Abstract
Insulin stimulates the translocation fatty acid transport protein 1 (FATP1) to plasma membrane, and thus greater free fatty acid (FFA) uptake, in adipocyte cell models. Whether insulin stimulates greater FFA clearance into adipose tissue in vivo is unknown. We tested this hypothesis by comparing direct FFA storage in subcutaneous adipose tissue during insulin versus niacin-medicated suppression of lipolysis. We measured direct FFA storage in abdominal and femoral subcutaneous fat in 10 and 11 adults, respectively, during euglycemic hyperinsulinemia or after oral niacin to suppress FFA compared with 11 saline control experiments. Direct palmitate storage was assessed using a [U-13C]palmitate infusion to measure palmitate kinetics and an intravenous palmitate radiotracer bolus/timed biopsy. Plasma palmitate concentrations and flux were suppressed to 23 ± 3 and 26 ± 5 µmol ⋅ L−1 (P = 0.91) and 44 ± 4 and 39 ± 5 µmol ⋅ min−1 (P = 0.41) in the insulin and niacin groups, respectively, much less (P < 0.001) than the saline control group (102 ± 8 and 104 ± 12 µmol ⋅ min−1, respectively). In the insulin, niacin, and saline groups, abdominal palmitate storage rates were 0.25 ± 0.05 vs. 0.25 ± 0.07 vs. 0.32 ± 0.05 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively (P = NS), and femoral adipose storage rates were 0.19 ± 0.06 vs. 0.20 ± 0.05 vs. 0.31 ± 0.05 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively (P = NS). In conclusion, insulin does not increase FFA storage in adipose tissue compared with niacin, which suppresses lipolysis via a different pathway.
Introduction
Body fat distribution provides important information regarding cardiovascular risk and metabolic derangements (1,2). Whereas regional lipolysis rates (3,4) and normal meal fat storage patterns (5,6) do not relate strongly to differences in fat accumulation, the redistribution of free fatty acid (FFA) between depots via the direct uptake pathway (7–9) does explain some of the interindividual differences in body fat distribution. FFAs are stored in subcutaneous adipose tissue in both the postabsorptive (7,9–12) and postprandial state (8,10,13–15), with much greater fractional FFA storage in the postprandial state (8,13). The findings that insulin stimulates the translocation of fatty acid transport protein 1 (FATP1) (16) and CD36 (17) to adipocyte plasma membranes provides a potential explanation for greater proportional direct FFA storage in the postprandial state.
Insulin also suppresses FFA concentrations by inhibiting adipose tissue lipolysis, which in turn can create a favorable concentration gradient for FFA uptake. In order to assess whether insulin specifically enhances adipocyte FFA uptake independent of its effect to reduce FFA efflux, it is necessary to compare the hyperinsulinemic condition to one with equivalent FFA concentrations without hyperinsulinemia. Niacin, acting via GPR109A, also suppresses lipolysis. We hypothesized based on in vitro (16) data and our in vivo observation of enhanced FFA storage in the postprandial state that insulin would enhance adipocyte FFA storages not only by suppressing lipolysis but also by facilitating protein-mediated transmembrane transport. To test this hypothesis, we measured FFA storage in subcutaneous adipose during a hyperinsulinemic-euglycemic clamp compared with that seen with niacin-suppressed lipolysis.
Research Design and Methods
Subjects
The study was approved by the Mayo Clinic Institutional Review Board. Participants were healthy men and women with a BMI ≤30 kg/m2, who were weight stable for ≥3 months and taking no medications that could affect lipid metabolism and, for women, were premenopausal. After signing informed consent, a history and physical examination was performed, dietary instructions were given, and a blood sample was collected for laboratory screening to exclude chronic medical conditions. Participants were randomized to an insulin clamp group or a niacin group. Contemporaneous with these experiments, we conducted saline control studies measuring direct FFA storage rates in adipose tissue using the identical experimental design. Data from these volunteers, who were of comparable age and percent body fat, are provided to offer context for the insulin and niacin data.
Body Composition
Participants underwent DXA and a computed tomography scan of L2–3 interspace to measure fat-free mass (FFM), total body fat, and regional fat masses as previously described (18).
Protocol
Volunteers received meals from the Metabolic Kitchen of the Clinical Research Unit for 5 days prior to admission to provide consistent intake and macronutrient composition (45% carbohydrate, 35% fat, and 20% protein). The meal energy content was based on estimated energy needs with a correction for physical activity; the amount of food was adjusted if necessary to maintain body weight ± 0.3 kg. The participants were admitted to the Clinical Research Unit the evening prior to the study and received their evening meal at ∼1800 h. The next morning, two intravenous catheters were placed: an antecubital catheter used for administration of study infusates and a retrograde hand vein catheter used for sampling of arterialized blood using the “hotbox” approach (19). Subjects received a single, 325-mg dose of aspirin at ∼0630 h to reduce the flushing associated with niacin administration and as a control for the insulin group; saline control volunteers did not receive aspirin. A baseline blood sample was collected at ∼0700 h and was used to measure palmitate concentration and background palmitate enrichment. If randomized to the niacin group, the participant received a 1-g dose of extended-release niacin (Niaspan) at 0700 h and another at 0800 h. Volunteers randomized to the insulin clamp group received a primed, continuous infusion (0.5 µU ⋅ kg−1 ⋅ min−1) of insulin in addition to 50% dextrose to maintain blood glucose concentrations at 100 mg/dL. The saline control volunteers received an infusion of 0.9% NaCl. At 0700 h, a continuous infusion of [U-13C]palmitate bound to human albumin was started to allow measurement of steady-state palmitate flux. The rates were the 0.6 nmol ⋅ kg FFM−1 ⋅ min−1 in the insulin and niacin groups and 2 nmol ⋅ kg FFM−1 ⋅ min−1 in the saline control group. The lesser rate in the intervention group is to maintain plasma palmitate enrichment in the optimal assay range. To measure direct palmitate storage in subcutaneous adipose tissue, an intravenous bolus of ∼60 µCi of [1-14C]palmitate or, if the participant had previously received [1-14C]palmitate, ∼200 µCi of [9,10-3H]palmitate was administered at 0800 h. Exactly 30 min later, simultaneous periumbilical and anterior thigh subcutaneous fat biopsies were performed (9). Blood samples were collected throughout the study at frequent intervals to assess palmitate concentration and enrichment, as well as plasma insulin, cortisol, growth hormone, epinephrine, and norepinephrine concentrations. Blood glucose was measured at 10-min intervals to guide the glucose infusion rate to maintain euglycemia.
Methods
Adipose tissue biopsy samples were immediately thoroughly rinsed with 0.9% NaCl. A portion of the sample was flash-frozen to be used for additional assays, and a minimum of 500 mg was placed in HEPES solution. From the latter, adipocytes were isolated by collagenase digestion, fat cell size was measured (20), and adipocyte lipid was extracted with chloroform:methanol (2:1) and counted for specific activity to <2% counting error. For protein content and enzyme assays, ∼500 mg of the flash-frozen adipose tissue was homogenized in 2 mL of standard homogenization buffer (20 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 255 mmol/L sucrose) with antiprotease tablets (Roche, Indianapolis, IN). Supernatant was collected after centrifugation at 2,100 rpm at 4°C for 10 min. The lipid was extracted (chloroform:methanol) and weighed to allow normalization of protein content and enzymatic assays per unit lipid weight.
Adipose CD36, Acyl-CoA Synthetase, and Diacylglycerol Acetyltransferase
Adipose tissue CD36 content was measured using an ELISA assay as previously described (21). Acyl-CoA synthetase (ACS) and diacylglycerol acetyltransferase (DGAT) were assayed using modifications (22) of established assays (23).
FATP1 Assay
Adipocytes were isolated using collagenase digestion and processed as per Gargiulo et al. (24). In brief, the adipocytes were homogenized and centrifuged to remove the lipid. The remaining total homogenate was ultracentrifuged, and the pellet, containing total membrane proteins, was resuspended and layered on top of a sucrose cushion and ultracentrifuged. The interface, containing plasma membrane proteins, was further ultracentrifuged and then resuspended and stored at −70°C until assayed. Plasma membrane proteins were released by adding SDS to a final concentration of 0.06%, and samples were boiled for 10 min. Total protein concentrations were calculated using Pierce BCA total protein kit per the manufacturer’s instruction. Western blotting was performed by running 25 µg of total protein on an Invitrogen NuPAGE Novex 10% Bis-Tris gel in a Midi apparatus per the manufacturer’s instructions. Proteins were transferred onto nitrocellulose using an Invitrogen semidry apparatus at 20 V for 1 h. The blot was blocked in PBS with 1% milk for 15 h at 4°C. Primary antibody from Santa Cruz Biotechnology (SC-31955) was added at 1:100 in blocking buffer and incubated for 30 h at 4°C. Three 10-min washes with PBST (PBS with 0.1% Tween-20) were performed followed by incubation with secondary antibody (LI-COR 926-68024) at 1:10,000 in PBST with 0.01% SDS and 1% milk at room temperature in the dark for 1 h. Three 10-min washes with PBST in the dark were performed followed by image scan using a LI-COR Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE). A plasma membrane sample obtained from surgical waste fat was run on each gel as a standard, and each sample band intensity was calculated relative to this standard using the LI-COR software.
ERK1/2 Phosphorylation
Stahl et al. (16) found that the MAP kinase inhibitor PD98059 markedly reduced insulin-stimulated FFA uptake, suggesting a role for this pathway in FATP1 translocation. Niacin, acting via the β-arrestin 1 pathway (25), may also activate ERK/MAPK. To understand whether insulin or niacin stimulates this pathway relative to a saline control condition in vivo, in humans, we measured the ratio of phosphorylated ERK1/2 (phospho-ERK1/2) to total ERK1/2 using capillary Western blotting approaches. In brief, adipose tissue from needle biopsies was washed with saline, frozen in liquid nitrogen, and stored at −80°C. For this assay, samples were weighed and 20–150 mg was placed in a tube containing Omni ceramic homogenization beads (Omni International, Kennesaw, GA). Two microliters of homogenization buffer (20 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 255 mmol/L sucrose, Roche mini-tabs antiprotease cocktail, and Thermo Scientific Halt Phosphatase Inhibitor Cocktail) per mg tissue was added while on ice. Tissue was homogenized using an Omni Bead Ruptor (speed = 2.10, cycles = 2, time = 15 s, and delay = 10 s). Homogenates were centrifuged at 1,000g at 4°C for 10 min. The subnatant (below the fat cake) was needle aspirated into a new tube. An additional 2 µL of homogenization buffer per mg tissue (for a total of 4 µL per mg tissue) was added to the tube, and samples were vortexed briefly and centrifuged again. The subnatant was again needle aspirated and mixed with the first homogenate and stored at −80°C. These homogenates were analyzed using the ProteinSimple Wes System (capillary Western) using Cell Signaling Technology anti-ERK1/2 antibody (no. 9102) and anti–phospho-ERK1/2 antibody (no. 9101) and anti–rabbit-HRP secondary antibody. A control sample was extracted with 2 µL per mg tissue so as to be more concentrated than the samples to be tested. A twofold dilution series from this control was created and run with each assay to ensure that values from the study adipose samples were in the linear range of the assay. To assess ERK/MAPK activation, we used the ratio of the area of the phospho-ERK1/2 peak to total ERK1/2 peak. Any samples not within the linear range were diluted appropriately and reanalyzed. Interassay variability of the ratio of phospho-ERK1/2 to ERK1/2 was 10%, and intra-assay variability was 5%.
Calculations
Steady-state palmitate flux was calculated as previously described (26). At steady state, whole-body palmitate appearance and disappearance rates are equal, allowing us to use flux and disappearance rates interchangeably. To calculate the rates of palmitate storage in adipose tissue, we used the fractional storage of radiolabeled palmitate (dpm/g of adipocyte lipid/dpm administered) times the total palmitate disappearance rate (7).
Statistics
Values are provided as means ± SEM. Based upon the result of Stahl et al. (16), we anticipated a threefold increase in the efficiency of direct FFA uptake/storage in adipose tissue in the insulin clamp group compared with the niacin group. Although we expected the variability to be greater (a pooled SD of 5% of direct FFA uptake), the effect size we predicted based upon the available literature and our previous studies was sufficiently large that with 10 subjects in each group (5 women and 5 men) we had >90% power to detect the anticipated differences. Between-group differences were analyzed using a one-way ANOVA, and if significant differences were detected, the groups were then compared using a nonpaired Student t test to identify which groups differed significantly. Paired Student t test was used for comparison of results between depots within the same individuals. When testing for between-group or between-depot differences of factors that were not considered in our primary analysis, we used a Bonferroni correction to reduce the risk of a type 1 statistical error. Univariate regression analyses were used to test for correlations between palmitate storage and lipogenic factors.
Results
Subject Characteristics
The participants in the three groups were comparable in terms of age, percent body fat, lower body subcutaneous (LBSQ) fat, visceral fat, FFM, and fat cell size (Table 1). Upper body subcutaneous (UBSQ) fat mass was greater in the insulin group than the niacin or saline control groups (P < 0.05). Thigh fat cell size was greater than abdominal fat cell size in all groups. The abdominal and femoral adipose tissue contents of CD36 and ACS were not different between the three groups; however, DGAT activity was less (P < 0.05) in the saline control group than in the insulin and the niacin groups (Table 2). For those insulin/niacin participants from whom we could collect sufficient adipocytes to measure plasma membrane FATP1, we did not detect statistically significant differences in protein content (Table 2). We did not measure adipocyte plasma membrane FATP1 in saline control volunteer samples. As intended, plasma insulin concentrations were greater in the insulin clamp group (P < 0.01 vs. other groups) than the saline control and niacin groups (Table 3).
Table 1.
Subject characteristics
| Insulin (n = 10, men = 5) | Niacin (n = 11, men = 5) | Control (n = 11, men = 5) | |
|---|---|---|---|
| Age (years) | 28 ± 2 | 33 ± 3 | 32 ± 3 |
| BMI (kg/m2) | 27.8 ± 1.0 | 26.5 ± 1.0 | 24.1 ± 0.6‡ |
| Percent body fat | 35 ± 3 | 32 ± 2 | 30 ± 2 |
| FFM (kg) | 54.6 ± 4.0 | 52.0 ± 4.9 | 49.9 ± 3.6 |
| UBSQ fat (kg) | 16.7 ± 1.7† | 12.2 ± 1.0 | 10.8 ± 0.7‡ |
| LBSQ fat (kg) | 10.0 ± 0.8 | 9.3 ± 0.7 | 8.1 ± 1.8 |
| Visceral fat (kg) | 2.0 ± 0.4 | 2.2 ± 0.5 | 2.0 ± 0.3 |
| Average abdominal fat cell size (µg lipid/cell) | 0.58 ± 0.070* | 0.59 ± 0.08* | 0.43 ± 0.05* |
| Average thigh fat cell size (µg lipid/cell) | 0.80 ± 0.08 | 0.82 ± 0.06 | 0.64 ± 0.08 |
Values are means ± SEM.
*P < 0.02 vs. thigh fat cell size.
†P < 0.05 vs. niacin group.
‡P < 0.05 vs. insulin group.
Table 2.
Adipose tissue fatty acid storage factors
| Insulin | Niacin | Control | |
|---|---|---|---|
| Abdomen CD36 (arbitrary units/mg lipid) | 18 ± 3 | 21 ± 3 | 21 ± 3 |
| Thigh CD36 (arbitrary units/mg lipid) | 19 ± 2 | 22 ± 3 | 23 ± 2 |
| Abdomen ACS (pmol/mg lipid/min) | 69 ± 10 | 64 ± 9 | 68 ± 11 |
| Thigh ACS (pmol/mg lipid/min) | 73 ± 8 | 76 ± 12 | 66 ± 9 |
| Abdomen DGAT (pmol/mg lipid/min) | 6 ± 1 | 7 ± 1 | 4 ± 1†,‡ |
| Thigh DGAT (pmol/mg lipid/min) | 5 ± 1 | 6 ± 1 | 3 ± 1†,‡ |
| Abdomen FATP1 (arbitrary units/mg lipid) | 1.1 ± 0.3 (n = 6) | 1.7 ± 0.7 (n = 6) | NA |
| Thigh FATP1 (arbitrary units/mg lipid) | 0.8 ± 0.2 (n = 5) | 0.9 ± 0.3 (n = 6) | NA |
Values are means ± SEM.
†P < 0.05 vs. niacin group.
‡P < 0.05 vs. insulin group.
Table 3.
Plasma hormone and catecholamine concentrations
| Insulin | Niacin | Control | |
|---|---|---|---|
| Insulin (µU/mL) | 18.9 ± 3.1* | 6.1 ± 1.3 | 5.3 ± 0.8 |
| Growth hormone (ng/mL) | 2.38 ± 1.14 | 1.47 ± 0.68 | 1.27 ± 0.35 |
| Epinephrine (pg/mL) | 21 ± 3 (n = 9)† | 30 ± 3 (n = 9) | 33 ± 7 |
Values are means ± SEM.
*P = 0.002 vs. niacin group.
†P = 0.03 vs. niacin group without Bonferroni correction.
Palmitate Responses to Insulin and Niacin
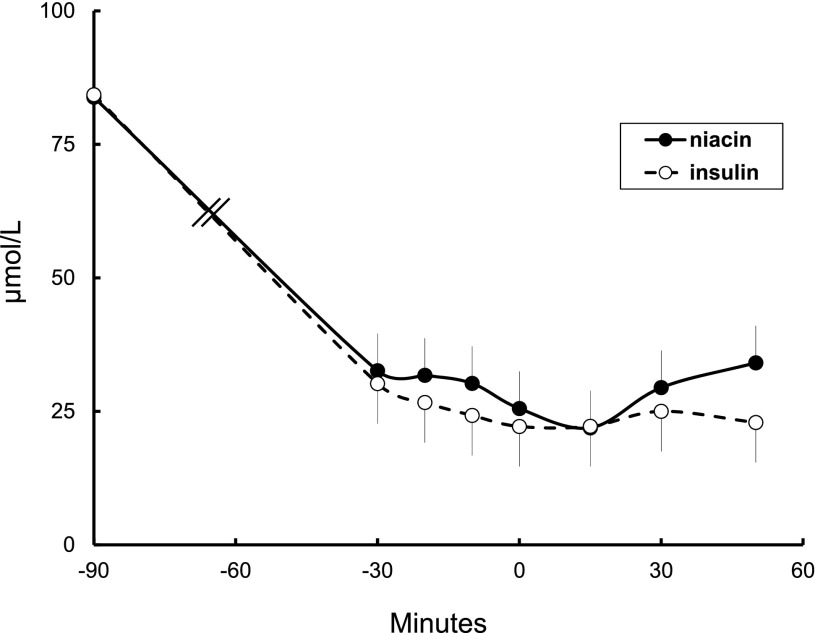
Baseline palmitate concentrations and the concentrations during the measurement of direct FFA storage rates (bolus at time 0 min and biopsy at time +30 min) were virtually identical (Fig. 1). Palmitate concentrations averaged 23 ± 3 and 26 ± 5 µmol ⋅ L−1 (P = 0.91) in the insulin and niacin groups, respectively, compared with 102 ± 8 µmol ⋅ L−1 (P < 0.001 vs. other groups) in the saline control group. Palmitate flux averaged 44 ± 4 and 39 ± 5 µmol ⋅ min−1 (P = 0.41) in the insulin and niacin groups and 104 ± 12 µmol ⋅ min−1 in the saline control group (P < 0.001 vs. other groups). Palmitate clearance rates were 1.74 ± 0.15 and 1.56 ± 0.20 L ⋅ min−1 (P = 0.47) in the insulin and niacin groups and 1.02 ± 0.08 L ⋅ min−1 in the saline control group (P < 0.05 vs. both other groups).
Figure 1.
Time course of plasma palmitate concentrations before (time −90 min) insulin or niacin administration and during the time of the radiotracer palmitate bolus (time 0 min) and adipose biopsies (time 30 min).
Direct FFA Storage Rates
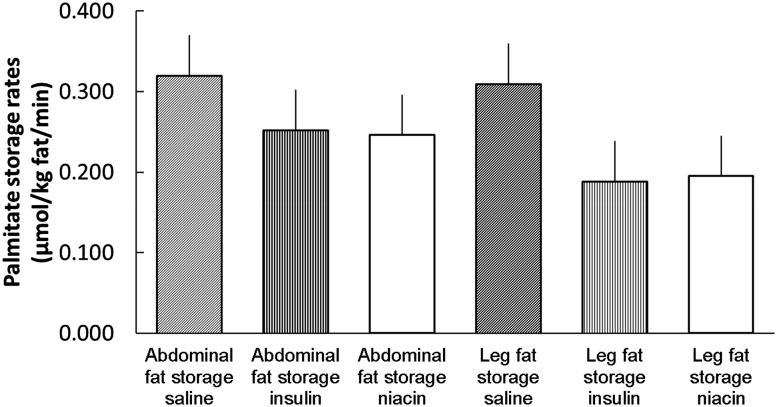
Palmitate storage rates in UBSQ fat were not different between the insulin, niacin, and saline control groups (0.25 ± 0.05 vs. 0.25 ± 0.07 vs. 0.32 ± 0.05 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = NS) (Fig. 2). Likewise, palmitate storage rates in LBSQ fat were not different between the insulin, niacin, and saline control groups (0.19 ± 0.06 vs. 0.20 ± 0.05 vs. 0.31 ± 0.05 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = NS). When examined by sex, palmitate storage rates in men were greater in UBSQ than LBSQ adipose tissue in both insulin (0.21 ± 0.05 vs. 0.07 ± 0.02 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = 0.006) and niacin (0.14 ± 0.03 vs. 0.05 ± 0.01 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = 0.006) groups. In women, palmitate storage rates per kg adipose lipid were similar in UBSQ and LBSQ adipose tissue in both insulin (0.29 ± 0.08 vs. 0.29 ± 0.08 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = 0.9) and niacin (0.34 ± 0.11 vs. 0.32 ± 0.06 µmol ⋅ kg adipose lipid−1 ⋅ min−1, respectively; P = 0.8) groups. Palmitate storage rates in LBSQ fat were greater in women than men both under the insulin and niacin condition (both P < 0.05).
Figure 2.
Direct palmitate storage rates in abdominal subcutaneous and femoral adipose tissue in male and female participants in the saline control group and in those with suppressed FFA via a hyperinsulinemic-euglycemic clamp vs. oral niacin administration. There were no significant differences between saline, insulin, and niacin or between abdomen and thigh for the combined male/female group.
Effects of Insulin and Niacin on ERK1/2 Phosphorylation
To understand whether insulin or niacin at these doses activates the MAPK pathway to levels above background, abdominal adipose tissue from all insulin and niacin participants and 10 of the 11 saline control participants was assayed for the ratio of phospho-ERK1/2 to total ERK1/2. The phospho-ERK/ERK ratio in insulin, niacin, and saline control conditions was 0.40 ± 0.07, 0.30 ± 0.08, and 0.34 ± 0.06 (P = 0.58 by ANOVA), respectively.
Predictors of Palmitate Storage Rates
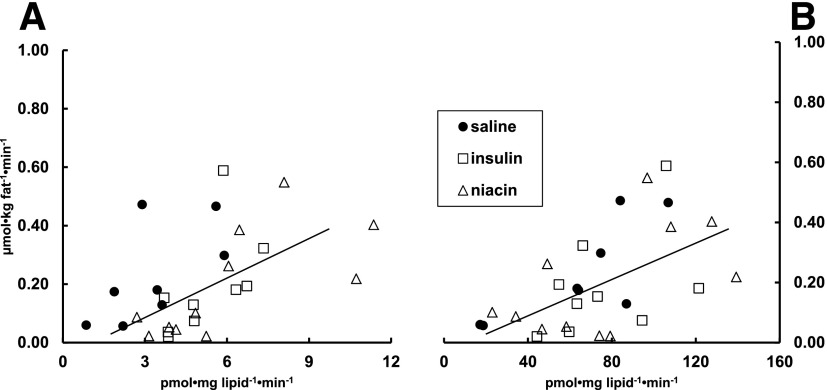
Whether examined for the two FFA-lowering interventions (insulin vs. niacin), sex, or combining all subject data, we found no relationship between UBSQ palmitate storage rates (µmol ⋅ kg adipose lipid−1 ⋅ min−1) and plasma palmitate concentrations, adipocyte plasma membrane FATP1 content, or adipose tissue CD36, ACS, or DGAT. For LBSQ fat, if all observations from men and women, insulin, and niacin were included, DGAT (P = 0.003) (Fig. 3A), ACS (P = 0.02) (Fig. 3B), and activities were correlated with palmitate storage rates, whereas plasma palmitate concentrations, adipocyte plasma membrane FATP1 content, and CD36 were not. The relationship between LBSQ palmitate storage rates and DGAT (Fig. 3A) and ACS (Fig. 3B) are also provided. Whereas the relationship between ACS and LBSQ palmitate storage rates in saline control participants essentially overlapped the insulin and niacin participants, the DGAT/palmitate storage rate relationship appeared to be shifted up and to the left for the saline control group. This suggests greater storage rates for any given DGAT activity in the saline control group.
Figure 3.
The relationship (r = 0.63, P = 0.003) between DGAT activity and direct palmitate storage rates in femoral adipose tissue for the niacin and insulin groups is shown in A. These same data from the saline control group are also provided. The regression line is for the insulin and niacin groups only. The relationship (r = 0.53, P = 0.02) between ACS activity and direct palmitate storage rates in femoral adipose tissue for the combined groups (same symbols) is shown in B; the regression line is for the insulin and niacin groups only. The data from the saline control group are also depicted.
Discussion
The fractional direct FFA storage in adipose tissue is approximately threefold greater when FFAs are suppressed during postprandial conditions (13) than in the postabsorptive state. Furthermore, the rates of direct adipose tissue FFA storage are not suppressed during meal ingestion compared with the postabsorptive state (8) as we might expect they would be given the relationship between fasting FFA concentrations and direct FFA storage rates (7). The reports that insulin promotes the localization of FATP1 (16) and CD36 (17) to adipocyte plasma membranes provided a potential mechanism for these observations. We tested the hypothesis that hyperinsulinemia is responsible for the greater fractional FFA storage in subcutaneous fat by comparing direct FFA storage rates in humans whose FFA had been suppressed to the same levels with insulin versus niacin. To our surprise, storage rates of plasma palmitate in subcutaneous fat were virtually identical in both groups, implying that hyperinsulinemia does not stimulate direct FFA storage rates in adipose tissue more so than suppression of lipolysis by an alternate pathway. There were no differences in plasma palmitate clearance rates between the two conditions, which suggests that any extra-adipose tissue effects of insulin (compared with niacin) to stimulate tissue uptake under these conditions are modest.
Our measurement of adipose tissue FFA storage is specific to the re-esterification of FFAs that have entered the systemic circulation and are then taken up and stored in different cells. Our approach cannot detect re-esterification of fatty acids that are released into the intracellular environment by lipolysis but never exit the cell. However, our approach recapitulates the experimental conditions used by Stahl et al. (16) to describe the effect of insulin on an adipocyte cell model and thus to test the hypothesis that insulin stimulates adipocyte uptake of extracellular fatty acids independent of its effects on lipolysis. Under postprandial conditions, both plasma glucose and insulin increase and it is possible that this combination is needed for enhanced adipocyte FFA storage. However, our experimental design (stable glucose and high insulin) recapitulates the conditions of the experiments (16), demonstrating the dynamic properties of FATP1 in adipose tissue.
A previous study of FFA clearance during high FFA concentrations and hyperinsulinemia was interpreted as indicating that insulin increased FFA clearance independent of its ability to suppress lipolysis (27). At high FFA concentrations, FFA entry into most tissues likely occurs via the flip-flop mechanism and, because only a small fraction of FFAs are taken up by adipose tissue under these conditions (7,8), any enhanced clearance would be in lean tissue. Bickerton et al. (14) and McQuaid and colleagues (11,12) have reported on adipose tissue FFA uptake using the arterio-venous balance/FFA tracer study design. This elegant experiment approach can detect the transcapillary removal of FFA from blood as it traverses adipose tissue in the fed state (11,12,14), but not adipocyte FFA storage that occurs in the fasting state (7,9). This is most likely because the lesser fractional FFA uptake in the fasting state (9) makes adipose tissue FFA removal more difficult to detect using arterio-venous balance approaches. These investigators found that abdominal subcutaneous fat in men takes up FFA in the fed state (14), that postprandial VLDL fatty acid and FFA uptake per unit abdominal subcutaneous fat mass is less in abdominally obese than lean men, and that femoral and abdominal subcutaneous fat have similar postprandial FFA uptake (12). In contrast, we found that FFA storage rates were greater in abdominal than femoral subcutaneous fat in men but not women. To the extent that euglycemic hyperinsulinemia can be used to infer postprandial adipose metabolism behavior, it is possible that the apparent similarity between femoral and abdominal FFA uptake reported by McQuaid et al. (12) was the result of combining data from men and women.
Insulin, like niacin, suppresses lipolysis by reducing intracellular cAMP concentrations. Niacin acts via GPR109A, a Gi/Go-protein–coupled receptor that inhibits adenylyl cyclase. In contrast, insulin, acting through its signaling pathway, activates Akt, which in turn activates phosphodiesterase 3-B, thereby accelerating the degradation of cAMP. Stahl et al. (16) presented evidence that the FATP1 translocation to the plasma membrane was independent of insulin’s ability to inhibit lipolysis and may involve the MAP kinase cascade. Niacin is reported to induce an activating conformational change in β-arrestin and β-arrestin–dependent signaling to ERK1/2 MAPK (25). In theory, this could also induce FATP1 translocation and explain our findings. Our findings that the relative phosphorylation of ERK1/2 was very similar in the adipose tissue of the saline control, insulin, and niacin groups implies that, at the doses we used, this pathway is not activated by insulin or niacin.
There are several potential explanations as to why we found no difference in direct FFA storage rates between the hyperinsulinemic-FFA– and niacin-FFA–suppressed states. Previous studies of cells have compared zero insulin to high insulin conditions (16). It is possible that even the relatively low insulin concentrations seen in the niacin group were sufficient to translocate enough FATP1 to the adipocyte plasma membrane to maximize facilitated fatty acid transport. Alternatively, it may be that the intracellular, rather than plasma membrane, content of FATP1 is responsible for adipocyte fatty acid accumulation (28,29). Perhaps adipocyte FATP1 plays only a minor role in adipose fatty acid storage in humans.
Our interest in the direct FFA storage pathway is stimulated by the observation that the patterns of direct storage of FFA in adipocytes correlate with body fat distribution patterns. Consistent with our previous observations (7,8), in these studies, palmitate storage rates in LBSQ fat were greater in women than men and greater in UBSQ than LBSQ fat in men. Similar to our findings in the postprandial state (8), when palmitate concentrations were low and spanned only a narrow range, palmitate concentrations did not predict adipose palmitate storage rates. In contrast to our postprandial results, where none of the fatty acid storage factors we measured correlated with LBSQ adipose tissue, ACS and DGAT activities were positively correlated with palmitate storage rates per unit mass when both conditions and sexes were included in the analysis. Of interest, DGAT activity was greater in the insulin group than the saline group (Table 2), which was surprising given the brief exposure to insulin (2 h) compared with previous studies showing that insulin increases DGAT2 transcription after 24 h (30). The fact that DGAT activity was also increased in the niacin group may suggest an allosteric modulation of DGAT enzyme activity in response to suppressed intracellular lipolysis. Despite the lesser adipose DGAT activity in the saline control than insulin and niacin groups, adipose FFA storage rates were not less in the control group. This may be because the higher fasting plasma FFA concentrations synergize with intracellular fatty acid storage factors to drive fatty acid storage (7). One of our hypotheses was that proteins involved in facilitated fatty acid transport (CD36 and FATP1) would be stronger predictors of direct FFA storage rates at low extracellular FFA concentrations. The data from this study are not consistent with that hypothesis.
Although direct adipose tissue storage of FFA is of interest because as a fatty acid redistribution mechanism that may modulate body fat distribution, meal fat is the greatest source of fatty acids for adipose tissue. Circulating chylomicron triglycerides are hydrolyzed by lipoprotein lipase in adipose tissue, and the resultant fatty acids are readily stored in the underlying adipocytes. The same proteins and enzymes shepherd triglyceride-derived fatty acids and FFA into adipocyte triglycerides. Although the pattern of meal fatty acid storage and direct FFA storage are similar in men (greater storage in abdominal than femoral fat) (5), in women the patterns are reversed; meal fatty acid storage is greater in abdominal than femoral fat (5), and direct FFA storage is greater in femoral than abdominal fat (7).
There are some limitations to this study. For example, the BMI was different between groups (Table 1), but percent body fat was not (ANOVA, P = 0.37). The insulin group had more UBSQ (Table 1). However, neither abdominal nor thigh fat cell size were different between groups (ANOVA, P = 0.17 for both). It is possible that these between-group differences in body fat confounded our results, but we are reassured that percent body fat and fat cell size were not different between groups. Finally, based upon the literature and the mechanism of action of niacin, we predicted that adipocyte plasma membrane FATP1 and direct FFA storage rates would be greater in the insulin compared with the niacin condition. Because of this assumption, we did not isolate the adipocyte plasma membrane fraction from saline control participants and thus cannot exclude the possibility that adipocyte plasma membrane FATP1 content was greater in the insulin and niacin compared with saline control. It is possible that FATP1 translocates to human adipocyte cell membranes in response both to insulin and niacin in the absence of ERK activation. If so, this would suggest that the original studies (16) failed to identify the correct signaling pathway, perhaps because of the cell model used. However, if there are uncertainties regarding the utility of the cell model to identify the correct signaling pathway for FATP1 translocation, we might be equally skeptical regarding whether this model correctly recapitulates the pathways for human adipocyte FFA uptake.
In summary, we hypothesized that direct FFA storage rates in subcutaneous adipose tissue would be significantly greater under hyperinsulinemic than euinsulinemic (niacin) conditions in the context of similar FFA concentrations. Instead we found the storage rates to be virtually identical. Although facilitated FFA transport does seem to be important for adipose and muscle under low FFA conditions (10), the current data suggest this is not necessarily an insulin effect per se.
Article Information
Acknowledgments. The authors are indebted to the research volunteers for their participation. The authors are also grateful to Barbara Norby and Carley Vrieze for assistance with nursing care and adipose biopsies and Christy Allred, Debra Harteneck, Darlene Lucas, and Lendia Zhou (Mayo Clinic, Rochester, MN) for assistance with assay development and performance.
Funding. This work was supported by grants DK-40484, DK-45343, and DK-50456 from the U.S. Public Health Service and by the Mayo Foundation. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant 1-UL1-RR-024150-01.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.H.A., M.M., and C.K. performed experiments; analyzed data; interpreted results of experiments; prepared figures and tables; and drafted, edited, revised, and approved the final version of the manuscript. D.A.B. conceived and designed the research; provided guidance on laboratory assays; interpreted results of experiments; and edited, revised, and approved the final version of the manuscript. M.D.J. conceived and designed the research; analyzed data; interpreted results of experiments; prepared figures and tables; and drafted, edited, revised, and approved the final version of manuscript. M.D.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Björntorp P. Adipose tissue distribution and function. Int J Obes 1991;15(Suppl. 2):67–81 [PubMed] [Google Scholar]
- 2.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994;74:761–811 [DOI] [PubMed] [Google Scholar]
- 3.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 1995;96:2297–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 2000;279:E455–E462 [DOI] [PubMed] [Google Scholar]
- 6.Votruba SB, Jensen MD. Insulin sensitivity and regional fat gain in response to overfeeding. Obesity (Silver Spring) 2011;19:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60:2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsari C, Mundi MS, Ali AH, Jensen MD. Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes 2012;61:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 10.Hames KC, Vella A, Kemp BJ, Jensen MD. Free fatty acid uptake in humans with CD36 deficiency. Diabetes 2014;63:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010;59:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 2008;51:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007;56:168–176 [DOI] [PubMed] [Google Scholar]
- 15.Kalant D, Phélis S, Fielding BA, Frayn KN, Cianflone K, Sniderman AD. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women. J Lipid Res 2000;41:1963–1968 [PubMed] [Google Scholar]
- 16.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2002;2:477–488 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Van Oort MM, Yao M, Van der Horst DJ, Rodenburg KW. Insulin and chromium picolinate induce translocation of CD36 to the plasma membrane through different signaling pathways in 3T3-L1 adipocytes, and with a differential functionality of the CD36. Biol Trace Elem Res 2011;142:735–747 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 19.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism 1991;40:406–409 [DOI] [PubMed] [Google Scholar]
- 20.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–1801 [DOI] [PubMed] [Google Scholar]
- 21.Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res 2011;52:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring) 2009;17:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol 1992;209:98–104 [DOI] [PubMed] [Google Scholar]
- 24.Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE. Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J Lipid Res 1999;40:881–892 [PubMed] [Google Scholar]
- 25.Walters RW, Shukla AK, Kovacs JJ, et al. Beta-arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 2009;119:1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51:2761–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpentier AC, Frisch F, Cyr D, et al. On the suppression of plasma nonesterified fatty acids by insulin during enhanced intravascular lipolysis in humans. Am J Physiol Endocrinol Metab 2005;289:E849–E856 [DOI] [PubMed] [Google Scholar]
- 28.Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res 2007;48:609–620 [DOI] [PubMed] [Google Scholar]
- 29.Zhan T, Poppelreuther M, Ehehalt R, Füllekrug J. Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS ONE 2012;7:e45087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun 2002;298:317–323 [DOI] [PubMed] [Google Scholar]