stages (mature bud, early anthesis, anthesis, post-anthesis) to analyse male and female development (anther dehiscence, style length and the presence of pollen grains in the style) under the dissecting microscope. In addition, we collected 12 flowers at PUMB to correlate style–stigma length with number of pollen grains adhered.
Floral visitors, abundance and visitation rates
In order to find out the main activity of pollinators, we carried out a total of 29·75 h (16·25 h for S. ramosissima and 13·5 h for S. niceensis) of direct observations during one whole day in April–May of 2013. We did not observe any activity during the day, and some nocturnal floral visitors were influenced by our presence. Thus, in May 2014, we decided to concentrate our censuses from 0630 to 1030 h and from 2000 to 0030 h (GMT+2), using video observation (Lortie et al., 2012). We used a Bushnell NatureView Cam (model 119440; Bushnell Corporation, Overland Park, KS, USA) that incorporated a no-glow ‘black’ infrared LED flash, which allows recording in night light conditions. Each census day, nine cameras fixed to a tripod (20–30 cm height) were used. Cameras were set to continuous recording, with a lens with 42 mm of focal distance and video size of 1280 × 720 pixels. The camera stopped recording when the file size reached 1 Gb (approx. 36 min with our image resolution); we consider this period a pollination census. The total time of video observation was 53 and 42 h in the BARR population (44 and 38 censuses) and 41 and 28 h in the PUMB population (114 and 72 censuses) for S. niceensis and S. ramosissima, respectively. Censuses were carried out in areas with intermixed individuals of S. niceensis and S. ramosissima. At the period of censuses, the temperature and relative humidity ranged from 17 to 30 °C and from 70 to 100 % , respectively.
Based on selected video recordings, lepidopteran floral visitors were identified by the specialist Jose L. Yela (Universidad de Castilla-La Mancha, Toledo, Spain). Identified species were: the noctuids Autographa gamma (L), Mythimna vitellina (Hübner) and Heliothis peltigera (Sehiff.); the sphingids Hyles euphorbiae (L) and Hyles spp., and the syrphid Eristalis tenax L. However, a clear recognition of the insects was not possible in all videos; thus, we considered for analyses two groups of nocturnal pollinators: sphinx moths (Sphingidae; see Supplementary Data Video S1) and noctuid moths (Noctuidae) (Video S2). The a priori different foraging behaviour of both insect groups (Pettersson, 1991) led us to consider them two functional groups (Wilson et al., 2004). In order to discover the quantitative component of pollination effectiveness, we noted for each floral visitor: (1) the number of visits; (2) the duration of each visit; and (3) the number of flowers visited. For each functional pollinator group, we calculated their ‘abundance’ as the number of individuals per hour (standardized by number of flowers in each census that were within the camera’s field of vision; Rodríguez-Rodríguez et al., 2013) and the ‘visitation rate’ as the number of flowers visited per foraging bout duration (in seconds) (modified from Herrera, 1989).
Statistical analysis
The significance of the difference between the proportion of fruits in the autonomous selfing treatment and the proportion of fruits in controls was analysed using Pearson’s χ2 test for count data, or Fisher’s exact tests when at least one observed frequency was found to be <5. The effects of bagging (autonomous selfing vs. controls) and population (BARR vs. PUMB) on seed set were measured by generalized linear models (GLMs). We assumed binomial errors and logit link, including factors and interactions. Due to overdispersion, we adjusted the models with quasibinomial errors and simplified the models to the minimal adequate model (Crawley, 2007). The same procedure was applied to measure the effect of supplementary hand pollination and population on seed set. The effect of species, populations and interaction on the P/O ratio was tested with linear models because errors were normal (tested by means of a quantile–quantile plot and Shapiro–Wilk normality test). Pollinator abundance and visitation rates were modelled using GLMs with Gaussian or Gamma distribution with a log link function. Some statistical comparisons among functional groups of pollinators were not possible, due to the low number of visits (i.e. sample size <5). Finally, interspecific differences in colour parameters (brightness, chroma and hue) were tested with the independent two-group Mann–Whitney U-test. In the boxplots, outliers are values with >1·5 times the interquartile range above or below the third quartile and the first quartile, respectively (Crawley, 2007). All of the analyses were performed using R version 3·1·0 (http://www.R-project.org).
RESULTS
Levels of autonomous selfing, pollen deficit and P/O ratio
In Silene niceensis, fruit set after autonomous selfing was 47 % in BARR against 100 % in open-pollinated flowers (controls). In PUMB, fruit set after autonomous selfing was 17 %, against 65 % in controls. The proportion of fruits after autonomous selfing was significantly different from the proportion of fruits in controls in both BARR (n1 = 30, n2 = 49, P < 0·001) and PUMB (n1 = 65, n2 = 81, P < 0·001) populations. In S. ramosissima, fruit set after autonomous selfing and controls was 100 % in BARR, whereas in PUMB it was 97 % after autonomous selfing and 94 % for controls. The proportion of fruits in autonomous autogamy was not significantly different from the proportion of fruits in controls both in BARR (n1 = 17, n2 = 17, P = 1·00) and in PUMB populations (n1 = 35, n2 = 83, P = 0·67).
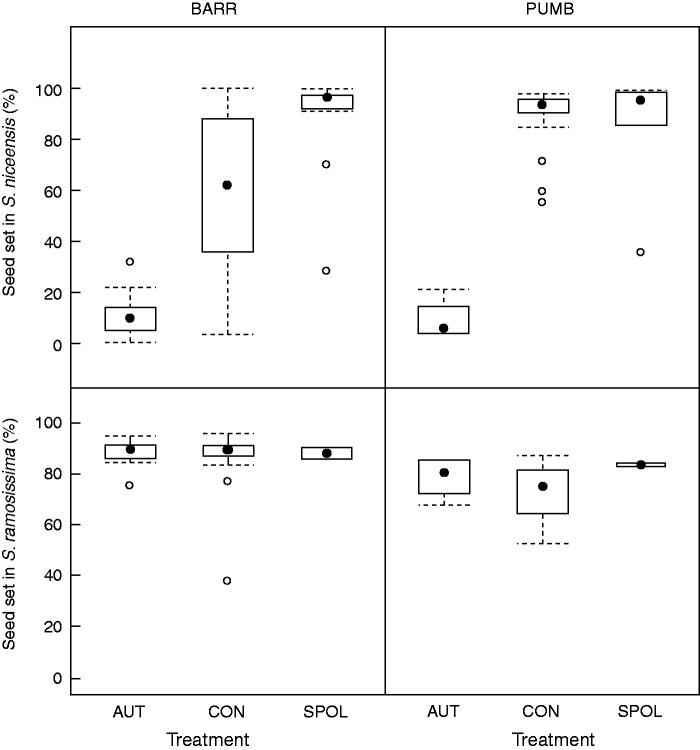
In S. niceensis, mean ( ± s.e.) seed set in the BARR population was 11·5 ± 2·3 % and 61·6 ± 4·5%, for autonomous selfing and control treatment, respectively. In the PUMB population, mean seed set for autonomous selfing was 9·3 ± 2·1 % and 89·8 ± 1·9 % for controls. As shown in Fig. 2A, the distribution of values for seed set in the autonomous selfing treatment was very similar in both populations, but dispersion was higher in the controls of BARR. Thus, the generalized linear model showed a significant effect of treatment and interaction, but not population (Table 1). The number of seeds per fruit for autonomous selfing was also smaller than that of controls (14·3 ± 3·1 vs. 73·8 ± 5·8 for BARR and 9·3 ±1·7 vs. 106·6 ± 3·6 for PUMB, respectively). Supplementary pollinated flowers had a mean seed set of 87·6 ± 6·4 % and 84·9 ± 10·1 %, and a mean number of seeds per fruit of 106 ± 8·7 and 92 ± 10·7 in BARR and PUMB, respectively. Generalized linear models comparing supplementary pollinated flowers with controls showed a significant effect of treatment, population and interaction (Table 2).
Fig. 2.
Boxplots of seed set produced by autonomous selfing (bagged flowers) and controls (natural pollinated flowers) of S. niceensis and S. ramosissima in BARR and PUMB populations. The central point shows the median, the bottom and top of the box are the first and third quartiles, the whiskers are either the maximum value or 1·5 times the interquartile range, whichever is the smaller, and the points are outliers.
Table 1.
Minimal adequate model resulting of simplification of generalized linear models for the effect of treatment (autonomous selfing vs. controls), population (BARR vs. PUMB) and the interactions on seed set
| Species | Source of variation | Estimate | s.e. | t | P-value |
|---|---|---|---|---|---|
| S. niceensis | Intercept | 0·40 | −5·10 | *** | |
| Population | −2·05 | 0·70 | −0·46 | 0·65 | |
| Treatment | 2·58 | 0·43 | 6·02 | *** | |
| Population × treatment | 2·00 | 0·78 | 2·56 | * | |
| S. ramosissima | Intercept | 1·93 | 0·14 | 13·72 | *** |
| Population | −0·77 | 0·19 | −4·11 | *** |
Because of overdispersion, we refitted the models using quasibinomial.
Significance values: ***P < 0·001, *P < 0·05.
Table 2.
Minimal adequate model resulting of simplification of generalized linear models for the effect of treatment (supplementary pollination vs. controls), population (BARR vs. PUMB) and the interactions on seed set in Silene niceensis
| Source of variation | Estimate | s.e. | t | P-value |
|---|---|---|---|---|
| Intercept | 0·53 | 0·17 | 3·10 | ** |
| Population | 1·68 | 0·39 | 4·30 | *** |
| Treatment | 1·44 | 0·54 | 2·66 | ** |
| Population × treatment | –1·99 | 0·92 | –2·16 | * |
Because of overdispersion, we refitted the models using quasibinomial.
Significance values: ***P < 0·001, **P < 0·01, *P < 0·05.
Silene ramosissima plants had similar very high seed sets between autonomous selfing and controls, in BARR as well as in PUMB (Fig. 2B). Seed set values in BARR were higher and less dispersed than in PUMB; thus, the GLM shows population as the only significant factor (Table 1). In BARR, the mean (±s.e.) seed/fruit was 113 ± 2·8 in autonomous selfing and 107·4 ± 4·4 in controls. In PUMB, the mean seed/fruit was 111·6 ± 4·3 in autonomous selfing and 103·4 ± 5·5 in controls. Hand-pollinated flowers of S. ramosissima had high mean seed sets in both populations (Fig. 2B), with a mean of 89 ± 9·0 seeds per fruit in BARRR and 120 ± 18·0 seeds per fruit in PUMB. We did not include statistical analyses due to the low number of hand-pollinated flowers.
Flowers of S. niceensis had a mean (±s.e.) number of pollen grains of 9929 (±588·6) and 112 (±4·7) ovules. Flowers of S. ramosissima had a three times lower mean number of pollen grains (3112 ± 337·7), but the number of ovules was higher (128 ± 6·9). Thus, S. niceensis had a mean P/O ratio (± s.e.) of 89·9 (± 5·4), compared with 27·0 (± 3·7) for S. ramosissima (Fig. 3). The effect of species was significant (P < 0·001), but those of population and interaction were not (Table 3).
Fig. 3.
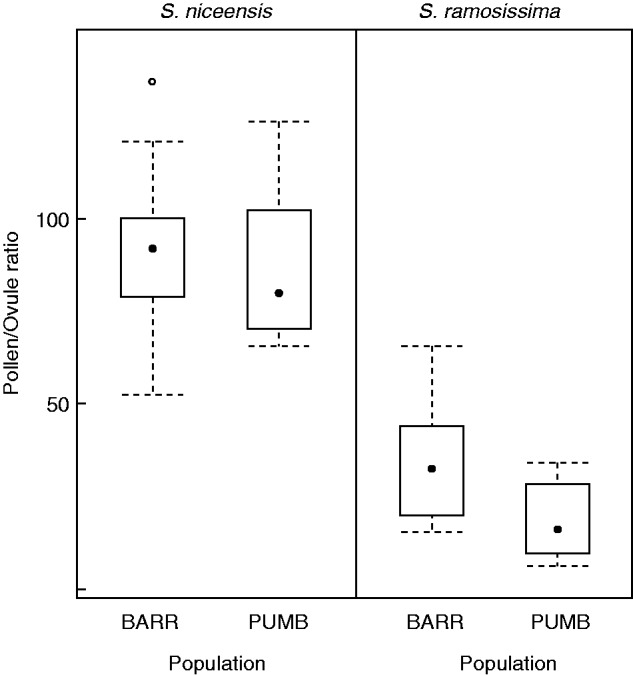
Boxplots of the pollen/ovule ratio for Silene ramosissima and Silene niceensis in BARR and PUMB populations. The central point shows the median, the bottom and top of the box are the first and third quartiles, the whiskers are either the maximum value or 1·5 times the interquartile range, whichever is the smaller, and the points are outliers.
Table 3.
Results of the linear model for the effect of explanatory variables species, population and their interaction on the response variable P/O ratio
| Source of variation | Estimate | s.e. | t | P-value |
|---|---|---|---|---|
| Intersection | 92·03 | 6·56 | 14·03 | *** |
| Species | –56·39 | 9·05 | –6·23 | *** |
| Population | –4·11 | 9·05 | –0·45 | 0·65 |
| Species × population | –13·26 | 12·62 | –1·05 | 0·30 |
Significance values: ***P < 0·001.
Reflectance spectra measurement and segment classification
Upper surface petal limbs of S. niceensis showed typical reflectance spectra of white petals with no reflectance in the UV region, whereas lower surface petal limbs appear grey to humans, with a gradual increase in reflectance from 400 nm with a valley at 670 nm (Supplementary Data Fig. S1). In S. ramosissima, upper and lower surfaces of petal limbs look pink to humans, with a spectra defined by a reflectance minimum in the green region. Lower surface petal limbs showed a similar pattern, but with a less defined reflectance minimum. Overall, the differences among plants in every species were mainly due to brightness variations (Fig. S1).
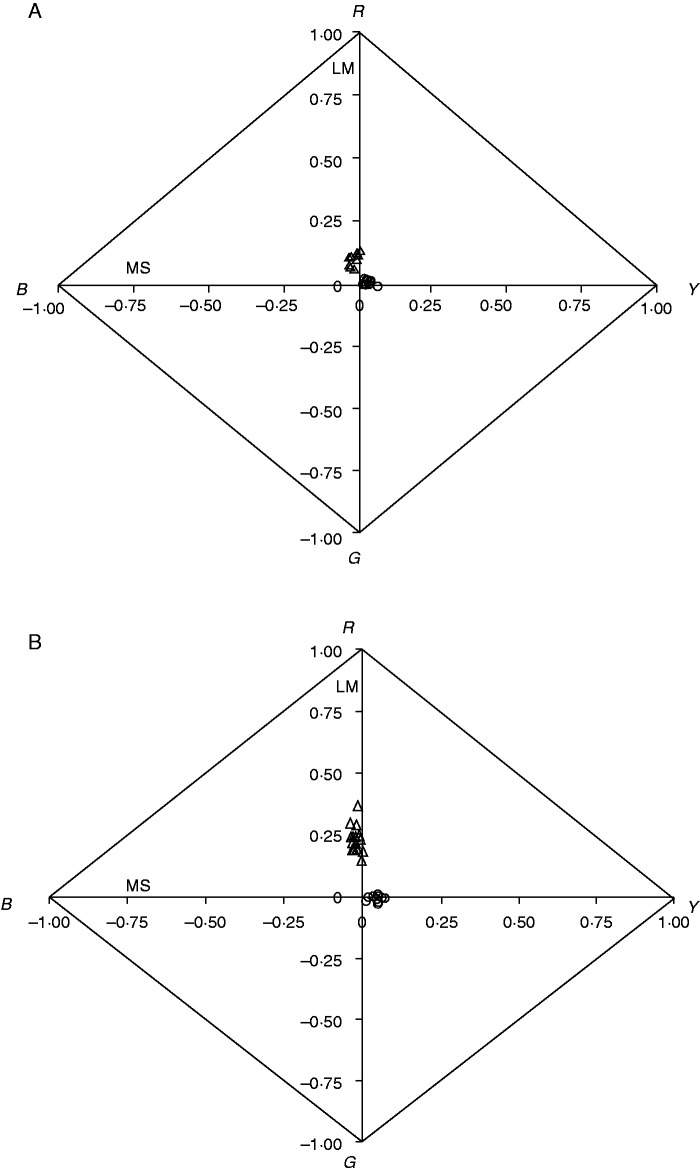
All S. niceensis upper surface petals showed very similar patterns in colour space in both populations (Fig. 4), with chroma close to zero (mean ± s.e.; 0·05 ± 0·003 in BARR, 0·03 ± 0·003 in PUMB). Mean values of brightness were 24·9 (± 1·27) and 26·7 (± 1·66), whereas mean values of hue were 98·65 (± 3·61) and 81·14 (± 5·17) in BARR and PUMB populations, respectively. Upper surface petals of S. ramosissima showed mean chroma values of 0·23 (± 0·01) and 0·10 (± 0·01), mean brightness values of 10·32 (± 0·54) and 14·18 (± 1·78), and mean hue values of 332·62 (± 22·10) and 308·67 (± 38·50) in BARR and PUMB populations, respectively. Upper surface petals of both species were differentially represented in the colour space (Fig. 4), with significant interspecific differences for brightness (H = 729, P < 0·0001), chroma (H = 2, P < 0·0001) and hue (H = 60, P < 0·0001).
Fig. 4.
Colour space with the segment classification system (Endler, 1990; Smith, 2014) for S. niceensis (open circles) and S. ramosissima (open triangles), for PUMB (A) and BARR (B) populations. Plotted values correspond to LM (the difference between long and medium wavelengths) and MS (the difference between medium and short wavelengths).
Comparative floral phenology
The mean floral longevity of S. niceensis flowers was 99·6 h (± 4·7); with male and female phase longevity of 55·2 h (± 2·7) and 52·8 h (± 4·1), respectively. A total of 41 % of flowers showed a male–female overlapping phase, with a mean of 14·6 h (± 1·8) duration of overlap.
The flowers of S. ramosissima lasted a mean of 19 h (± 4·5). The flowers analysed at mature bud stage had indehiscent anthers and no pollen grains in the style, whereas flowers in early anthesis, anthesis and post-anthesis had both dehiscent anthers and pollen grains at the style. The styles of the flowers at any developmental stage were 4–5 mm long, and the anthers were located at the same place or a little above the style. Observation of the styles under the dissection microscope showed that stigmatic papillae are distributed along their entire surface. No correlation was found between style length and number of pollen grains (R = 0·009, P = 0·98, n = 12).
Floral visitors and visitation rates
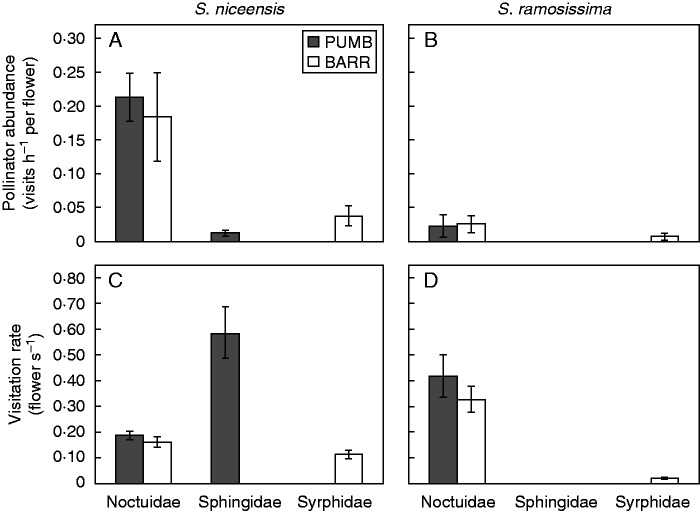
In our censuses, S. niceensis received a total of 320 visits, 306 at dusk/night and 15 at dawn/morning, whereas S. ramosissima received only nine visits (seven at dusk/night and two at dawn/morning). In S. niceensis, the most abundant floral visitors were noctuids in both PUMB and BARR populations (92·2 and 81·5 % of total visits, respectively; Fig. 5A). Sphingids and Eristalis tenax only occasionally visited S. nicaeensis flowers at the PUMB and BARR population, respectively. In S. ramosissima, the main floral visitors were also noctuids (100 and 71·4 %; PUMB and BARR, respectively; Fig. 5B). The standardized abundance of the noctuid functional group was nine and seven times higher in S. niceensis than in S. ramosissima in both PUMB and BARR populations, respectively (Fig. 5A, B). The abundance of noctuids visiting flowers of S. niceensis at PUMB and BARR populations was statistically similar (t1,157 = 0·41, P = 0·68; Fig. 5A).
Fig. 5.
Comparing functional groups of pollinators between S. niceensis and S. ramosissima at PUMB and BARR populations (see key in A). (A, B) Mean (±s.e.) pollinator abundance, calculated as the visits per flower per hour and relative to the available flowers in that video recording. (C, D) Mean (± s.e.) visitation rate, calculated as the number of flowers visited per the foraging bout duration (seconds).
In the PUMB population of S. niceensis, the functional group of sphingids showed a visitation rate almost three times higher than those of noctuids, and this difference was significant (t1,238 = –4·31, P < 0·0001; Fig. 5C). In contrast, noctuids showed a statistically similar visitation rate to E. tenax in PUMB (t1,80 = 1·24, P = 0·22). There were no significant differences between visitation rates of noctuids between PUMB and BARR populations in both S. niceensis (t1,294 = –0·80, P = 0·42) and S. ramosissima (t1,6 = –0·89, P = 0·41; Fig. 5C, D). Visitation rates of noctuids in S. ramosissima were nearly double that of S. niceensis in both BARR and PUMB populations. This is because noctuids visited a low number of flowers per bout in S. ramosissima (mean = 1·4, range = 1–3) whereas in S. niceensis this number was higher (mean = 3·5, range = 1–23). In addition, the time spent in the bout was much lower in S. ramosissima (mean = 5·4 s, range = 2–16 s) than in S. niceensis (mean = 32·6 s, range = 2–234 s; see also Supplementary Data Videos S2 and S3).
DISCUSSION
Silene ramosissima shares habitat and phenology with the co-occurring S. niceensis, which has a longer blooming period and wider distribution area. Flowers also open from dusk/night until dawn/morning and share pollinators (noctuids and syrphids). However, the number of visits to S. ramosissima flowers was extremely low. In spite of low pollinator visitation, S. ramosissima produces high proportions of seeds by autonomous selfing. Conversely, S. niceensis showed low levels of autonomous selfing and thereby a higher dependence on pollinators, with frequent visits of pollinators to both populations. Therefore, pollinator-mediated competition could be a potential problem for the less abundant and completely overlapped S. ramosissima. Bell et al. (2005) demonstrated that competition for pollination reduced the number of seeds and the outcrossing rate of Mimulus ringens growing with Lobelia siphilitica, and similar results were found by Tokuda et al. (2015). In our study system, we have found no pollinator competition because, even sharing pollinators, they almost exclusively visited S. niceensis flowers. Thus, the selfing strategy showed by S. ramosissima allows both species to coexist in the specialized habitat with a similar flowering phenology and night-pollination syndrome.
Competition for visits is not the only form of pollinator-mediated competition; another is interspecific pollen transfer, which also has two components: heterospecific pollen deposition and conspecific pollen loss (Morales and Traveset, 2008). Interspecific pollen transfer may have negative consequences even in unrelated species. In addition, if the sympatric congeneric species are closely related, an additional consequence of interspecific pollen transfer could be the formation of hybrids. If this is the case, selfing may contribute to reproductive isolation. For example, Matallana et al. (2010) proposed that in 40 species of Bromeliaceae analysed, self-compatibility could be a reproductive isolating mechanism, since they found self-compatible species more frequently associated with congeneric species, and with a higher flowering overlap. In the present study, selfing in S. ramosissima avoids interspecific pollen transfer and its negative consequences.
Silene niceensis showed inter-population differences in pollen limitation, with lower and more variable seed set of controls vs. supplementary pollinated plants in one of the populations studied. Thus, the results suggest that pollinators may fluctuate with the changing conditions of the coastal dunes, which may also decrease pollen availability. Restricted pollinator activity was found in other plant communities, such as forest spring wild flowers (Motten, 1986), and has been found to be dependent on plant density (Zorn-Arnold and Howe, 2007). The strategy selected to avoid limited availability of pollinators and reach reproductive assurance may be autonomous selfing, as has been suggested in the three closely related species Centaurium erythraea, C. littorale and C. pulchellum (Brys and Jacquemyn 2011).
Reproductive assurance has also been proposed for the evolution of autonomous selfing in other species of Silene, such as S. noctiflora (Davis and Delph, 2005), where 40 % of seeds were produced by prior selfing. Similarly to S. noctiflora, the stigmatic papillae in S. ramosissima are distributed all along the surface of the styles, which facilitates self-fertilization, since the anthers are located at the level of the style. Moreover, the flowers lasted a mean of 19 h, with total overlap between male and females phases. On the other hand, S. niceensis flowers had a five times longer life span, with an overlap between male and female phases of 15 h. In Silene, mature anthers and stigmas are physically close, which facilitates the shift to autogamy when dichogamy disappears (Jürgens et al., 2002a). A shift from outcrossing to selfing has been demonstrated at different taxonomic levels: in different sister species, such as Clarkia unguiculata (outcrosser) and C. exilis (facultative selfer), and in subspecies, as is the case of C. xanthiana ssp. xantiana (outcrosser) and the selfing ssp. parviflora (Mazer et al., 2009).
According to the observations of the mating systems experiments, S. ramosissima had a P/O ratio within the range established by Cruden (1977) as obligate autogamy. As expected, the less autogamous S. niceensis had a higher P/O ratio, although the values were lower than would correspond to a facultative xenogamous plant. However, high variability in P/O ratios is expected in xenogamous breeding systems, i.e. lower P/O ratios had been reported for xenogamous species with large stigma areas (Cruden, 2000). Moreover, the P/O ratio values we found in S. niceensis are very similar to those presented by Jürgens et al. (2002a) for this plant. On the other hand, S. ramosissima presented the lowest P/O ratio values found in Silene to date (mean = 27), only comparable with Silene apetala (mean = 36, in Jürgens et al. 2002a), a highly selfing plant that varies between autogamy and cleistogamy (Jürgens et al. 2002a).
We have found that upper surface petals of S. niceensis and S. ramosissima differ in brightness, hue and chroma. Brightness was higher in the predominantly outcrossing S. niceensis, but chroma and hue were lower. In Camissoniopsis cheiranthifolia, Button et al. (2012) found higher values of brightness and chroma in predominantly outcrossing populations, whereas higher values of hue, corresponding to a shift towards the red end of the spectrum, were found in the selfing populations of this species. In contrast to diurnal honey-bees and diurnal hawkmoths, nocturnal hawkmoths can see colours at dim starlight levels (Kelber and Roth, 2006). In addition, some hawkmoth species may use achromatic (brightness) vision under dim conditions (Goyret and Yuan, 2015). Therefore, these abundant nocturnal visitors found in the two populations studied could potentially differentiate between both species.
The analysis of the main functional groups of pollinators in three sister species of Silene showed that S. virginica is hummingbird pollinated (red petals), S. stellata is pollinated by nocturnal moths (white petals), and S. caroliniana showed a less consistent pattern (Reynolds et al., 2009). In the same way, the sister species S. latifolia (white) and S. dioica (pink) have been included as examples of moth and bumble-bee as primary pollinators, respectively (Kephart, 2006). Other studies have found both diurnal and nocturnal pollinators in S. latifolia, although nocturnal pollinators were more effective (Barthelmess, 2006). In the narrow endemic S. sennenii, which also has a nocturnal pollinator syndrome, Martinell et al. (2010) found mainly night pollination, although there were also day visitors. Conversely, S. ciliata is effectively pollinated by diurnal and nocturnal pollinators in spite of its nocturnal pollination syndrome (Giménez-Benavides et al., 2007). Silene niceensis follows the predictions for night-flowering Caryophylloideae; the more abundant pollinators were noctuids. Sphingids were less abundant, but visited a higher number of flowers per bout. Although we did not measure pollinator effectiveness, sphingids probably do not contact anthers and style as frequently as noctuids (Supplementary Data Videos S1 and S2). A study of pollinator fauna of night flowering in S. vulgaris showed that the most long-tongued moths (sphingids) acted as pollinators, but were less efficient than short-tongued moths (noctuids) (Pettersson, 1991). On the other hand, Jürgens (2006) demonstrated that many nocturnally pollinated species of Caryophylloideae have the filaments and the style exserted beyond the calyx, ensuring contact of moths and sphingids with floral parts. Silene ramosissima had much lower visitation rates. The small flowers of this species may contribute to low attraction. Moreover, prior selfing in this species reduces the time the flower is available to pollinator visitation. In S. ramosissima, folding of petals during the day could be a remnant of previous adaptation to nocturnal pollinators. An alternative explanation for the folding of petals during the day is a protective function against diurnal water loss (Greuter, 1995; Galen et al., 1999). The evolution of selfing Caryophylloideae has been proposed from narrow tubes adapted to long-tongued pollinators, a breakdown of dichogamy and finally the cessation of exsertion of reproductive organs (Jürgens, 2006).
In conclusion, our findings demonstrate that the different mating systems in S. ramosissima (autonomous prior selfing) and S. niceensis (facultative xenogamy) are found to be effective in assuring reproduction in both species. The potentially less attractive S. ramosissima (reduced flower size, petals with low brightness, no exserted styles, less abundant population) was rarely visited by insects; in contrast, high levels of fruit and seed set were achieved by autonomous pollination. Although our data may suggest that coexistence with S. niceensis could be the driving force leading to selfing, future experiments should examine populations of S. ramosissima without S. niceensis. On the other hand, we have found that the petal colour of both species is different, which could suggest a priori that pollinators may differentiate between them. However, it might be interesting to analyse flower scent composition in these species, since scent has been shown to play a important role in night-pollinated species of Silene (Jürgens et al., 2002b; Waelti et al. 2008).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: spectral reflectance of the petal upper and lower surfaces of Silene niceensis and S. ramosissima. Video S1: short film showing a visit by Hyles spp. (Sphingidae) to the flowers of S. niceensis. Video S2: short film showing a visit by Noctuidae to the flowers of S. niceensis. Video S3: short film showing a visit by Noctuidae to the flowers of S. ramosissima. Video S4: short film showing a visit by Syrphidae to both S. ramosissima and S. niceensis flowers.
ACKNOWLEDGEMENTS
We thank José Luis Yela, who kindly identified moths and sphingids from the videos. We also thank Ágata Cardoso, Silvio Calgaro and Mónica Míguez for field assistance, Ana Ma Macías-Augustín for help in spectra data, and Justen B. Whittall for valuable discussion of species reproductive strategies. We thank the Handling Editor, Donald A. Levin and three anonymous reviewers for constructive comments on an earlier draft. This research was supported by the Spanish Government MINECO project (CGL2012-37646).
LITERATURE CITED
- Armbruster WS, Edwards ME, Debevec EM. 1994. Floral character displacement generates assemblage structure of western australian triggerplants (Stylidium). Ecology 75: 315–329. [Google Scholar]
- Barthelmess EL, Richards CM, McCauley DE. 2006. Relative effects of nocturnal vs diurnal pollinators and distance on gene flow in small Silene alba populations. New Phytologist 169: 689–698. [DOI] [PubMed] [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. 2005. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology 86: 762–771. [Google Scholar]
- Briscoe Runquist R, Moeller DA. 2014. Floral and mating system divergence in secondary sympatry: testing an alternative hypothesis to reinforcement in Clarkia. Annals of Botany 113: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe Runquist R, Stanton ML. 2013. Asymmetric and frequency-dependent pollinator-mediated interactions may influence competitive displacement in two vernal pool plants. Ecology Letters 16: 183–190. [DOI] [PubMed] [Google Scholar]
- Brys R, Jacquemyn H. 2011. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany 107: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch JW, Delph LF. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button L, Lopez Villalobos A, Dart SR, Eckert CG. 2012. Reduced petal size and color associated with transitions from outcrossing to selfing in Camissoniopsis cheiranthifolia (Onagraceae). International Journal of Plant Sciences 173: 251–260. [Google Scholar]
- Chater AO, Walters SM. 1993. Silene L. In: Tutin TG, Bungers NA, Chater AO, et al., eds Flora Europea, Vol. 1 Cambridge: Cambridge University Press, 158–181. [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170: 533–543. [Google Scholar]
- Crawley MJ. 2007. The R book. Chichester , UK: John Wiley & Sons Ltd. [Google Scholar]
- Cruden RW. 1977. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- Cruden RW. 2000. Pollen grains: why so many? Plant Systematics and Evolution 222: 143–165. [Google Scholar]
- Davis SL, Delph LF. 2005. Prior selfing and gynomonoecy in Silene noctiflora L. (Caryophyllaceae): opportunities for enhanced outcrossing and reproductive assurance. International Journal of Plant Sciences 166: 475–480. [Google Scholar]
- Eckert CG, Samis KE, Dart S. 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. New York: Oxford University Press, 183–203. [Google Scholar]
- Endler JA. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biological Journal of the Linnean Society 41: 315–352. [Google Scholar]
- Erixon P, Oxelman B. 2008. Reticulate or tree-like chloroplast DNA evolution in Sileneae (Caryophyllaceae)? Molecular Phylogenetics and Evolution 48: 313–325. [DOI] [PubMed] [Google Scholar]
- Fægri K, Iversen J. 1975. Textbook of pollen analysis. Copenhagen: Scandinavian University Books. [Google Scholar]
- Feinsinger P, Tiebout HM., III 1991. Competition among plants sharing hummingbird pollinators: laboratory experiments on a mechanism. Ecology 72: 1946–1952. [Google Scholar]
- Fishman L, Wyatt R. 1999. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution 53: 1723–1733. [DOI] [PubMed] [Google Scholar]
- Frajman B, Oxelman B. 2007. Reticulate phylogenetics and phytogeographical structure of Heliosperma (Sileneae, Caryophyllaceae) inferred from chloroplast and nuclear DNA sequences. Molecular Phylogenetics and Evolution 43: 140–155. [DOI] [PubMed] [Google Scholar]
- Galen C, Sherry RA, Carroll AB. 1999. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia 118: 461–470. [DOI] [PubMed] [Google Scholar]
- García-Mora MR, Gallego-Fernández JB, García-Novo F. 1999. Plant functional types in coastal foredunes in relation to environmental stress and disturbance. Journal of Vegetation Science 10: 27–34. [Google Scholar]
- Gibson AK, Hood ME, Giraud T. 2012. Sibling competition arena: selfing and a competition arena can combine to constitute a barrier to gene flow in sympatry. Evolution 66: 1917–1930. [DOI] [PubMed] [Google Scholar]
- Giménez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. 2007. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene–Hadena interaction. Oikos 116: 1461–1472. [Google Scholar]
- Goyret J, Yuan ML. 2015. Influence of ambient illumination on the use of olfactory and visual signals by a nocturnal hawkmoth during close-range foraging. Integrative and Comparative Biology (in press). [DOI] [PubMed] [Google Scholar]
- Greuter W. 1995. Silene (Caryophyllaceae) in Greece: a subgeneric and sectional classification. Taxon 44: 543–581. [Google Scholar]
- Grill CP, Rush VN. 2000. Analysing spectral data: comparison and application of two techniques. Biological Journal of the Linnean Society 69: 121–138. [Google Scholar]
- Grossenbacher DL, Whittall JB. 2011. Increased floral divergence in sympatric monkeyflowers. Evolution 65: 2712–2718. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1989. Pollinator abundance, morphology, and flower visitation rate: analysis of the ‘quantity’ component in a plant–pollinator system. Oecologia 80: 241–248. [DOI] [PubMed] [Google Scholar]
- Hopkins R. 2013. Reinforcement in plants. New Phytologist 197: 1095–1103. [DOI] [PubMed] [Google Scholar]
- Jürgens A. 2006. Comparative floral morphometrics in day-flowering, night-flowering and self-pollinated Caryophylloideae (Agrostemma, Dianthus, Saponaria, Silene, and Vaccaria). Plant Systematics and Evolution 257: 233–250. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2002a. Pollen grain numbers, ovule numbers and pollen–ovule ratios in Caryophylloideae: correlation with breeding system, pollination, life form, style number, and sexual system. Sex Plant Reproduction 14: 279–289. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2002b. Flower scent composition in night-flowering Silene species (Caryophyllaceae). Biochemical Systematics and Ecology 30: 383–397. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2012. Pollen grain size variation in Caryophylloideae: a mixed strategy for pollen deposition along styles with long stigmatic areas? Plant Systematics and Evolution 298: 9–24. [Google Scholar]
- Kelber A, Roth LSV. 2006. Nocturnal colour vision – not as rare as we might think. Journal of Experimental Biology 209: 781–788. [DOI] [PubMed] [Google Scholar]
- Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR. 2006. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: evaluating a model system to study the evolution of mutualisms. New Phytologist 169: 667–680. [DOI] [PubMed] [Google Scholar]
- Lepers C, Dufay M, Billiard S. 2014. How does pollination mutualism affect the evolution of prior self-fertilization? A model. Evolution 68: 3581–3598. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1971. The origin of reproductive isolating mechanisms in flowering plants. Taxon 20: 91–113. [Google Scholar]
- Levin DA. 2010. Environment-enhanced self-fertilization: implications for niche shifts in adjacent populations. Journal of Ecology 98:1276–1283. [Google Scholar]
- Lloyd DG. 1979. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist: 113: 67–79. [Google Scholar]
- Lortie CJ, Budden AE, Reid AM. 2012. From birds to bees: applying video observations techniques to invertebrate pollinators. Journal of Pollination Ecology 6: 125–128. [Google Scholar]
- Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68–82. [DOI] [PubMed] [Google Scholar]
- Martinell MC, Dötterl S, Blanché C, Rovira A, Massó S, Bosch M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. [Google Scholar]
- Matallana G, Godinho MAS, Guilherme FAG, Belisario M, Coser TS, Wendt T. 2010. Breeding systems of Bromeliaceae species: evolution of selfing in the context of sympatric occurrence. Plant Systematics and Evolution 289: 57–65. [Google Scholar]
- Mazer SJ, Dudley LS, Delesalle VA, Paz H, Galusky P. 2009. Stability of pollen–ovule ratios in pollinator-dependent versus autogamous Clarkia sister taxa: testing evolutionary predictions. New Phytologist 183: 630–648. [DOI] [PubMed] [Google Scholar]
- Morales CL, Traveset A. 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences 27: 221–238. [Google Scholar]
- Motten AF. 1986. Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecological Monographs 56: 21–42. [Google Scholar]
- Muchhala N, Potts MD. 2007. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proceedings of the Royal Society B: Biological Sciences 274: 2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxelman B. 1996. RAPD patterns, nrDNA ITS sequences and morphological patterns in Silene section Sedoideae (Caryophyllaceae). Plant Systematics and Evolution 201: 93–116. [Google Scholar]
- Palmer TM, Stanton ML, Young TP. 2003. Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds. American Naturalist 162: S63–S79. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Barrett SCH. 1998. Baker’s law revisited: reproductive assurance in a metapopulation. Evolution 52: 657–668. [DOI] [PubMed] [Google Scholar]
- Petri A, Oxelman B. 2011. Phylogenetic relationships within Silene (Caryophyllaceae) section Physolychnis. Taxon 60: 953–968. [Google Scholar]
- Pettersson MW. 1991. Pollination by a guild of fluctuating moth populations: option for unspecialization in Silene vulgaris. Journal of Ecology 79: 591–604. [Google Scholar]
- Popp M, Oxelman B. 2001. Inferring the history of the polyploid Silene aegaea (Caryophyllaceae) using plastid and homoeologous nuclear DNA sequences. Molecular Phylogenetics and Evolution 20: 474–481. [DOI] [PubMed] [Google Scholar]
- Popp M, Erixon P, Eggens F, Oxelman B. 2005. Origin and evolution of a circumpolar polyploid species complex in Silene (Caryophyllaceae) inferred from low copy nuclear RNA polymerase introns, rDNA, and chloroplast DNA. Systematic Botany 30: 302–313. [Google Scholar]
- Ramsey J, Bradshaw HD, Jr, Schemske DW. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57: 1520–1534. [DOI] [PubMed] [Google Scholar]
- Reynolds RJ, Westbrook MJ, Rohde AS, Cridland JM, Fenster CB, Dudash MR. 2009. Pollinator specialization and pollination syndromes of three related North American Silene. Ecology 90: 2077–2087. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez MC, Jordano P, Valido A. 2013. Quantity and quality components of effectiveness in insular pollinator assemblages. Oecologia 173: 179–190. [DOI] [PubMed] [Google Scholar]
- Santamaría L, Rodríguez-Girones MA. 2007. Linkage rules for plant–pollinator networks: trait complementarity or exploitation barriers? PLoS Biology 5: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent RD, Ackerly DD. 2008. Plant–pollinator interactions and the assembly of plant communities. Trends in Ecology and Evolution 23: 123–130. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology and Evolution 28: 307–315. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Schlüter PM. 2009. Floral isolation, specialized pollination, and pollinator behaviour in orchids. Annual Review of Entomology 54: 425–46. [DOI] [PubMed] [Google Scholar]
- Smith SD. 2014. Quantifying color variation: improved formulas for calculating hue with segment classification. Applications in Plant Sciences 2: 1300088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Rausher MD. 2007. Close clustering of anthers and stigma in Ipomoea hederacea enhances prezygotic isolation from Ipomoea purpurea. New Phytologist 173: 641–647. [DOI] [PubMed] [Google Scholar]
- Smith RA, Rausher MD. 2008. Experimental evidence that selection favors character displacement in the ivyleaf morning glory. American Naturalist 171: 1–9. [DOI] [PubMed] [Google Scholar]
- Talavera S. 1990. Silene L. In: Castroviejo S, Laínz M, López González G, et al., eds. Flora Iberica. Madrid: Real Jardín Botánico, CSIC, 313–406. [Google Scholar]
- Tokuda N, Hattori M, Abe K, Shinohara Y, Nagano Y, Itino T. 2015. Demonstration of pollinator-mediated competition between two native Impatiens species, Impatiens noli-tangere and I. textori (Balsaminaceae). Ecology and Evolution 5: 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser NM. 1983. Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 277–293. [Google Scholar]
- Waelti MO, Muhlemann JK, Widmer A, Schiestl FP. 2008. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology 21: 111–121. [DOI] [PubMed] [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. 2004. A multivariate search for pollination syndromes among penstemons. Oikos 104: 345–361. [Google Scholar]
- Zorn-Arnold B, Howe HF. 2007. Density and seed set in a self-compatible forb, Penstemon digitalis (Plantaginaceae), with multiple pollinators. American Journal of Botany 94: 1594–1602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.