Abstract
The electroencephalography (EEG) is a widely used diagnostic tool for a number of clinical applications, such as diagnosis of epilepsy and study of sleep. Traditionally, to acquire a single channel of EEG signal, at least three electrodes must be installed on the skin separated at certain distances. They must also be connected to an amplifier by electrode leads. These basic requirements are acceptable in most clinical laboratories, but are unacceptable in certain point-of-care applications, such as during patient transportation. In order to remove these requirements, we are designing a single-unit EEG sensor in the size of a U.S. penny. It contains multiple closely spaced dry electrodes that can hook onto the skin, an electronic circuitry for signal amplification, digitization and wireless transmission, and a battery providing power. In this paper, we answer two key questions regarding the feasibility of the single-unit design: 1) can the closely-spaced electrodes obtain EEG signal reliably? and 2) will the electrodes orientated in certain ways improve signal quality? We conducted experiments utilizing closely spaced electrodes to record the alpha wave in the EEG. Our results have shown positive answers to the two feasibility questions.
Keywords: Electroencephalography (EEG), Single-unit EEG sensor, Alpha rhythm, Analysis of variance (ANOVA)
I. Introduction
The electroencephalography (EEG), first studied specifically by a German psychiatrist Hans Berger in 1929 [1], provides a unique window to observe the functional activity within the brain. In recent years, the EEG becomes increasingly popular because of the development of modern technologies to acquire EEG signal. In clinical practice, EEG is a common diagnostic tool for certain neurological diseases, such as epilepsy [2] and acute stroke [3]. EEG is also an important tool in brain-computer interface (BCI) for patients who are paralyzed or with communication disabilities [4]. In addition, EEG becomes increasingly viable for consumer applications such as game control and drowsy driving monitoring in vehicles [5].
Over the past 85 years, the basic method of acquiring EEG has never been changed. A single-channel EEG requires installations of three electrodes (more for additional channels) using a standard, but time-consuming procedure. Two of the electrodes (signal electrodes) must be installed on the scalp, and the remaining one (reference electrode) can be installed either on the scalp or at an electrically neutral location, such as the mastoid. Each electrode must be connected by a wire (called electrode lead) to an amplifier. The tedious procedure for skin preparation/electrode affixation, tangling of electrode leads, and need of a separate amplification unit cause considerable problems in many point-of-care scenarios, such as in the Intensive Care Unit (ICU) and during an emergency patient transportation.
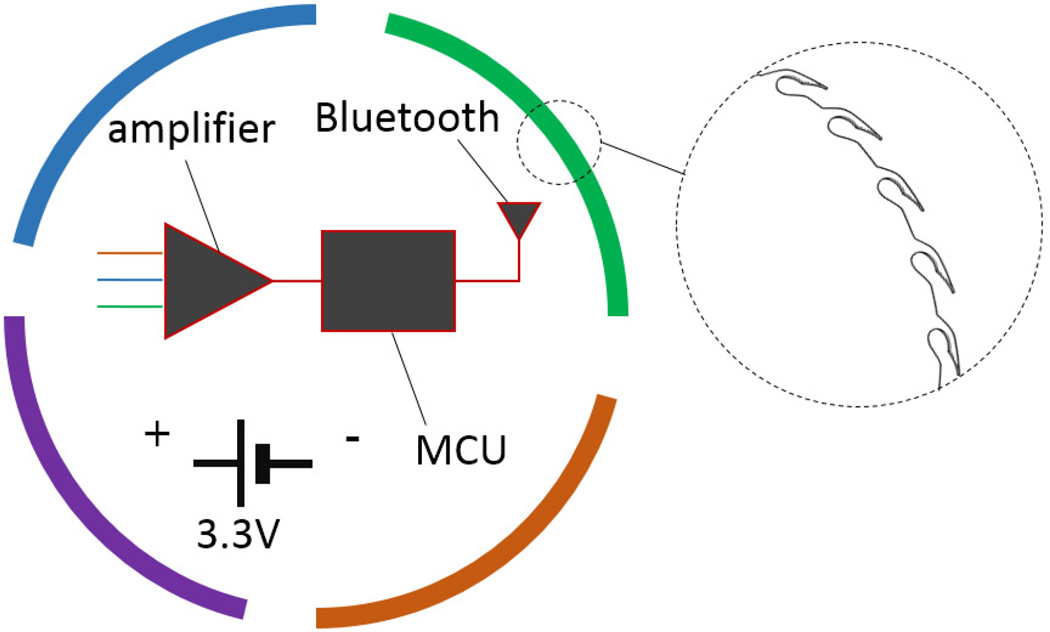
Responding to these problems, we have been investigating the feasibility of a disruptive change in the way of acquiring EEG. We have envisioned the design of a new device by combining separate electrodes, electrode leads and the amplifier into a single miniature sensor, and eliminating all leads and cords making the device completely wireless (Fig. 1). Specifically, we are designing a single cylindrically shaped sensor with a diameter close to a U.S. penny, leveraging on the skin screw electrode that we developed previously [6]. Before the implementation of this bold design, we must answer a key feasibility question of whether EEG can be recorded from closely spaced electrodes with a separation of at most 20mm. If yes, we need to answer the second question of whether it is possible to maximize signal energy by orienting the recording electrodes in a certain way. This paper addresses these two feasibility questions by installing electrodes in a close proximity. We observe the well-known alpha wave as a signature signal from the EEG. We change orientations of recording electrodes and compare the energy values of alpha wave. Our experimental results indicate that EEG with features comparable to the alpha wave can be recorded reliably within a close proximity of electrodes, and that there exists at least one favorable orientation to optimize signal quality. Our results provide strong support for the design and construction of a single-unit EEG sensor.
Fig. 1.
The single-unit sensor will acquire the EEG signal and wirelessly transmit it to a cellphone for monitoring and further processing.
II. Single-Unit EEG Sensor
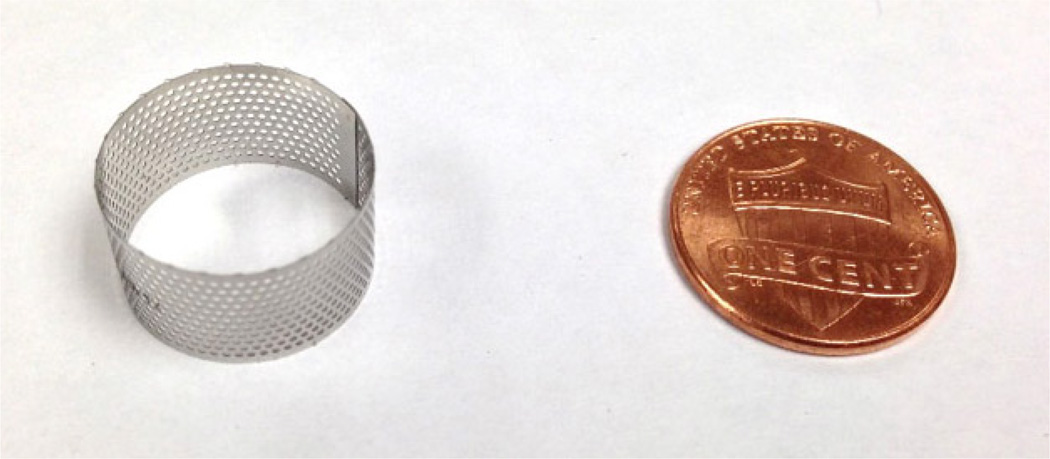
In order to clarify our study, in this section, we briefly explain the envisioned single-unit EEG sensor. It is in a cylindrically shaped sensor of approximately 20mm in diameter and 13mm in height. A partially completed sensor body is shown in Fig. 2. This part will be cut into four arc pieces in the form of Fig. 3. These quarter-circle pieces act as four separate electrodes. There are numerous microscopic teeth along the bottom edge of the sensor (see the close-up view) which hooks on the skin firmly and makes low-impedance electrical contacts by a clockwise twisting [6]. The hollow interior of the electrode body fits a coin battery and sophisticated electronic circuits for signal amplification, filtering, digitization, and wireless transmission. Previously, it was not possible to fit these components into the small space of the penny size EEG sensor. The electronic technology that enables this level of compactness became available only recently.
Fig. 2.
The body of the EEG sensor has a size of the U.S. Penny.
Fig. 3.
The skin-contact side of the cylindrically shaped EEG sensor has four electrically insulated electrodes in the form of arcs. Each arc has microscopic teeth (amplified view) that hook the skin when the EEG sensor is twisted slightly. Electronics and a battery are placed inside the hollow space within the sensor body.
Among the four electrodes in Fig. 3, two of them are connected to a differential amplifier as the signal inputs, and the remaining two electrodes are connected together and further connected to the reference input of the same amplifier. Since any two of the arc pieces can be used as signal inputs, there are thus total ways of connections corresponding to different orientations of the electrodes.
III. Methods
Before a full-scale implementation of the single-unit EEG electrode design, we must study its feasibility. The most fundamental question is whether the EEG signal can be recorded reliably when electrodes are placed so closely with a separation around 20mm. Since the EEG signal is generated by functional neural substrates within the brain, the EEG signal at the surface of the scalp can be considered as the result of volume conduction in response to the current sources at locations of activated neural substrates. It is thus possible that some of the six orientations described previously provide better signal qualities than other orientations. Addressing these issues are important because they determine the feasibility of the new single-unit EEG sensor design.
A. Study Design
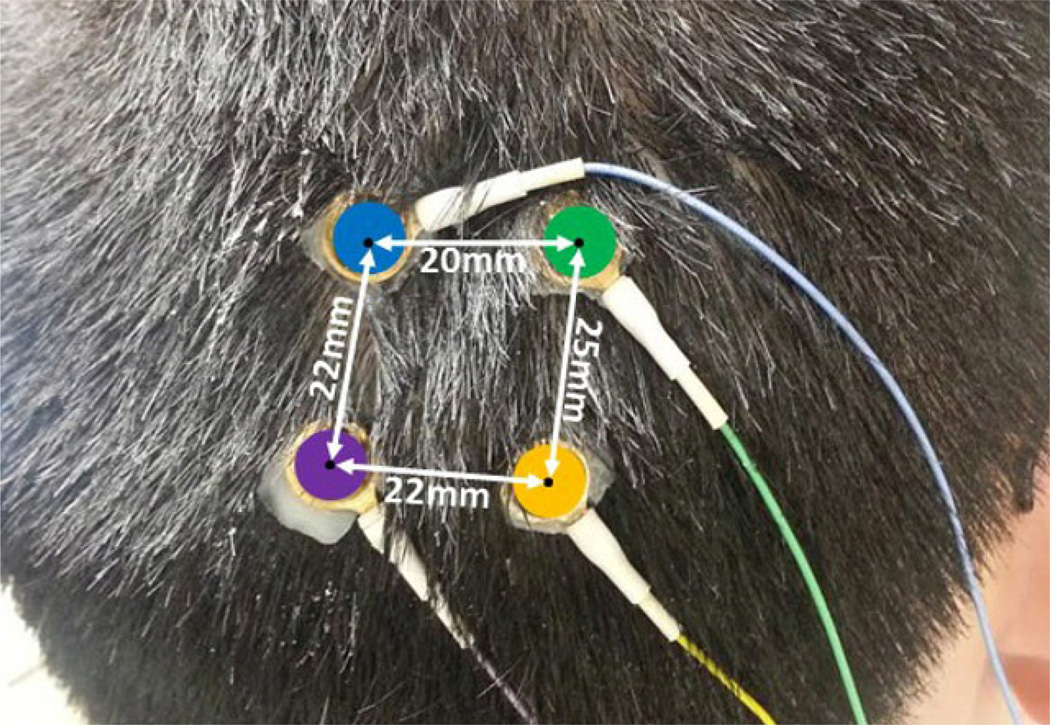
We installed four standard disc electrodes on the scalp of a male, healthy subject. The inter-distances of the electrodes were measured 20, 22, 22 and 25mm, respectively (Fig. 4). The electrodes are color-coded for easy identification. There were six ways to connect these electrodes as described previously. In any of these connections, two electrodes were connected to the pair of signal inputs of a differential amplifier, and the rest two electrodes were tied together and connected to the ground of the same amplifier. The EEG data were acquired using a self-built amplifier circuit. It contains an instrumentation amplifier, gain amplifiers and sets of high-pass and low-pass filters. Details of the amplifier design are described in [7]. The LabVIEW 2010 software (National Instruments, Inc, Austin, TX) was used to record data at a sampling rate of 500Hz with 16-bit resolution. The software is able to display the recorded EEG signal in real-time and save raw data for further processing.
Fig. 4.
Four electrodes were closely placed in the occipital region of the scalp.
B. Choice of EEG Signal
In order to verify that a true EEG signal has been recorded, we must identify the signal and noise in the acquired raw data. However, for the real-world recording, it is impossible to separate the two components exactly. We approached this problem by focusing on the alpha wave in the EEG [8]. This wave has been known to be closely associated with human alertness and wakefulness. It emerges into a strong rhythm when the subject is relaxed with eyes closed, and almost disappears when the subject becomes alert with eyes open. For most people, the frequencies of the alpha wave are in the range of 8–13 Hz [9]. These valuable properties enabled us to use the alpha wave as an indicator signal to study the presence of the EEG and compare its energy value among different electrode orientations.
C. Signal Processing
The simple but effective spectral analysis was used to process raw data. We divided each data, which was sampled at 500Hz, into 5-second segments (2,500 data points). The number of segments divided depends on the length of data. In order to suppress spectral leakage, the Hamming window with 5-second length was applied to each segment, and the discrete Fourier transform (DFT) was computed for each windowed segment padded with zeros so that the length of the DFT was 4,096. The energy value of alpha band, which was defined by the magnitude squared of DFT within 8–13Hz, was calculated for each segment to study the effect of electrode orientation.
D. Statistical Analysis
In each 5-second segment, an energy value for alpha band was computed from DFT result. Therefore, for each electrode orientation, we were able to compute a set of energy values for all segments of data. We performed such analysis for all cases of electrode orientations. In total, six sets of energy values were computed. The null hypothesis was that the energy values of six sets of data acquired using different electrode orientations were indifferent. The standard one-way analysis of variance (ANOVA) was utilized to test the null hypothesis.
IV. Experimental Results
During experiments, the subject was instructed to sit back, relax and move as little as possible. Four electrodes were placed on the occipital lobe location of the scalp, as seen in Fig. 4. The reason of selecting occipital lobe to collect data was because previous EEG studies have shown that the alpha wave can be observed clearly at this location [9]. The EEG signal were recorded for at least 120 seconds as the subject sit quietly. After this resting state, the subject was given an instruction to close eyes for at least 180 seconds. In the first session, blue and orange EEG leads were connected together to the ground of the differential amplifier. In the following five sessions, the position of reference and input electrodes altered in the ways shown in the 3rd row in Table 1 so that the effects of different electrode orientations on the acquired EEG signal energy could be investigated.
Table 1.
Schematic of electrode connections in this experiment.
| Experiment session |
1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Electrodes orientation | ||||||
| Reference connection | Blue, Orange | Green, Purple | Green, Orange | Blue, Green | Blue, Purple | Purple, Orange |
| Peak frequency (Hz)* | 11.72 | 11.84 | 11.72 | 11.23 | 11.72 | 11.72 |
| Power (dB)** | −23.35 | −22.9 | −23.76 | −29.86 | −25.47 | −29.14 |
The frequency where maximum magnitude of power is observed within 8–13Hz alpha frequency band.
Power at the peak frequency.
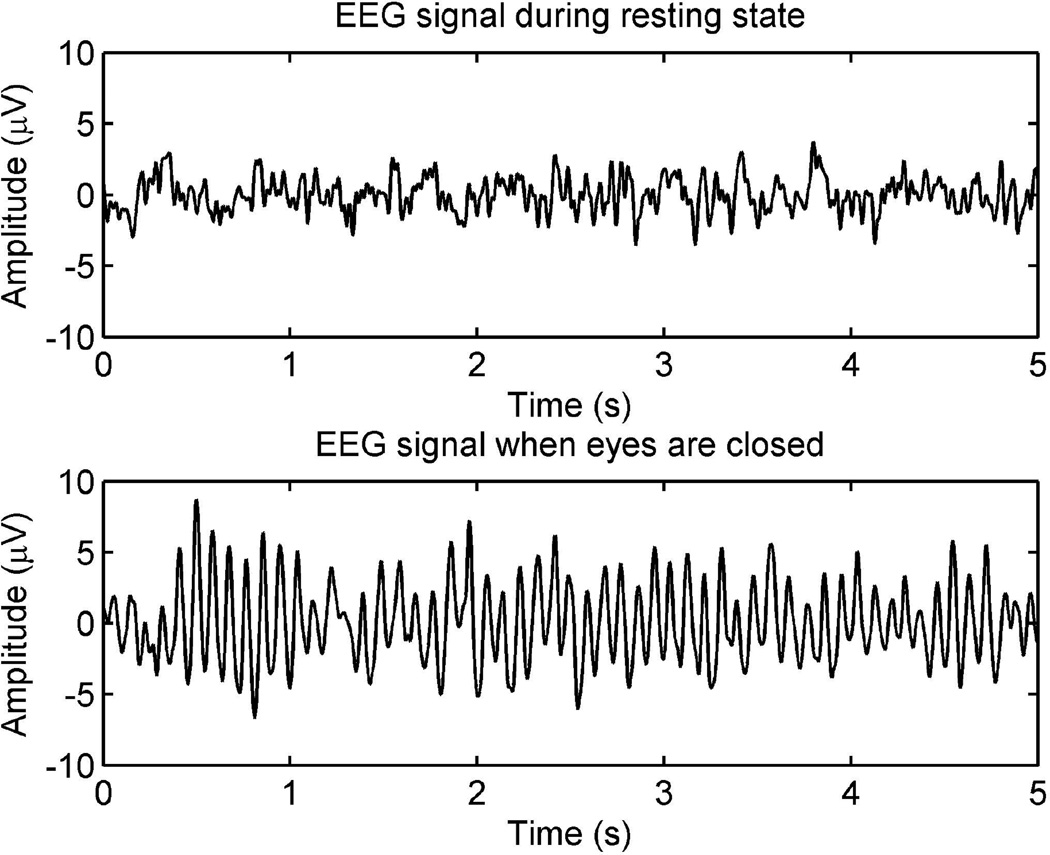
The amplifier circuit was able to acquire and amplify signal with a gain of 7,320 and a common-mode rejection ratio (CMRR) over 110dB. In each experiment, we were able to observe EEG at subject’s resting-state with eyes open. Then, the alpha wave was observed after the subject closed eyes. Fig. 5 shows two typical recorded signals when subject’s eyes open (top panel) and closed (bottom panel). Fig. 6 shows the averaged power spectra over all segments of data for six forms of electrode connections. From this figure, the alpha wave can be clearly observed.
Fig. 5.
Typical EEG waveforms when the subject’s eyes open (top panel) and closed (bottom panel).
Fig. 6.
Spectral plots of six electrode orientations. The alpha wave components with frequencies within 8–13Hz can be observed in all cases.
In order to determine whether the change of electrode orientation affects the energy of the alpha wave, a one-way ANOVA test was conducted to test the truthfulness of the null hypothesis stated previously. Our calculation revealed that not all the averaged energy of alpha wave calculated from the siz electrode orientations are equal. At least the means of alpha-wave energy from two orientations are different (p < 0:01). Therefore, the null hypothesis was rejected and we conclude that the orientation of electrodes does affect the alpha wave energy.
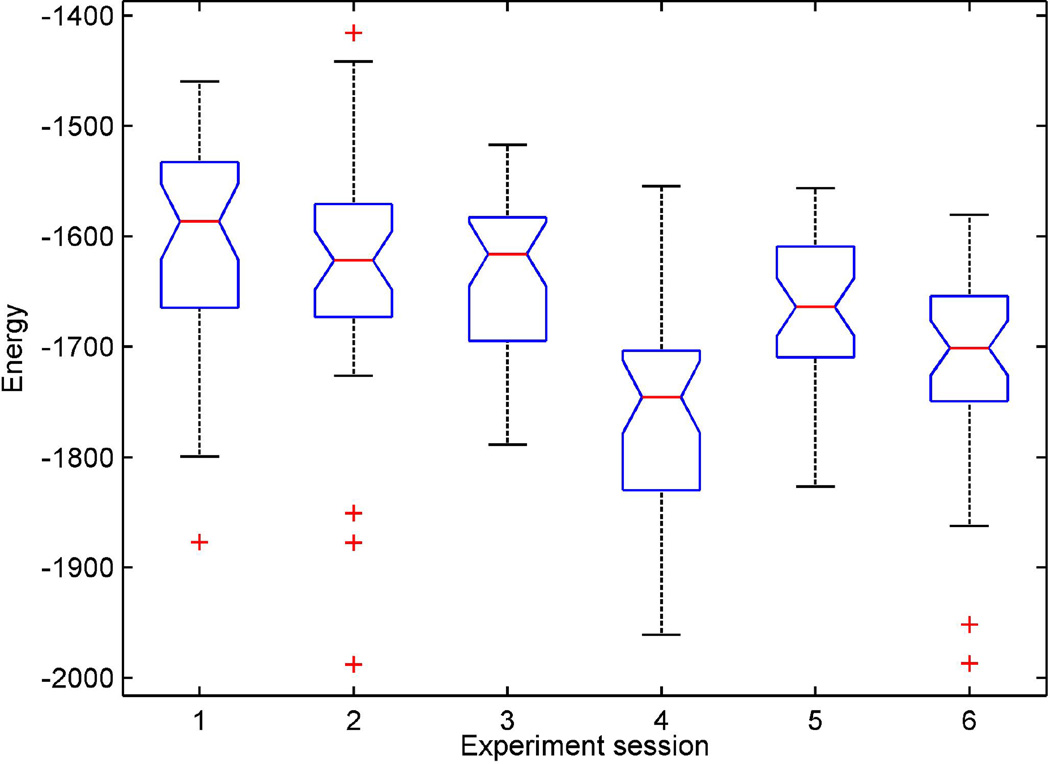
The box plot of the alpha-wave energy values are shown in Fig. 7. It can be seen that the energy values at the six orientations differ between orientation groups (1, 2, 3, 5) and (4, 6). The energy values for group 4 and group 6 were smaller than other orientations. Table 1 summarizes the six connections and the corresponding signal central frequencies and energy. Note that at these two orientations the reference channels were placed horizontally, either linked between blue and green electrodes, or purple and orange electrodes. Along with observations from Table 1, it was suggested that the direction of the maximum gradient of the alpha wave on the occipital site did not lie horizontally.
Fig. 7.
Box plot of alpha-wave energy in six electrode orientations shown in Table 1. Median values are labeled as the dash lines in the center of boxes. The margins at two sides of the box represent 25th and 75th percentiles. The criss-cross marks in the plot represent outliers.
V. Conclusion
Our study has demonstrated that EEG signal with an amplitude scale similar to the alpha wave can be recorded reliably when electrodes are placed at a separation of approximately 20mm. Our study has also found that the signal energy is significantly related to the orientation of the recording electrodes on scalp. Both findings provide valuable feasibility data for the design of a single-unit wireless EEG sensor.
Acknowledgments
This work was supported by the National Institutes of Health Grants No. R01EB013174, U54EB007954, Point-of- Care Center for Emerging Neuro-Technologies (POC-CENT), and the Center for Medical Innovation (CMI), Swanson School of Engineering, University of Pittsburgh.
Contributor Information
Bo Luan, Email: bol12@pitt.edu.
Wenyan Jia, Email: wej6@pitt.edu.
Parthasarathy D. Thirumala, Email: thirumalapd@upmc.edu.
Jeffrey Balzer, Email: balzerjr@upmc.edu.
Di Gao, Email: gaod@pitt.edu.
Mingui Sun, Email: drsun@pitt.edu.
References
- 1.Niedermeyer E, da Silva FL. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Salinsky M, Kanter R, Dasheiff RM. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: an operational curve. Epilepsia. 1987;28(4):331–334. doi: 10.1111/j.1528-1157.1987.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 3.Luu P, Tucker DM, Englander R, Lockfeld A, Lutsep H, Oken B. Localizing acute stroke-related EEG changes: Assessing the effects of spatial undersampling. Journal of Clinical Neurophysiology. 2001;18(4):302–317. doi: 10.1097/00004691-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Wolpaw JR, McFarland DJ. Multichannel EEG-based brain-computer communication. Electroencephalography and Clinical Neurophysiology. 1994;90(6):444–449. doi: 10.1016/0013-4694(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin C-T, Wu R-C, Liang S-F, Chao W-H, Chen Y-J, Jung T-P. EEG-based drowsiness estimation for safety driving using independent component analysis. IEEE Transactions on Circuits and Systems I: Regular Papers. 2005;52(12):2726–2738. [Google Scholar]
- 6.Sun M, Sclabassi RJ, Liang W, Marcanio J. Skin screw electrode. 8,112,139. US Patent. 2012
- 7.Luan B, Sun M, Jia W. Portable amplifier design for a novel EEG monitor in point-of-care applications; 38th Annual Northeast Bioengineering Conference (NEBEC); 2012. pp. 388–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan AH, McDonald PJ, Macdonald H. Differences in bilateral alpha activity as a function of experimental task, with a note on lateral eye movements and hypnotizability. Neuropsychologia. 1971;9(4):459–469. doi: 10.1016/0028-3932(71)90011-x. [DOI] [PubMed] [Google Scholar]
- 9.Klimesch W. EEG-alpha rhythms and memory processes. International Journal of Psychophysiology. 1997;26(1–3):319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]