Abstract
Background and Aims During evolution, plants have acquired and/or lost diverse sugar residues as cell-wall constituents. Of particular interest are primordial cell-wall features that existed, and in some cases abruptly changed, during the momentous step whereby land-plants arose from charophytic algal ancestors.
Methods Polysaccharides were extracted from four charophyte orders [Chlorokybales (Chlorokybus atmophyticus), Klebsormidiales (Klebsormidium fluitans, K. subtile), Charales (Chara vulgaris, Nitella flexilis), Coleochaetales (Coleochaete scutata)] and an early-diverging land-plant (Anthoceros agrestis). ‘Pectins’ and ‘hemicelluloses’, operationally defined as extractable in oxalate (100 °C) and 6 m NaOH (37 °C), respectively, were acid- or Driselase-hydrolysed, and the monosaccharides analysed chromatographically. One unusual monosaccharide, ‘U’, was characterized by 1H/13C-nuclear magnetic resonance spectroscopy and also enzymically.
Key Results ‘U’ was identified as 3-O-methyl-d-galactose (3-MeGal). All pectins, except in Klebsormidium, contained acid- and Driselase-releasable galacturonate, suggesting homogalacturonan. All pectins, without exception, released rhamnose and galactose on acid hydrolysis; however, only in ‘higher’ charophytes (Charales, Coleochaetales) and Anthoceros were these sugars also efficiently released by Driselase, suggesting rhamnogalacturonan-I. Pectins of ‘higher’ charophytes, especially Chara, contained little arabinose, instead possessing 3-MeGal. Anthoceros hemicelluloses were rich in glucose, xylose, galactose and arabinose (suggesting xyloglucan and arabinoxylan), none of which was consistently present in charophyte hemicelluloses.
Conclusions Homogalacturonan is an ancient streptophyte feature, albeit secondarily lost in Klebsormidium. When conquering the land, the first embryophytes already possessed rhamnogalacturonan-I. In contrast, charophyte and land-plant hemicelluloses differ substantially, indicating major changes during terrestrialization. The presence of 3-MeGal in charophytes and lycophytes but not in the ‘intervening’ bryophytes confirms that cell-wall chemistry changed drastically between major phylogenetic grades.
Keywords: Charophytic algae, charophytes, Embryophyta, Streptophyta, Chlorokybus, Klebsormidium, Chara, Coleochaete, plant cell-wall evolution, pectin, pectic polysaccharides, rhamnogalacturonan-I, 3-O-methyl-d-galactose
INTRODUCTION
All land-plants, from liverworts to angiosperms, comprise a single taxon, the Embryophyta, believed to have evolved from a single aquatic green-algal ancestor approximately 460 Mya (Leliaert et al., 2012). That ancient aquatic is presumably extinct, but phylogenetic studies imply that its closest living relatives are freshwater algae of the division Charophyta. The charophytes plus the embryophytes together constitute the Streptophyta. Nevertheless, a major evolutionary chasm separates the earliest-diverging land-plants from their closest charophytic relatives. It remains uncertain which of three extant charophytic orders (Charales, Coleochaetales or Zygnematales) is closest related to the land-plants (Karol et al., 2001; Wodniok et al., 2011; Zhong et al., 2013); two earlier-diverging orders of charophytes (Klebsormidiales and Chlorokybales) are clearly less closely related to them.
Adapting to life on land must have necessitated numerous changes; among the most important, enabling an upright growth habit in a windy atmosphere that lacks buoyancy, and conferring resistance to the desiccation and new herbivores and pathogens encountered on land, would have involved cell walls. We are interested in the primordial wall components that were seconded into new roles when charophytic algae adapted to terrestrial life.
The chemistry of charophyte cell walls has been relatively little explored. At least some charophytes are known to contain cellulose (Hotchkiss and Brown, 1987) and α-d-galacturonate-rich polysaccharides (Cherno et al., 1976; Proseus and Boyer, 2006; Domozych et al., 2014), but few charophytic polysaccharides have been described in detail. Recent studies of charophyte cell walls have relied heavily on immunochemistry (Domozych et al., 2009, 2014; Ikegaya et al., 2008). The immunochemical approach depends on assumptions about the specificity of the antibodies used; however, it is difficult to predict the ability of an antibody to recognize a postulated carbohydrate epitope that is not available in pure form for testing. Thus, as an alternative, we have adopted the chemical strategy of comparing the monosaccharide residues that comprise the wall polysaccharides of charophytes and embryophytes, aiming to discover primordial features of land-plant cell walls by characterizing the walls of their closest living algal relatives. New data on primordial wall polysaccharides may also offer new insights into how angiosperm cell walls function, e.g. in their growth-controlling roles. Thus, evolutionary knowledge of charophyte polysaccharides could inform and re-focus agriculturally relevant research on the mechanisms and control of crop-plant growth.
Land-plant primary walls consist mainly of polysaccharides – categorized into cellulose, pectins and hemicelluloses – whose chemistry has been studied in some detail (Albersheim et al., 2011; Fry, 2011). Primary cell-wall polysaccharides are often assumed to be built of a common set of major monosaccharide residues. However, distinct taxonomically defined differences in wall chemistry are emerging (Popper and Fry, 2003, 2004; Nothnagel and Nothnagel, 2007; Fry et al., 2008; Brennan and Harris, 2011; Sørensen et al., 2011). Here we have extended this knowledge, focusing on charophyte ‘pectic’ polysaccharides.
In land-plants, pectins may play structural, hydrating, lubricating and porosity-defining roles in the primary cell wall. Pectins can be chemically defined as polysaccharides rich in 4-linked α-d-galacturonic acid residues (often partially methyl- and/or acetyl-esterified); they are often also rich in α-l-rhamnose, β-d-galactose and α-l-arabinose residues (Albersheim et al., 2011; Fry, 2011; Peaucelle et al., 2012). An alternative (operational) definition would characterize pectins as the polysaccharide fraction that can be solubilized from the plant cell wall by hot solutions of chelating agents, e.g. oxalate at pH∼4 or EDTA at pH∼6·5. This operational definition will be employed here, avoiding unwarranted assumptions about the chemical nature of charophyte ‘pectins’. The present paper explores the monosaccharide composition of the operationally defined ‘pectins’ of charophytes.
MATERIALS AND METHODS
Source of plant material
Chara vulgaris L. was collected from Blackford Pond, Edinburgh, UK, and Nitella flexilis (L.) C. Agardh from an unnamed moorland pond near Heriot, Scottish Borders, UK. Both were manually freed of fragments of angiosperm pondweeds and any extraneous material such as snails’ eggs. Cultures of Coleochaete scutata Brébisson, Klebsormidium subtile (Kütz.) Tracanna ex Tell, K. fluitans (F. Gay) Lokhorst and Chlorokybus atmophyticus Geitler were purchased from the Culture Collection of Algae and Protozoa (CCAP), Dunstaffnage, UK, and maintained on 3N-BBM+V medium (http://www.ccap.ac.uk/media/documents/3N_BBM_V.pdf); these cultures were not axenic, but the named alga was the only photosynthetic organism present. Axenic cell-suspension cultures of the hornwort Anthoceros agrestis Paton (Vogelsang et al., 2006) were a generous gift of Dr Maike Petersen, University of Marburg, Germany. Lycopodium clavatum L. was from Harehope Hill, near Peebles, UK.
All plant and cell samples were was washed extensively in water, removing any free mucilage, then stirred in 70–77 % (v/v) ethanol (acidified with 1 % formic acid) at 20 °C for 16 h, and centrifuged at 5000 g for 10 min. The resulting cell-wall-rich alcohol-insoluble residue (AIR) was washed several times in 70 % ethanol, then acetone, and finally dried.
For the experiment described in Fig. 1, the AIR was freed of non-covalently bound proteins by stirring in phenol/acetic acid/water (ΦAW; 2:1:1, w/v/v) at 70 °C for 1 h, followed by rinsing in ethanol.
Fig. 1.

Fractionation of charophyte and hornwort cell walls into broad polymer classes. The bar chart shows the yield of each polymer class, after fractionation by the method shown in the scheme.
For the experiments described in Figs 2– 4 and 6, the samples were de-starched as follows. The AIR was suspended at 10 mg mL–1 in 40 mm lutidine (OAc–) buffer, pH 6·7, in 0·25 % (w/v) chlorobutanol, stirred at 100 °C for 15 min (gelatinizing any starch), and cooled to 60 °C. Next, 0·1 volumes of a solution of heat-stable α-amylase (Bacillus amyloliquifaciens; Sigma A7595 (Sigma-Aldrich, Poole, Dorset, UK); 10 mL of the commercial solution dialysed against water then diluted to 45 mL with the lutidine buffer) was added, and incubation was continued at 60 °C for 72 h. Ethanol and ammonium formate were then added to give final concentrations of 70 % (v/v) and 1 % (w/v), respectively, and the suspension was incubated at 20 °C for 16 h, precipitating any water-soluble polysaccharides among the cell walls, which were thoroughly rinsed with 70 % ethanol and dried.
Fig. 2.
Paper chromatography of monosaccharide constituents of matrix polysaccharide fractions from charophytes and a hornwort. Chromatography was on Whatman No. 20 paper in BAW (12:3:5) followed by EPW (8:2:1). Stain: AgNO3. Polysaccharide fractions are ‘pectins’ (P1, P2), hemicelluloses a and b (Ha, Hb), and the wash after alkali extraction (W). MM, monosaccharide marker mixture; GlcN, glucosamine; ‘U’, unfamiliar monosaccharide in Chara hydrolysate. Rhamnose was allowed to run off the end of the paper, thus improving the resolution of the slower-migrating monosaccharides.
Fig. 3.
Thin-layer chromatography of monosaccharide constituents of matrix polysaccharide fractions from charophytes and a hornwort. TLC was performed on silica-gel in ethyl acetate/pyridine/acetic acid/H2O (6 : 3 : 1 : 1). Stain: thymol/H2SO4. Polysaccharide fractions are ‘pectins’ (P1, P2), hemicelluloses a and b (Ha, Hb), and the wash after alkali extraction (W). MM, monosaccharide marker mixture; GlcN, glucosamine; ‘U’, unfamiliar monosaccharide in Chara pectin hydrolysates.
Fig. 4.
Semi-quantification of monosaccharide residues of matrix polysaccharide fractions from charophytes and a hornwort, as revealed by acid hydrolysis. Polysaccharide fractions are ‘pectins’ (P1, P2) and hemicelluloses a and b (Ha, Hb). The coloured horizontal bars indicate staining intensity of each monosaccharide on an arbitrary scale. The +, ± and – symbols to the right of each bar indicate release, partial release or no release, respectively, of the corresponding monosaccharide upon Driselase digestion (cf. Fig. 6). In some cases (especially mannose), the Driselase yield was difficult to assess owing to the presence of a trace of the monosaccharide (or a co-eluting peak) in the Driselase-only blanks, so no symbol is shown. In other cases, acid hydrolysis results were not available, but Driselase digestions were performed and a high (+) or low (±) yield of the monosaccharide was recorded.
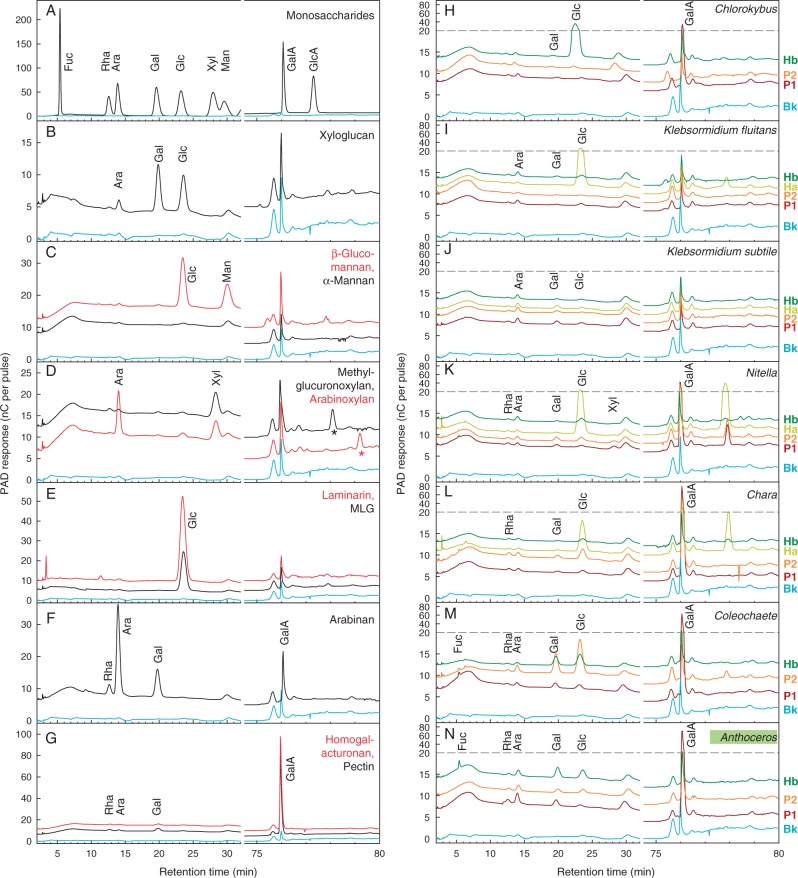
Fig. 6.
HPLC of Driselase digests of charophyte polysaccharide fractions and standard polysaccharides. Authentic polysaccharides (A–G) and various charophyte and hornwort fractions (H–N) were digested with Driselase and the products analysed by HPLC on a Dionex PA1 column; cyan curve (Bk) = blank showing Driselase autolysis products. In D, the acidic oligosaccharides indicated by asterisks are probably MeGlcA-Xyl2 (black) and GlcA-Xyl2 (red); Driselase does not release free GlcA or MeGlcA from heteroxylans. The ∼77·9-min peak in several charophyte samples represents an unidentified acidic disaccharide. Polysaccharide fractions analysed are ‘pectins’ (P1, P2) and hemicelluloses a and b (Ha, Hb), obtained as in Fig. 1. Curves have been arbitrarily slid up or down the y-axis so that samples can be distinguished easily. The horizontal dashed line indicates a change in the y-axis scale.
Source of enzymes and authentic carbohydrates
α-Amylase from Bacillus licheniformis (specific activity >500 U (mg protein)–1; Sigma-Aldrich) was used at a final concentration of 4 U mL–1, where one unit liberates 1 mg of maltose from starch in 3 min at pH 6·9 and 20 °C. d-Galactose oxidase from Dactylium dendroides (specific activity ∼500 U mg–1; Sigma-Aldrich) was used at a final concentration of 4 U mL–1, where 1 U produces a ΔA425 of 1 min–1 (in an o-tolidine–peroxidase system) at pH 6·0 and 25 °C. Driselase, a mixture of hydrolytic enzymes from the basidiomycete Irpex lacteus, was freed of carbohydrates as described (Fry, 2000).
Tamarind xyloglucan was a generous gift from Dainippon Pharmaceutical Co., Osaka, Japan; konjac glucomannan, beet arabinan, wheat arabinoxylan and barley mixed-linkage glucan were from Megazyme, Bray, Ireland. Yeast α-mannan was isolated in our laboratory. Methylglucuronoxylan, homogalacturonan (‘polygalacturonic acid’), citrus pectin, laminarin and monosaccharides were from Sigma-Aldrich. Authentic 3-O-methyl-d-galactose (3-MeGal) was obtained from Lycopodium clavatum AIR as described (Popper et al., 2001).
Extraction of polysaccharides
‘Pectin’ was solubilized from AIR with 0·2 m ammonium oxalate (pH 4·0–4·3), at 100 °C, for 2 h followed by a further 16 h. The 2-h and 16-h pectic extracts (P1 and P2) were dialysed and freeze-dried.
In some experiments, hemicellulose was then extracted from the oxalate-insoluble residue in 6 m NaOH, at 37 °C for 72 h. The extract was neutralized with acetic acid and dialysed against water; material that precipitated during these operations (termed hemicellulose a; Ha) was sedimented by centrifugation at 5000 g for 10 min, rinsed in water and freeze-dried. The remaining solution (hemicellulose b; Hb) was also freeze-dried. The NaOH-inextractable material was rinsed several times with pH 4 buffer, and the pooled washings (wash ‘W’) were dialysed and freeze-dried. The final residue (‘α-cellulose’) was rinsed in water and freeze-dried.
Polysaccharide hydrolysis and chromatography of sugars
AIR or polysaccharide fractions (5 mg) were hydrolysed with 1 mL of 2 M trifluoroacetic acid (TFA) at 120 °C for 1 h. The hydrolysate was dried, re-dissolved in water and chromatographed. Analytical paper chromatography was performed on Whatman No. 1 or No. 20, typically in butan-1-ol/acetic acid/water (BAW; 12:3:5, v/v/v) for 16 h and/or in ethyl acetate/pyridine/water (EPW; 8:2:1, v/v/v) for 16–24 h; sugars were stained with aniline hydrogen-phthalate or AgNO3 (Fry, 2000). Thin-layer chromatography (TLC) was on Merck silica-gel plates, usually in BAW (4:1:1) or ethyl acetate/pyridine/acetic acid/water (EPAW; 6:3:1:1); sugars were stained with thymol/H2SO4 (Jork et al., 1994).
Other samples of AIR were digested in 1 % Driselase (purified) in pyridine/acetic acid/water (PyAW; 1:1:98, pH ≈ 4·7) containing 0·5 % chlorobutanol at 37°C for 48 h. Ethanol (three volumes) was then added, and the digest was incubated at 80°C for 30 min. After centrifugation at 13000 g for 5 min, the supernatant was dried in vacuo and redissolved in water for chromatographic analysis. Driselase digests were analysed by high-pressure liquid chromatography (HPLC) on a Dionex CarboPac PA1 column in a NaOH/H2O gradient at 1 mL min–1: 0–2 min, 20 mm NaOH; 2–40 min, water; 40–75 min, water → 800 mm NaOH (linear gradient); 75–82 min, 800 mm NaOH; 82–90 min, 20 mm NaOH.
Purification and HPLC of an unfamiliar sugar (‘U’) from Chara
Hydrolysate from Chara vulgaris AIR (25 mg) was applied as a 20-cm streak on Whatman No. 3 paper, and the unknown (‘U’) was partially purified by chromatography in, sequentially, BAW (12:3:5) for 40 h, phenol/water (ΦW; 4:1, w/w) for 24 h, and EPW (8:2:1) for 40 h. After each preparative run, only the fringes were stained with aniline hydrogen-phthalate, then the unstained majority of the ‘U’ zone was eluted according to Eshdat and Mirelman (1972).
Partially purified ‘U’ (∼100 µg) from the third chromatogram was freed of traces of soluble, paper-derived polysaccharides on a 100-mL Bio-Gel P-2 column in PyAW (1:1:98 by vol., pH 4·7), and 10 % of each 2-mL fraction was analysed by TLC in BAW (4:1:1). Authentic 3-MeGal was used as a marker.
Partially purified ‘U’, with and without a spike of authentic 3-MeGal (∼50 µg), was subjected to analytical HPLC on a Dionex CarboPac PA1 column in a NaOH/H2O gradient at 1 mL min–1: 0–0·1 min, 20 mm NaOH; 0·1–50 min, 20 mm NaOH – 2 mm NaOH, linear gradient; 5–45 min, 2 mm NaOH; 45–75 min, 2 mm NaOH – 800 mm NaOH, concave gradient; 75–81 min, 800 mm NaOH; 81–82 min, 800 mm NaOH – 20 mm NaOH, linear gradient; 82–90 min, 20 mm NaOH.
Nuclear magnetic resonance (NMR) methods
The approach used was essentially that previously reported by Popper et al. (2001). A 4 -µg sample of ‘U’ in D2O was examined on a Bruker AVANCE III 800 MHz spectrometer operating at 799·72 MHz for protons and 201·10 MHz for 13C nuclei. The composition of the ‘U’ preparation was determined by a series of one-dimensional (1D) and 2D NMR spectroscopy experiments as described in the Results section.
Enzymic determination of enantiomerism of 3-MeGal
Samples of authentic d-galactose, l-galactose, methyl β-d-galactopyranoside and ‘U’ (each at ∼0·25 mg mL–1) were incubated with d-galactose oxidase (4 U mL–1) in 0·3 % collidine (OAc–) buffer, pH 6·0, for up to 96 h. At intervals, 16 µL of the reaction mixture (≡ 4 µg substrate) was added to 10 µL of 50 % formic acid, dried and analysed by TLC in BAW (4:1:1).
RESULTS
Yield of operationally defined polysaccharide fractions from diverse charophytes and Anthoceros
AIR samples from several charophytes and a cell-culture of the hornwort Anthoceros were fractionated into polysaccharide classes (Fig. 1). In each case, a substantial proportion (35–63 %) of the AIR was extractable in hot oxalate, and thus operationally defined as ‘pectic’. Of the total ‘pectin’, a high proportion was solubilized quickly (as fraction P1) in Anthoceros and the ‘higher’ charophytes (Chara, Nitella and Coleochaete). In the ‘lower’ charophytes (Klebsormidium and Chlorokybus), most of the pectin was extracted only by prolonged heating.
All organisms tested yielded hemicelluloses (extractable in 6 m NaOH at 37 °C), the great majority of which was Hb (i.e. remained soluble when the NaOH was removed). An appreciable amount of material (up to 15 % of the AIR) was subsequently solubilized from the residual (NaOH-inextractable) wall by a mildly acidic buffer. A final residue (‘α-cellulose’) was found in all cases, although this was a highly variable proportion of the AIR, ranging from <4 % in Chlorokybus to 20–30 % in Klebsormidium, Chara and Nitella.
Monosaccharide residue composition of ‘pectins’
The polysaccharide fractions reported in Fig. 1 were acid-hydrolysed under conditions which hydrolyse matrix polysaccharides but not cellulose, and the monosaccharide products were resolved by paper chromatography (Fig. 2) and TLC (Fig. 3). The spots were semi-quantified by staining intensity (Fig. 4). The following description collates information from both paper chromatography and TLC.
Classically, the pectic polysaccharides of land-plants are rich in GalA, Rha, Gal and Ara, with a trace of 2-O-methylxylose (2-MeXyl) and several other minor sugars – a pattern confirmed in our representative land-plant, Anthoceros. The pectins of the ‘higher’ charophytes generally also followed this trend, except for a notably low yield of Ara and 2-MeXyl residues. The co-occurrence of GalA, Rha and Gal supports the possible presence in higher charophytes of rhamnogalacturonans in addition to homogalacturonan. The pectins of higher charophytes contained little or no Ara and 2-MeXyl, although Coleochaete did produce a spot identified as 3-O-methylrhamnose (3-MeRha; acofriose), which has been observed before in charophytes and lower land-plants (Popper and Fry, 2004).
Among the lower charophytes, Klebsormidium ‘pectin’ was rich in Xyl and Gal, and also contained Man, Ara and Rha; it thus resembled land-plant pectin in certain respects (Gal, Ara, Rha), but the presence of Man and the absence of GalA singled out Klebsormidium ‘pectin’ as differing fundamentally from that of land-plants. Klebsormidium fluitans and K. subtile resembled each other closely in ‘pectin’ composition. The ‘pectin’ of the other lower charophyte tested, Chlorokybus, resembled land-plant pectin surprisingly closely in some respects, containing GalA, Rha, Gal, Ara and 2-MeXyl, but differed in also possessing GlcA, Glc and a trace of Man.
Unexpectedly, Xyl was also a major component of the ‘pectins’ of Anthoceros, Chara, Nitella and Klebsormidium. A difference in the colour of staining distinguishes Fuc (reddish) from Xyl (purple/blue) on TLC (Fig. 3). Very little Xyl was present in Coleochaete (as noted before; Popper and Fry, 2003) and in Chlorokybus.
Chara pectin consistently yielded an unfamiliar monosaccharide, indicated on Figs 2 and 3 as ‘U’, which was not present in the other plants tested. ‘U’ was obtained from the AIR of Chara but not from that of the majority of land-plants, including Equisetum (a eusporangiate fern-ally), Zea (a poalean monocot) and Vinca (a dicot); in a hydrolysate of total Chara AIR, ‘U’ exceeded Ara (Fig. 5). Its colour of staining suggested that ‘U’ was a neutral hexose.
Fig. 5.
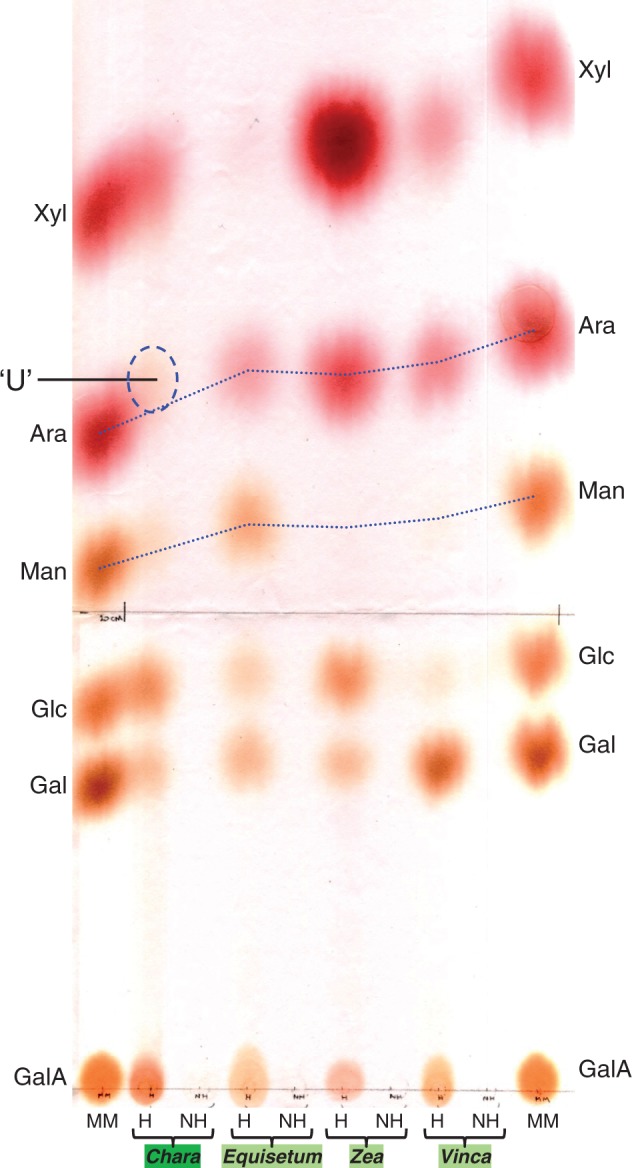
Paper chromatography of monosaccharide constituents of matrix polysaccharides from a charophyte, a eusporangiate fern, a poalean monocot and a eudicot. AIR from the young (growing) shoot tissue of each plant was hydrolysed (H) in trifluoroacetic acid (TFA) at 120 °C and the products were resolved by paper chromatography in EPW (8 : 2 : 1) and stained with aniline hydrogen-phthalate. Staining colours are: uronic acids, orange; neutral pentoses, red; neutral hexoses (including O-methylhexoses), brown. NH, non-hydrolysed control treated with TFA at 20 °C instead of 120 °C; MM, monosaccharide marker mixture; ‘U’, unfamiliar brown-staining monosaccharide in Chara hydrolysate.
Monosaccharide residue composition of ‘hemicelluloses’
The majority of the alkali-extractable hemicellulose remained soluble after neutralization (and was thus Hb). As expected, the Hb of the land-plant (Anthoceros) was rich in Glc, Xyl, Gal and Ara, compatible with the presence of xyloglucan and arabinoxylan. None of these four characteristic residues was consistently present in all charophyte Hb samples. Glc was abundant in them all except Klebsormidium; Xyl was abundant in all except Coleochaete and Chlorokybus; Gal was abundant only in Coleochaete and Klebsormidium; and Ara was abundant only in Coleochaete. The Hb of Chlorokybus was almost pure glucan, whose linkage remains to be elucidated: it is clearly not a (1→4)-β-glucan since it was water-soluble, and it was not a mixed-linkage (1→3), (1→4)-β-glucan since it was not digestible by lichenase. Very little Man was present in Anthoceros Hb, indicating negligible mannan; Chara and Nitella were the only charophytes with Man-rich Hb.
In the case of Klebsormidium, the Ha fraction was also analysed. The two Klebsormidium spp. differed greatly, their Ha compositions being K. fluitans, Glc >> Rha ≈ Xyl; and K. subtile, Gal ≈ Man > Ara ≈ Xyl >> Rha. Thus, surprisingly, the major component of K. fluitans Ha (Glc) was almost undetectable in the Ha of K. subtile.
Many charophyte polysaccharides are resistant to Driselase digestion
The polysaccharide fractions investigated by acid hydrolysis were also treated with ‘Driselase’, a mixture of hydrolytic enzymes from the basidiomycete Irpex lacteus. Driselase typically gives almost complete digestion of angiosperm primary cell walls to yield mono- and disaccharides (Fry, 2000), and the effectiveness of the present batch was verified on several authentic polysaccharides from land-plants (Fig. 6B–G). [Note that incubation of Driselase alone yields traces of autolysis products (cyan curves in Fig. 6), including Man, which must be taken into account in any evaluation of the polysaccharide products.] However, HPLC showed that, unlike land-plant polysaccharides, many of the charophyte samples gave only low yields of monosaccharides (Fig. 6H–N).
As a generalization, the ‘higher’ charophyte pectins were well digested to monosaccharides by Driselase, suggesting that they resembled land-plant pectins. In contrast, the pectins of the ‘lower’ charophytes were poorly digested to monosaccharides by Driselase, indicating that they differed fundamentally from land-plant pectins (e.g. in linkage, enantiomerism or anomerism), despite having some sugar residues in common. Exceptions to this generalization were the GalA of Chlorokybus, suggesting that this structurally simple and early-diverging charophyte possesses homogalacturonan; and the Ara of Klebsormidium ‘pectin’, suggesting that it may be present as non-reducing terminal α-l-Ara residues, which are targeted by Driselase in many land-plant polysaccharides (arabinoxylans and pectic arabinogalactans).
The major sugars of Hb in Coleochaete (Gal, Glc, Ara) were well digested to monosaccharides by Driselase, suggesting linkages reminiscent of those found in land-plant hemicelluloses. In contrast, the major Hb residues in all other charophytes, including Chara and Nitella, were not well released as monosaccharides by Driselase – Ara again being an exception. Curiously, the abundant Glc found in the Ha of K. fluitans, Nitella and Chara and in Hb of Chlorokybus (and Coleochaete) was very well released as the monosaccharide by Driselase. However, the abundant Glc in the Hb of Chara and Nitella was surprisingly resistant to Driselase. We conclude that the hemicelluloses of most charophytes differ starkly from those of land-plants, Coleochaete being closest to the land-plants in this respect.
Purification of ‘U’ from Chara AIR
The unfamiliar monosaccharide ‘U’, found in acid hydrolysates of Chara and Coleochaete AIR, migrated slightly faster than Ara on paper chromatography in EPW (8:2:1; Fig. 5). The yield of ‘U’ in Chara (∼10 µg (mg AIR)–1, estimated by paper chromatography) approximately equalled that of fucose and exceeded that of arabinose. It stained brown with aniline hydrogen-phthalate, and, when viewed under 366-nm UV light, the stained spot gave a bluish-white fluorescence – properties suggesting a neutral, reducing hexose, deoxyhexose or O-methylhexose.
‘U’ was partially purified by preparative paper chromatography in three successive solvents (Supplementary Data Fig. S1) followed by gel-permeation chromatography (GPC) on Bio-Gel P-2 (Fig. S2). TLC confirmed an acceptable purity (Fig. S2).
On TLC, ‘U’ co-migrated with authentic 3-MeGal. In addition, purified ‘U’ was run by HPLC alone and after spiking with authentic 3-MeGal obtained from Lycopodium. Under these conditions, ‘U’ co-eluted with 3-MeGal, suggesting that this was its identity (Fig. S3).
NMR spectroscopy results
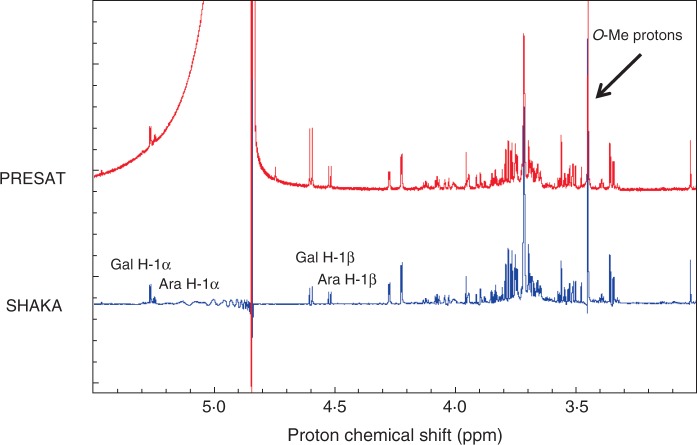
The structure of ‘U’ was investigated by NMR. Signals in the 4·4–5·4 δ region of the pre-saturation and SHAKA 1D proton NMR spectra (Fig. 7) showed the presence of α- and β-anomers of two monosaccharides. In addition, a strong singlet at 3·45 δ indicated the presence of an O-methyl group. The 2D proton–proton chemical shift spectrum (COSY) showed many signals which corresponded to those obtained (Popper et al., 2001) from a sample of 3-O-methyl-d-galactose. The presence of this compound in the ‘U’ sample was confirmed as described below (we arbitrarily assume the d-enantiomer, which is confirmed in the following section).
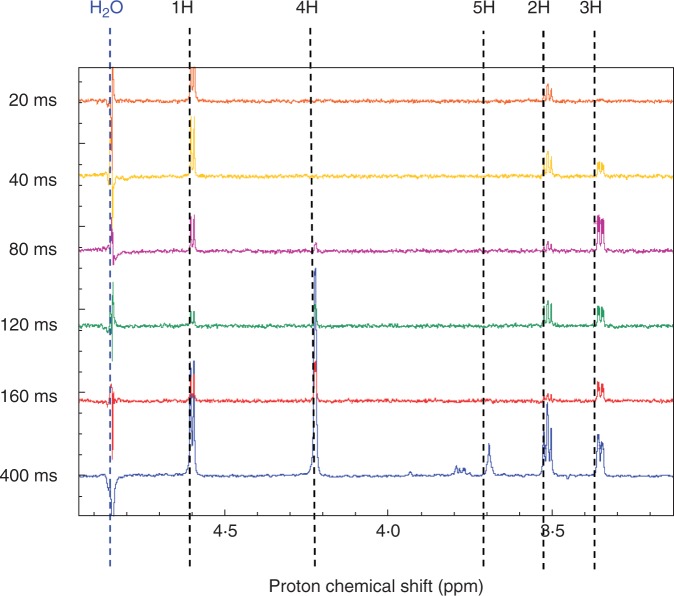
A series of selective 1D TOCSY experiments in which the signal corresponding to H-1 of the β-anomer and in which the mixing time was successively increased showed the successive appearance of the signals from H-2, H-3, H-4 and H-5 (Fig. 8). These signals occurred at the expected chemical shifts for 3-O-methyl-β-d-galactose. The coupling constants were also in agreement with those for MeGal, thus confirming the configuration of the ring protons and excluding the possibility of a glucose derivative (Supplementary Data Table S1).
A 1D-NOESY experiment in which the signal corresponding to the O-methyl protons was irradiated showed enhancement of only the H-3 and H-4 protons on the ring, demonstrating them to be spatially close to the protons of the O-methyl group (Fig. 9). If the O-methyl group were on O-4 or O-6, H-3 would not be enhanced and if the O-methyl group were on O-2, H-4 would not be enhanced.
A 2D proton–carbon (HSQC) chemical shift correlation spectrum gave signals in agreement with those obtained by Popper et al. (2001) for 3-MeGal.
Fig.7.
Pre-saturation and SHAKA proton NMR spectra of a sample containing ‘U’. These experiments were to suppress the residual water signal. Signals from anomeric protons and from an O-methyl group are clearly visible.
Fig. 8.
Selective total correlation spectrum (TOCSY) of a sample containing ‘U’, showing signals from the O-methyl β-galactose spin system only. Spectra obtained by selective excitation of the H-1 signal of the β-anomer at ≈ 4·59. The signals from the ring protons appear in sequence as the mixing time is increased from 20 to 400 ms.
Fig.9.
1D Nuclear Overhauser spectrum (NOESY) of a sample containing ‘U’, with irradiation of the signal from the O-methyl protons. Only proton signals which are enhanced appear in the spectrum. The signal from the irradiated protons appears strongly negative. The 400-ms 1D TOCSY spectrum showing the signals from protons H1–H5 in 3-O-methyl β-galactose is shown for comparison.
The identity of the other monosaccharide, present as a contaminant in ‘U’, was confirmed as arabinose from signals in the COSY spectrum by comparison with those from an authentic sample.
3-MeGal (‘U’) from Chara is the d-enantiomer
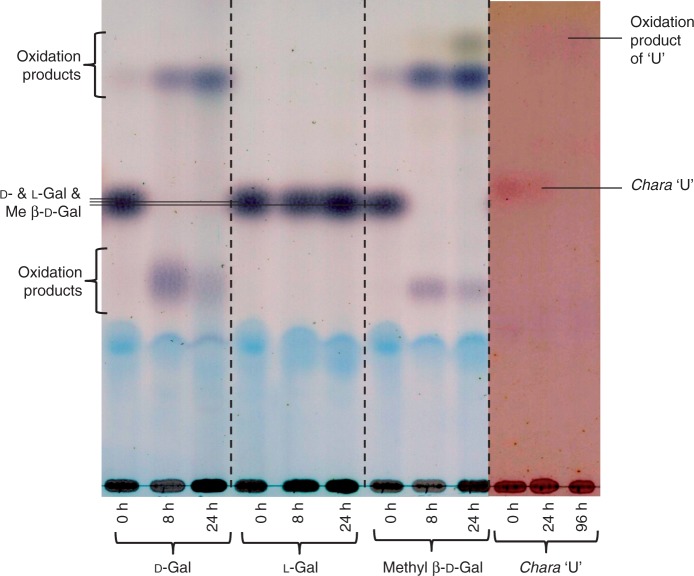
Both the d- and the l-enantiomers of 3-MeGal occur in polysaccharides of a red alga (Jania rubens; Navarro and Stortz, 2008), and we therefore investigated the optical isomerism of Chara ‘U’. d-Galactose oxidase oxidizes d- but not l-Gal derivatives, including 3-MeGal. After 8 h of incubation with d-Gal oxidase both d-Gal and methyl β-d-galactoside were oxidized to products including galactose dialdehyde or its methyl glycoside, whereas l-Gal was unchanged after 24 h (Fig. 10). Chara ‘U’ was partially oxidized within 24 h and completely within 96 h (Fig. 10). Its susceptibility to d-galactose oxidase showed that ‘U’ is a d-Gal derivative.
Fig. 10.
Enzymic evidence that Chara U is the d-enantiomer. Samples of authentic d-galactose, l-galactose, methyl β-d-galactopyranoside and the Chara unknown (‘U’) (∼4 µg of each) were incubated with d-galactose oxidase for up to 96 h. At the indicated intervals, a portion of the reaction mixture was analysed by TLC in BAW (4 : 1 : 1). [In this TLC system, galactose does not resolve from methyl β-d-galactoside; 3-MeGal runs slightly faster.]
DISCUSSION
This study shows that the three ‘higher’ charophytes examined (Coleochaete, Chara and Nitella) all possess pectic polysaccharides with sugar residue compositions broadly resembling those of land-plant pectins, except that the Ara content is distinctly lower in the charophytes. In contrast, the oxalate-extractable polymers (operationally ‘pectins’) of lower charophytes are more variable: those of the two Klebsormidium spp. studied lack detectable GalA, and thus do not include appreciable amounts of pectin in the generally accepted (chemical) sense of the term. In contrast, the oxalate-extractable ‘pectins’ of Chlorokybus, which is thought to be an extremely early-diverging charophyte, do possess GalA. Furthermore, Chlorokybus pectin releases free GalA on digestion with Driselase, suggesting the same linkage as in land-plant pectins [4-linked α-d-GalpA]. We conclude that α-d-GalpA-rich pectin is an ancient feature of the Streptophyta, albeit secondarily lost from one order: the Klebsormidiales. Although we have not tested the Zygnematales, an order of charophytes that may have a particularly close affinity to the bryophytes, it may therefore be assumed that when conquering the land 460 Mya, the first embryophytes already possessed α-d-GalpA-rich pectins – which have been retained in the primary walls of all subsequently evolving land-plants.
Pectins of the higher charophytes, like those of land-plants such as Anthoceros, also possess Driselase-releasable Rha, suggesting that rhamnogalacturonan-I (RG-I) may be present. [RG-I, but not RG-II, is digestible by Driselase.] It remains to be seen whether the charophytic RG closely resembles the well-characterized land-plant RG-I. Speaking in favour of such a resemblance, Driselase-releasable neutral Gal residues were also present in the higher-charophyte pectins. The Rha and Gal residues of both lower charophytes (Klebsormidium and Chlorokybus), in contrast, were not efficiently released by Driselase, indicating that they were not linked through land-plant RG-I-like glycosidic bonds.
We found that the pectins of the higher charophytes, especially Chara, also contained 3-MeGal residues – potentially replacing the diminished arabinose. 3-MeGal is undetectable in the cell walls of most land-plant taxa, a significant exception being that it is a major wall component of lycophytes (Popper et al., 2001), where it is thus an automorphy. 3-MeGal residues were not detected in bryophytes (Popper et al., 2001), although they are found taxonomically scattered across various kingdoms. For example, 3-MeGal has been found in a neutral polysaccharide extracted from the green alga Chlorella vulgaris (Ogawa et al., 1994; ∼40 µg (mg hydrolysate)–1), although it does not appear to have been reported from any other genera of the Chlorophyta. Among the non-lycopod land-plants, 3-MeGal has been found as a component of specific extraprotoplasmic polymers in two lamialean dicots including an arabinogalactan-protein of sage (Salvia sp.) (Capek, 2008) and a hot-water-extracted neutral (1→4)-galactan from Acanthus ebracteatus (a mangrove plant) which contained 26–33 mol % 3-MeGal (Hokputsa et al., 2004) but is otherwise absent. Among basidiomycete fungi, 3-MeGal residues occur in a soluble extracellular (1→4)-α-d-galactan from the oyster mushroom Pleurotus ostreatoroseus (Rosado et al., 2002) and in a hot-water-extractable polysaccharide from the bracket fungus Phellinus igniarius (Yang et al., 2009); both these fungal polysaccharides contained ∼33 mol % 3-MeGal. The corallinalean red seaweed Jania rubens possesses a hot-water-extractable sulphated xylogalactan, with a repeat unit […→3)-β-d-Gal-(1→4)-α-l-Gal-(1→…] in which both d-Gal and l-Gal residues are partially 3-O-methylated (Navarro and Stortz, 2008). The freshwater red alga Porphyridium also possesses small amounts of 3-MeGal in a soluble extracellular polysaccharide (Percival and Foyle, 1979). Paulsen et al. (1992) also found an extracellular polysaccharide of a cryptophycean soil alga (Cryptomonas sp.) to possess 1·5 mol % 3-MeGal residues. Among animals, 3-MeGal occurs in the haemocyanin (an N-glycosylated glycoprotein) of certain molluscs (Staudacher, 2012); however, it does not appear to have been reported from vertebrates or from bacteria (Staudacher, 2012).
The distribution of 3-MeGal among charophyte taxa (present work) indicates that many of the higher charophytes possess this sugar residue, and that it was thus quite probably present in the pectin of the immediate charophytic ancestors of the first land-plants. However, 3-MeGal appears to have been quickly lost as a major wall component by the land-plants, specifically the bryophytes (as judged by its absence from all modern liverworts, hornworts and mosses; Popper and Fry, 2003, 2004), though retained (or reintroduced) by the lycophytes (Popper et al., 2001). It is not known whether the 3-MeGal of lycophytes is a pectic component and thus whether it may occupy the same molecular niche as in the charophytes. There is little obvious physiological or anatomical resemblance between Chara and Lycopodium, precluding any simple interpretation of the functional significance of 3-MeGal. Indeed, the roles of 3-MeGal and other O-methyl sugars in the diverse polymers of the diverse organisms in which they occur remain a mystery (Staudacher, 2012).
Hemicelluloses were extractable from all six charophytes examined, but none of these charophytes contained high concentrations of all four monosaccharide residues that typify the major land-plant hemicelluloses (xyloglucans, heteroxylans) – Glc, Xyl, Gal and Ara. Although further work is required to characterize the charophytic hemicelluloses in detail, it appears likely that abrupt changes in hemicellulose chemistry occurred during terrestrialization.
CONCLUSIONS
Our survey shows that an acid- and Driselase-digestible α-d-GalA-rich polysaccharide, presumably homogalacturonan, was an ancient feature of the Streptophyta, albeit secondarily lost from the Klebsormidiales. Only the ‘higher’ charophytes and the embryophytes also possess pectic material from which Driselase is able to release the monomers rhamnose and galactose – presumably RG-I. Thus, when conquering the land, the first embryophytes probably already possessed homogalacturonan and RG-I. In contrast, none of the modern charophytes investigated possesses hemicelluloses that contain all four major residues (Glc, Xyl, Gal, Ara) characteristic of xyloglucan and arabinoxylan, indicating that major changes in the principal hemicelluloses occurred during terrestrialization. The discovery of 3-MeGal in higher charophyte pectins confirms that during their long evolutionary history the Streptophyta have experimented with numerous diverse sugar residues for the construction of their cell-wall polysaccharides. The occurrence of 3-MeGal in charophytes and lycophytes but not in a large ‘intervening’ grade of plants – the bryophytes – supports the hypothesis that major steps in plant evolution were accompanied by notable changes in cell-wall chemistry.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: 1H and 13C chemical shifts and coupling constants for 3-O-methyl-α-d-galactose. Figure S1: preparative paper chromatography of sugar ‘U’ from Chara. Figure S2: removal of polysaccharide contaminants from ‘U’ as isolated by preparative paper chromatography. Figure S3: Chara ‘U’ co-elutes with authentic 3-MeGal on HPLC.
ACKNOWLEDGEMENTS
We thank Dr Lenka Franková for valuable discussions and Mrs Janice Miller for technical assistance. We also thank the Leverhulme Foundation (sponsor reference F00158/CI) for funding this work.
LITERATURE CITED
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. 2011. Plant cell walls: from chemistry to biology. New York: Garland Science. [Google Scholar]
- Brennan M, Harris PJ. 2011. Distribution of fucosylated xyloglucans among the walls of different cell types in monocotyledons determined by immunofluorescence microscopy. Molecular Plant 4: 144–156. [DOI] [PubMed] [Google Scholar]
- Capek P. 2008. An arabinogalactan containing 3-O-methyl-d-galactose residues isolated from the aerial parts of Salvia officinalis L. Carbohydrate Research 343: 1390–1393. [DOI] [PubMed] [Google Scholar]
- Cherno NK, Dudkin MS, Areshidze IV. 1976. Pectin substances of Chara aculeolata. Khim Prir Soedin 6: 702–705. [Google Scholar]
- Domozych DS, Sørensen I, Willats WG. 2009. The distribution of cell wall polymers during antheridium development and spermatogenesis in the Charophycean green alga, Chara corallina. Annals of botany 104: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Popper ZA, et al. 2014. Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum . Plant Physiology 165: 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y, Mirelman D. 1972. An improved method for the recovery of compounds from paper chromatograms. Journal of Chromatography 65: 458–459. [Google Scholar]
- Fry SC. 2000. The growing plant cell wall: chemical and metabolic analysis, reprint edition Caldwell, NJ: Blackburn Press. [Google Scholar]
- Fry SC. 2011. Cell wall polysaccharide composition and covalent crosslinking. In Ulvskov P, ed. Annual Plant Review: plant polysaccharides, biosynthesis and bioengineering, vol. 41 Oxford: Wiley-Blackwell, pp. 1–42. [Google Scholar]
- Fry SC, Nesselrode BHWA, Miller JG, Mewburn BR. 2008. Mixed-linkage (1→3,1→4)-β-d-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytologist 179: 104–115. [DOI] [PubMed] [Google Scholar]
- Hokputsa S, Harding SE, Inngjerdingen K, Jumel K, Michaelsen TE, Heinze T, Koschella A, Paulsen BS. 2004. Bioactive polysaccharides from the stems of the Thai medicinal plant Acanthus ebracteatus: their chemical and physical features. Carbohydrate Research 339: 753–762. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AT, Brown RM. 1987. The association of rosette and globule terminal complexes with cellulose microfibril assembly in Nitella translucens var. axillaris (Charophyceae). Journal of Phycology 23: 229–237. [Google Scholar]
- Ikegaya H, Hayashi T, Kaku T, Iwata K, Sonobe S, Shimmen T. 2008. Presence of xyloglucan‐like polysaccharide in Spirogyra and possible involvement in cell–cell attachment. Phycological Research 56: 216–222. [Google Scholar]
- Jork H, Funk W, Fischer W, Wimmer H. 1994. Thin-layer chromatography: reagents and detection methods, Vol. 1b Weinheim, Germany: VCH Verlagsgesellschaft mbH. [Google Scholar]
- Karol KG, McCourt RM, Cimino MT, Delwiche CF. 2001. The closest living relatives of land plants. Science 294: 2351–2353. [DOI] [PubMed] [Google Scholar]
- Leliaert F, Smith DR, Moreau H, et al. 2012. Phylogeny and molecular evolution of the green algae. Critical Reviews in Plant Sciences 31: 1–46. [Google Scholar]
- Navarro DA, Stortz CA. 2008. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohydrate Research 343: 2613–2622. [DOI] [PubMed] [Google Scholar]
- Nothnagel AL, Nothnagel EA. 2007. Primary cell wall structure in the evolution of land plants. Journal of Integrative Plant Biology 49: 1271–1278. [Google Scholar]
- Ogawa K, Yamaura M, Maruyama I. 1994. Isolation and identification of 3-O-methyl-d-galactose as a constituent of neutral polysaccharide of Chlorella vulgaris. Bioscience, Biotechnology, Biochemistry 58: 942–944. [Google Scholar]
- Paulsen BS, Vieira AAH, Klaveness D. 1992. Structure of extracellular polysaccharides produced by a soil Cryptomonas sp. (Cryptophyceae). Journal of Phycology 28: 61–63. [Google Scholar]
- Peaucelle A, Braybrook S, Höfte H. 2012. Cell wall mechanics and growth control in plants: the role of pectins revisited. Frontiers in Plant Science 3: Article Number 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival E, Foyle RAJ. 1979. The extracellular polysaccharides of Porphyridium cruentum and Porphyridium aerugineum. Carbohydrate Research 72: 165–176. [Google Scholar]
- Popper ZA, Fry SC. 2003. Primary cell wall composition of bryophytes and charophytes. Annals of Botany 91: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. 2004. Primary cell wall composition of pteridophytes and spermatophytes. New Phytologist 164: 165–174. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Sadler I, Fry SC. 2001. 3-O-Methyl-d-galactose residues in lycophyte primary cell walls. Phytochemistry 57: 711–719. [DOI] [PubMed] [Google Scholar]
- Proseus TE, Boyer JS. 2006. Periplasm turgor pressure controls wall deposition and assembly in growing Chara corallina cells. Annals of Botany 98: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado FR, Carbonero ER, Kemmelmeier C, Tischer CA, Gorin PAJ, Iacomini M. 2002. A partially 3-O-methylated (1→4)-linked α-d-galactan and α-d-mannan from Pleurotus ostreatoroseus Sing. FEMS Microbiology Letters 212: 261–265. [DOI] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Bacic A, et al. 2011. The charophycean green algae provide insights into the early origins of plant cell walls. The Plant Journal 68: 201–211. [DOI] [PubMed] [Google Scholar]
- Staudacher E. 2012. Methylation – an uncommon modification of glycans. Biological Chemistry 393: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsang K, Schneider B, Petersen M. 2006. Production of rosmarinic acid and a new rosmarinic acid 3′-O-β-d-glucoside in suspension cultures of the hornwort Anthoceros agrestis Paton. Planta 223: 369–373. [DOI] [PubMed] [Google Scholar]
- Wodniok S, Brinkmann H, Glöckner G, et al. 2011. Origin of land plants: do conjugating green algae hold the key? BMC Evolutionary Biology 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ye LB, Zhang JS, Liu YF, Tang QJ. 2009. Structural analysis of a bioactive polysaccharide, PISP1, from the medicinal mushroom Phellinus igniarius. Bioscience Biotechnology and Biochemistry 73: 134–139. [DOI] [PubMed] [Google Scholar]
- Zhong B, Liu L, Yan Z, Penny D. 2013. Origin of land plants using the multispecies coalescent model. Trends in Plant Science 18: 492–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.