Abstract
β-Cell dysfunction in diabetes results from abnormalities of insulin production, secretion, and cell number. These abnormalities may partly arise from altered developmental programming of β-cells. Foxo1 is important to maintain adult β-cells, but little is known about its role in pancreatic progenitor cells as determinants of future β-cell function. We addressed this question by generating an allelic series of somatic Foxo1 knockouts at different stages of pancreatic development in mice. Surprisingly, ablation of Foxo1 in pancreatic progenitors resulted in delayed appearance of Neurogenin3+ progenitors and their persistence into adulthood as a self-replicating pool, causing a fourfold increase of β-cell mass. Similarly, Foxo1 ablation in endocrine progenitors increased their numbers, extended their survival, and expanded β-cell mass. In contrast, ablation of Foxo1 in terminally differentiated β-cells did not increase β-cell mass nor did it affect Neurogenin3 expression. Despite the increased β-cell mass, islets from mice lacking Foxo1 in pancreatic or endocrine progenitors responded poorly to glucose, resulting in glucose intolerance. We conclude that Foxo1 integrates cues that determine developmental timing, pool size, and functional features of endocrine progenitor cells, resulting in a legacy effect on adult β-cell mass and function. Our results illustrate how developmental programming predisposes to β-cell dysfunction in adults and raise questions on the desirability of increasing β-cell mass for therapeutic purposes in type 2 diabetes.
Introduction
Environmental and nutritional cues can affect developmental programming and organ plasticity in utero, resulting in the metabolic syndrome and type 2 diabetes in adults (1). Examples of such gene/environment interactions include 1) low concordance rate for type 2 diabetes among identical twins, 2) increased risk of diabetes in children of mothers with diabetes, 3) fetal malnourishment as a risk factor for diabetes, and 4) limited success of adult lifestyle modifications in preventing type 2 diabetes (2). Although there are multiple explanations for these disparate observations, one unifying feature is that fetal programming of organ plasticity partly dictates adult predisposition to metabolic diseases.
There is evidence that developmental cues can result in acquired abnormalities of β-cell function. For example, intrauterine growth retardation in rodents and humans results in reduced pancreas size that predisposes to diabetes (3), and obese adolescents that were exposed to gestational diabetes in utero exhibit a fourfold higher risk than control subjects of becoming glucose intolerant or diabetic (4).
Foxo1 integrates cell differentiation, proliferation, and response to metabolic and cellular stressors (5). In the adult pancreas, Foxo1 expression is restricted to β-cells (6,7), where it modulates metabolic flexibility (i.e., the ability to switch between glucose and lipids as a source of acyl-CoA for mitochondrial oxidative phosphorylation [8]) and enforces the β-cell fate, allowing β-cells to retain terminally differentiated features (9). Disruption of these activities results in β-cell dysfunction (8–10).
In contrast to the adult pancreas, Foxo1 expression in the fetal pancreas parallels that of the master regulator of pancreas specification, Pdx1 (11). Like the latter, Foxo1 is expressed widely at embryonic day (E) 12.5–13.5 and becomes restricted to Neurogenin3+ (Neurog3+) endocrine progenitors at E14.5 and to β-cells at birth (7). Here, we investigated whether Foxo1 determines the number and function of pancreatic or endocrine progenitors, using stage-specific genetic inactivation strategies. The surprising results of this investigation indicate that Foxo1 controls timing and size of the endocrine progenitor cell pool, such that embryonic Foxo1 ablation delays formation of endocrine progenitors and causes their persistence into adulthood (12). Accordingly, β-cell mass continues to expand in mice lacking Foxo1 in endocrine progenitors, but cells do not function properly, impairing insulin secretion. The notion that Foxo1 preprograms adult β-cell mass and function has important implications for the hypothesis of fetal programming of adult β-cell function and invites caution with regard to expanding β-cell mass as a treatment for diabetes.
Research Design and Methods
Antibodies and Immunohistochemistry
We fixed and processed tissues for immunohistochemistry as previously described (7,9). We used the following antibodies: rabbit primary antibodies to FoxO1, amylase and glucokinase (Santa Cruz Biotechnology), Pdx1 (gift from C. Wright, Vanderbilt University, Nashville, TN), Ki67 (Dako), H2B (Cell Signaling Technology), glucagon (Sigma-Aldrich, Phoenix Pharmaceuticals), GFP (Invitrogen), Sox9 (Chemicon); guinea pig primary antibodies to insulin (Dako), somatostatin (Chemicon), pancreatic polypeptide (Linco), GFP (Rockland Immunochemicals); goat primary antibodies to Neurog3, NeuroD1, Hnf6 (Santa Cruz Biotechnology), and Pdx1; and mouse primary antibody to cytokeratin 19 (Sigma-Aldrich). We used FITC (fluorescein isothiocyanate)-, Cy3-, and Alexa-conjugated donkey secondary antibodies (Jackson ImmunoResearch Laboratories, Molecular Probes), peroxidase staining, and DAPI for nuclear counterstaining (7,9). We used ApopTag Kit for TUNEL assays (Millipore) and stained nuclei with DAPI or DRAQ5 (Cell Signaling Technology). Image acquisition and morphometry were performed as previously described (7,9). The data are normalized to the wild type (WT) control (WT = 1).
Flow Cytometry
We digested and dissociated the pancreas as previously described (13) and incubated single cell suspensions with glucose prior to flow cytometry (14) or performed immunohistochemistry with antibodies to insulin, Neurog3, amylase, or Ki67 (13).
Physiological Studies
Neurog3-Cre, Ins-Cre, Rosa26-EGFP, Neurog3-GFP, Neurog3GFP/+, and Foxo1flox/+ mice have been previously described (9,15). Pdx1-Cre mice were made using the XbaI-SacI 4.3 kb fragment of the Pdx1 promoter (16). Mice were on a C57BL/6J × 129sv background. All mice were granted free access to food and water in a 12-h light cycle facility. We performed intraperitoneal glucose tolerance tests in overnight-fasted 8-month-old male mice (17) and static incubations of collagenase-purified islets as previously described (18). We prepared acid-ethanol extracts from adult pancreas as previously described (9). We measured glucagon by radioimmunoassay and insulin, C-peptide, and proinsulin by ELISA (Millipore, ALPCO Diagnostics).
RNA Procedures
We applied standard techniques for mRNA isolation and quantitative PCR (qPCR) (9). Primer sequences for Gck, Kir6.1, Sur1, Pdk1, Ucp, Pc1, Pc2, Glut2, Glut4, Neurog3, Pdx1, MafA, Nkx6.1, NeuroD1, Nkx2.2, Tubulin2, Hprt, Foxo1, Insulin1, Insulin2 (9), Sox9 (19), Hnf6 (20), cyclin D1, cyclin D2, cyclin A2, CDK4, CDK6, p18, p21, CDK5, cyclin G1, and cyclin H (RT2 Profiler PCR Array; Qiagen, Mississauga, ON, Canada) have been previously described (9,15). Tubulin2 and Hprt were used as standards. We normalized the data to WT = 1 for fold change.
Statistical Analysis
We analyzed data using Student t test and used the traditional threshold P < 0.05 to declare statistical significance.
Results
Developmental Stage–Specific Pancreatic Foxo1 Knockouts
Foxo1 is a negative regulator of β-cell mass (6,21,22) that is expressed in pancreatic and endocrine progenitors during fetal development and becomes restricted to β-cells as the latter become terminally differentiated (7). We investigated the mechanism by which Foxo1 limits β-cell mass and asked whether it does so by controlling β-cell or endocrine progenitor cell number, i.e., pre– or post–β-cell formation. To distinguish between these two possibilities, we inactivated Foxo1 at three distinct developmental stages: 1) in pancreatic precursors (using Pdx1-Cre–mediated gene knockout) (16), 2) in endocrine progenitors (using Neurog3-Cre) (23), and 3) in terminally differentiated β-cells (using Ins-Cre) (9) (Fig. 1A). For each cross, we generated two independent lines and line-specific littermates carrying Cre (denoted WT) to control for locus effects and ectopic recombination (24). Genotyping of DNA extracted from multiple organs, including collagenase-purified islets, confirmed the specificity of Cre-mediated excision (not shown).
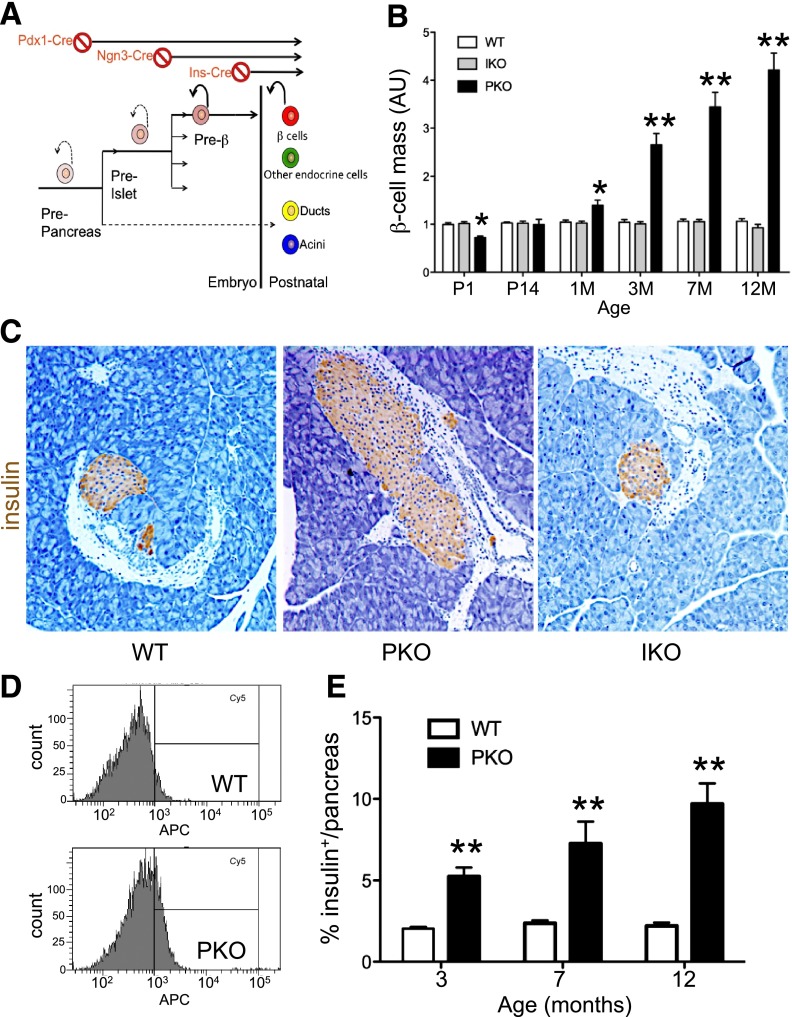
Figure 1.
Increased β-cell mass following pan-pancreatic Foxo1 ablation. A: Strategy to delete Foxo1 in all pancreatic cell types, endocrine progenitors, and differentiated β-cells. B: Analysis of β-cell mass by immunohistochemistry (n = 6 each genotype and each age). At each time point, β-cell mass in WT littermates was normalized to 1 for clarity. C: Representative images of insulin immunohistochemistry (brown) of pancreatic sections from 3-month-old mice used for the analysis in panel B. Original magnification ×100. Analysis of β-cell number by flow cytometry, showing a representative plot (D) and quantification of results (E). We digested pancreata into single cells and fixed and stained the cells with anti-insulin antibodies. Insulin+ cells were normalized by DNA concentration (n = 6 each genotype). *P < 0.05; **P < 0.01. AU, arbitrary units; M, month; P, postnatal day.
We first compared mice with pan-pancreatic or β-cell–specific Foxo1 ablation (PKO and IKO, respectively). qRT-PCR showed that Foxo1 mRNA was reduced by ∼90% in islets from PKO and ∼70% in islets from IKO mice, compared with WT (Supplementary Fig. 1A). Conversely, Foxo3 and Foxo4 transcripts increased three- to sevenfold in PKO and IKO compared with controls (Supplementary Fig. 1A–C), as seen before (9). Indeed, triple Foxo-deficient mice generated using a similar approach phenocopy PKO mice (J.Y. Kim-Muller and D. Accili, unpublished observations). In both lines, we introduced a Rosa26-GFP allele as a marker of Cre activity to identify cells in which recombination had occurred. Immunohistochemistry demonstrated GFP reactivity in all pancreatic cell types of PKO (Supplementary Fig. 1D) and only in β-cells of IKO mice (9). PKO and IKO mice had similar body weight and glucose levels as WT controls up to 8 months of age (Supplementary Fig. 1E and F). Thus, Foxo1 ablation occurred specifically and efficiently and did not result in overt metabolic abnormalities.
Altered β-Cell Mass After Foxo1 Ablation in Pancreatic Precursors
Homeostatic β-cell mass is the product of β-cell differentiation, survival, and self-renewal (25), whereas pancreas and islet size are predetermined by the number of pancreatic precursors (26). Therefore, the predicted outcome of Foxo1 deletion in pancreatic precursors or β-cells was that 1) if Foxo1 suppresses proliferation of differentiated β-cells, both IKO and PKO should show increased β-cell mass and 2) if Foxo1 acts on β-cell precursor differentiation and/or proliferation, but not on proliferation of mature β-cells, increased β-cell mass should be found in PKO, but not IKO. Histomorphometry of the pancreas showed that PKO mice were born with slightly lower β-cell mass but caught up rapidly, exhibiting an approximately twofold increase at 3 months, threefold at 7 months, and fivefold of WT at 12 months (Fig. 1B and C and Supplementary Fig. 1G). Other endocrine cell types showed commensurate increases (not shown). Analysis by flow cytometry in mice carrying a Cre-dependent Rosa-GFP reporter confirmed increased numbers of insulin-producing (GFP+) cells (Fig. 1D and E). In contrast, β-cell and pancreas mass in IKO mice remained unchanged throughout (Fig. 1B and C). These findings suggest that Foxo1 regulates β-cell mass through actions that precede β-cell formation.
Interestingly, total pancreas weight in PKO mice increased by ∼15% at 3 months and by 50% at 8 months (Supplementary Fig. 1H). Analysis of exocrine cell proliferation by flow cytometry of Ki67+/amylase+ cells and Ki67+ immunohistochemistry showed an ∼15% increase in 5-month-old PKO mice that petered out by 7 months (Supplementary Fig. 1I and J).
Adult Expansion of Endocrine Progenitors in PKO Pancreas
The preservation of β-cell mass in IKO mice focused our investigations on Foxo1 action in endocrine progenitors. Pan-pancreatic precursors and endocrine progenitors develop at distinct temporal windows (27). Neurog3+ progenitors give rise to endocrine cells (12). Their numbers peak at E13.5–15.5, decline after E17.5 (28), and are exceptionally rare in the adult, except in a duct ligation model (29). Thus, we investigated whether Foxo1 ablation in pancreatic progenitors affected generation and properties of endocrine progenitors, including their contribution to adult β-cell mass. We surveyed endocrine progenitor pool size during peak endocrine differentiation (E15.5). Surprisingly, in PKO pancreas, Neurog3 expression was hardly detectable by either qPCR or immunohistochemistry (Supplementary Fig. 2A and B). To increase the sensitivity of detecting Neurog3+ cells, we introduced a transgene encoding GFP driven by the Neurog3 promoter in PKO mice, yet failed to find pancreatic GFP+ cells at E15.5, while intestinal GFP+ cells were present (Supplementary Fig. 2B). Endocrine lineage development appeared to stall at E15.5 in PKO, as additional coeval markers of β-cell differentiation such as NeuroD1 (30) were also undetectable (Supplementary Fig. 2B). In contrast, we observed a 50-fold increase of Neurog3 mRNA at E17.5 that persisted into adulthood, reaching 18-fold over WT at P14 and remaining over twofold higher thereafter (Supplementary Fig. 2A).
These data suggest that Foxo1 ablation delays the appearance but promotes postnatal survival of endocrine progenitors. In adult animals, Neurog3 protein can be detected in hormone-producing cells using reporter genes and its function is required for endocrine maturation (31). To detect Neurog3+ cells in adult mice, we introduced two different genetically modified alleles in PKO mice in two separate experiments: one allele encodes a Neurog3-GFP transgene (12) and the other one a Neurog3-GFP knock-in (32). We took advantage of the longer half-life of GFP than endogenous Neurog3 (up to 1–2 days) (23) to increase the likelihood of detecting Neurog3+ cells.
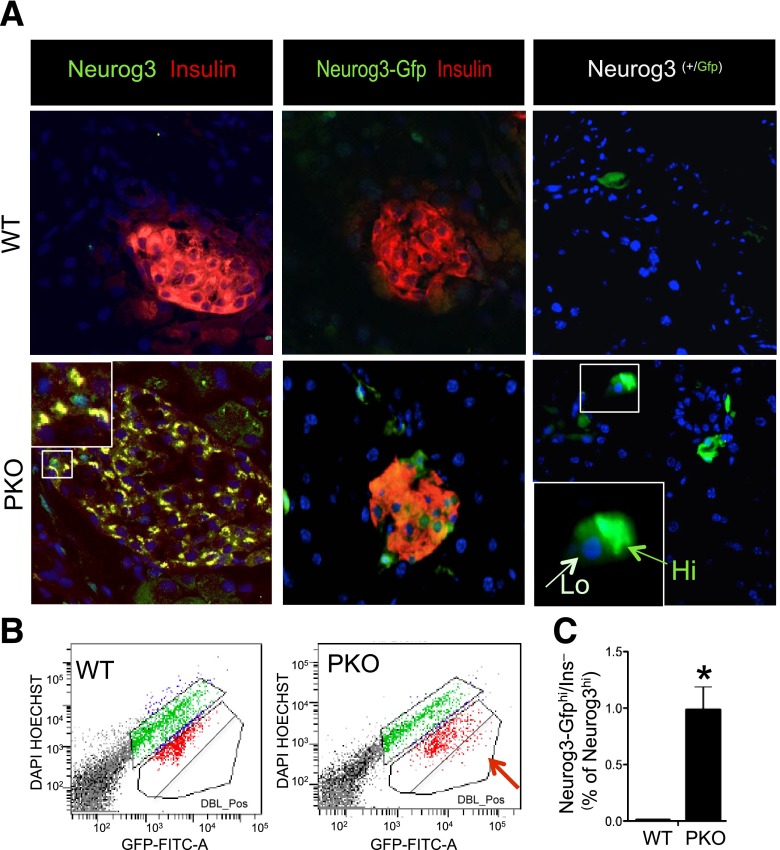
In 3-month-old PKO mice carrying transgenic or knock-in Neurog3 reporters, double immunohistochemistry with GFP and insulin revealed Neurog3-GFP+/insulin+ cells alongside with Neurog3-GFP+/insulin− cells. Neurog3-GFP+ cells resided within islets or near ducts (Fig. 2A) and differed by the levels of green fluorescence, consistent with the identification of distinct Neurog3hi and Neurog3low cell populations during development (27). We quantified Neurog3-GFP+/insulin− cells by flow cytometry (Fig. 2B), using glucose-induced autofluorescence to separate them from Neurog3-GFP+/insulin+ cells (14). The latter increased their fluorescence after incubation with glucose, whereas Neurog3-GFP+/insulin− cells did not. The population of Neurog3-GFP+/insulin− cells was enriched 15-fold in the PKO pancreas (Fig. 2B). The results from both GFP reporters were similar, confirming that there is an expanded Neurog3-GFP+ pool in adult PKO mice. These data show that Foxo1 ablation in pancreatic precursors leads to delayed expansion of Neurog3+ endocrine progenitors and their persistence into adulthood.
Figure 2.
Neurog3+ cells and β-cell neogenesis in adult PKO pancreas. A, left: Immunohistochemistry with antibodies against insulin (red) and Neurog3 (green) in WT and PKO mice. The inset in the lower panel shows representative double-positive cells (yellow). A, middle: Immunohistochemistry with anti-insulin (red) and anti-GFP antibodies (green) in WT and PKO carrying a Neurog3-GFP transgenic reporter. A, right: Immunohistochemistry with anti-GFP antibodies (green) in WT and PKO mice carrying a Neurog3-GFP knock-in reporter. The inset shows a representative Neurog3-GFPhi cell next to a Neurog3-GFPlow cell. Original magnification ×20 (n = 4 each genotype). B: Representative flow cytometry plots of dissociated live pancreatic cells treated with glucose to separate Neurog3-GFP+/insulin− cells (red arrow) from Neurog3-GFP+/insulin+ cells that are found in the central bracket. C: Quantification of the results of multiple experiments to determine the relative percentage of Neurog3+/insulin− cells in 3-month-old WT (Neurog3-GFP;Foxo1fl/fl) and PKO mice (Neurog3-GFP;Neurog3-Cre Foxo1fl/fl) (n = 6 each). *P < 0.05.
Juxta-Ductal β-Cells in PKO Mice
β-Cells are occasionally found near pancreatic ducts. We investigated whether the ductal milieu contributes to the enlarged β-cell mass in PKO mice. In 3-month-old WT mice, juxta-ductal insulin+ cells were rare, but in PKO pancreas, their frequency increased 100-fold (Supplementary Fig. 2C and D), with other hormone+ cells present at lower rates (Supplementary Fig. 2C) (7). Juxta-ductal insulin+ cells expressed Pdx1, confirming that they are bona fide β-cells (Supplementary Fig. 2E). We asked whether juxta-ductal insulin+ cells arise from Foxo1-deleted or -undeleted cells by comparing PKO and WT mice carrying Rosa26-GFP. All surveyed juxta-ductal insulin+ cells were GFP+ (Supplementary Fig. 2D). Thus, even considering the mosaicism of Pdx1-driven Rosa26-GFP expression (16), these cells should be considered descendants of Foxo1-ablated cells, indicating that juxta-ductal hormone+ cells in PKO mice arise cell-autonomously (7).
A Replicative Pool of Endocrine Progenitors in Adult PKO Mice
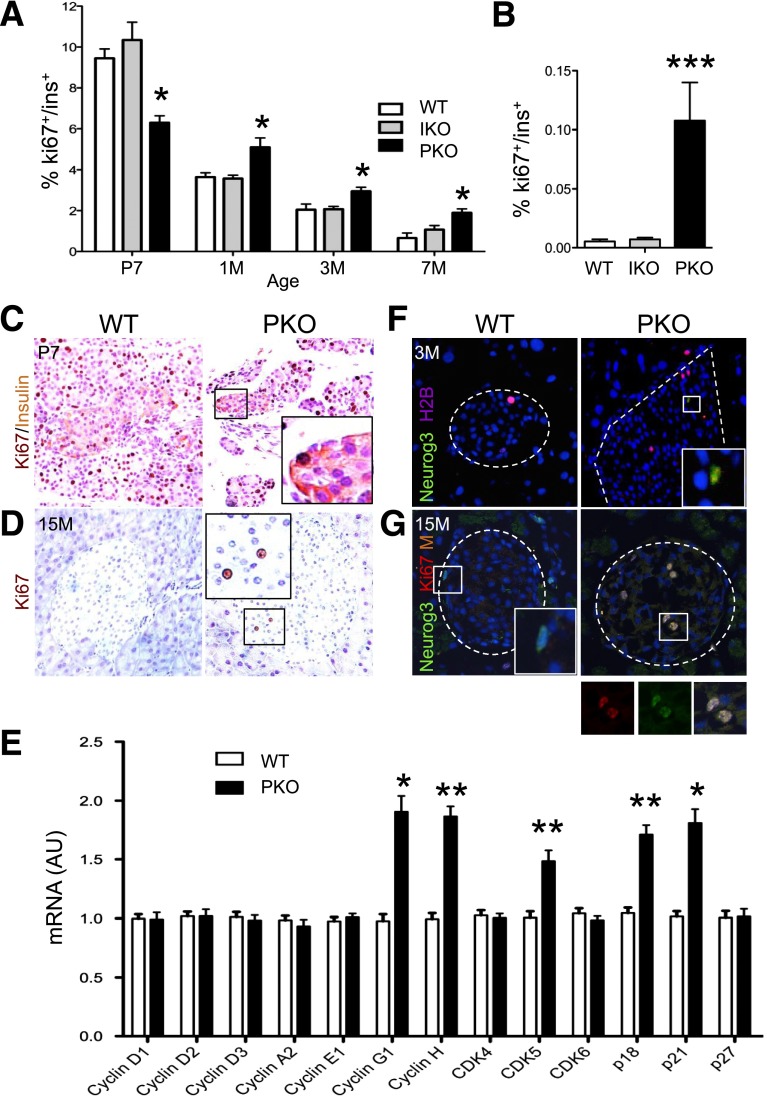
The data above indicate that Foxo1 ablation in pancreatic progenitors increases progenitor pool size and β-cell mass. We further investigated whether increased β-cell mass was also due to altered β-cell turnover. We have previously shown that β-cell turnover is normal in IKO mice throughout life (9). We surveyed β-cell death by TUNEL assay and found no difference between PKO and WT mice (Supplementary Fig. 3A and B). We assessed β-cell proliferation by immunohistochemistry or flow cytometry with the cell cycle marker Ki67. The percentage of Ki67+/insulin+ cells in PKO mice was slightly decreased at postnatal day 7 but rose at 1 month and remained elevated up to 1 year of age, a time when β-cell turnover was no longer detectable in WT mice (Fig. 3A and B). Immunohistochemistry yielded similar data (Fig. 3C and D). Next, we surveyed a panel of cell cycle genes (33). Quantitative mRNA measurements showed modest elevations of p18, p21, CDK5, and cyclin G1 and H in PKO but not IKO islets (Fig. 3E and Supplementary Fig. 3B). The small extent of these mRNA changes is commensurate with the increase in β-cell proliferation rates, but neither is sufficient to account for the large increase of β-cell mass in PKO mice. We therefore tested if there was an increase in endocrine progenitor proliferation, resulting in β-cell neogenesis. Strikingly, we identified Neurog3hi cells that were also positive for H2B, a marker of S phase cell division, and Ki67 in islets of 3- and 15-month-old PKO mice (Fig. 3F and G). These data indicate that Foxo1 ablation in β-cell precursors increases endocrine progenitor replication in the adult pancreas, contributing to bona fide postnatal β-cell neogenesis and providing an explanation for the lack of effects on β-cell mass in IKO mice.
Figure 3.
A self-replicating pool of endocrine progenitors in adult PKO mice. A: Quantification of Ki67+/insulin+ cells by flow cytometry in mice of the indicated ages and genotypes. We normalized the number of double-positive cells by the total number of insulin+ cells per pancreas (n = 6 each). B: Quantification of Ki67+/insulin+ cells by double immunohistochemistry in 12-month-old mice of the indicated genotype by manual counting, normalized by total number of insulin+ cells per pancreas (n = 4 each). C: Double immunohistochemistry with anti-Ki67 (dark brown) and anti-insulin antibodies (light brown) on pancreatic sections of 7-day-old mice (n = 4). Insets show representative insulin+ and/or Ki67+ cells. D: Immunohistochemistry with anti-Ki67 antibody (dark brown) on pancreas sections of 15-month-old mice (n = 4 each). Insets represent Ki67+ cells in islets. E: qPCR analysis of a panel of cell cycle regulatory genes in purified islets from control and PKO mice (n = 6 each). F: Immunofluorescence with anti-Neurog3 (green) and anti-H2B (purple) in pancreas sections from 3-month-old control and PKO mice (n = 4 each). Inset shows Neurog3+/H2B+ cells. G: Immunofluorescence with anti-Neurog3 (green) and anti-Ki67 (red) in pancreas sections from 15-month-old mice (n = 4 each). Insets show Neurog3+ and Neurog3+/Ki67+ cells. F and G: A dashed line is used to outline the islet. Data show means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student t test. AU, arbitrary units; M, month; P, postnatal day.
Abnormal Insulin Production and Release in PKO Mice
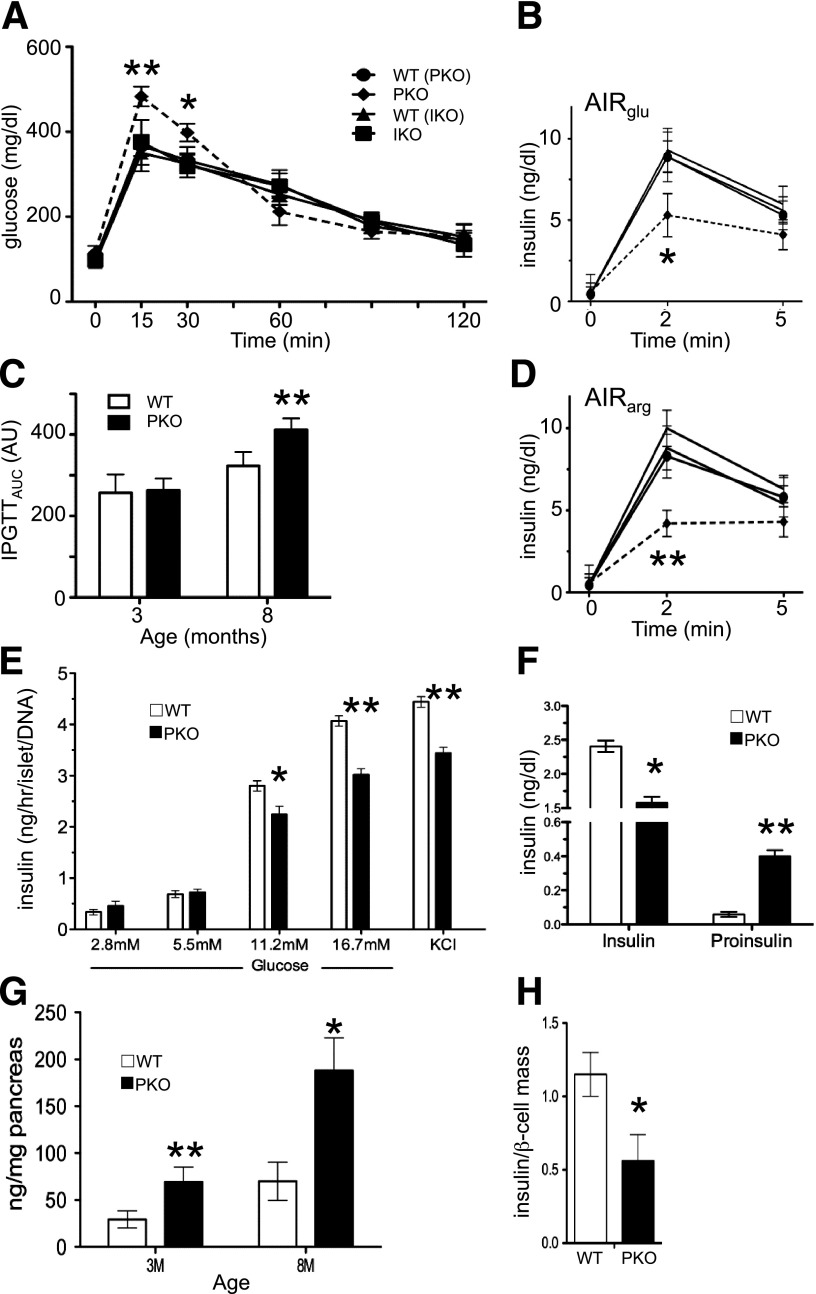
We next investigated the function of PKO β-cells in vivo. The expectation was that PKO mice would be more glucose tolerant, owing to larger islet size. But to our surprise, 8-month-old PKO mice showed greater glucose excursions during glucose tolerance tests (Fig. 4A and C), accompanied by impaired insulin release in response to glucose and arginine in vivo (Fig. 4B and D). These data indicate that PKO β-cells are dysfunctional, consistent with findings that Foxo1 ablation in pancreatic progenitors exacerbates diabetes in db/db mice (10).
Figure 4.
Metabolic analysis. A: Intraperitoneal glucose tolerance tests (IPGTTs) in 12 h–fasted 8-month-old male mice (n = 12 each genotype). B: Acute insulin response to glucose (AIRglu) (3 mg/kg) (n = 5 each genotype). Symbols are the same as in panel A. C: Calculated area under the curve (AUC) from IPGTT (n = 8 each genotype). D: Arginine-stimulated insulin secretion (AIRarg) in 8-month-old mice (n = 5 each). Symbols are the same as in panel A. E: Glucose-stimulated insulin secretion from static incubations of islets isolated from 3-month-old mice (n = 5). Three independent experiments were performed. Data show means ± SEM. F: Serum insulin and proinsulin levels in fed 8-month-old mice (n = 8). Insulin concentration (G) and ratio of insulin concentration to β-cell mass (H) in control and PKO pancreas, normalized by the concentration in WT mice (n = 6). Data are presented as means ± SEM. *P < 0.05 and **P < 0.01 by Student t test. M, month.
To determine whether PKO islet function was impaired due to cell-autonomous abnormalities of insulin secretion, we isolated islets and performed glucose-stimulated insulin secretion ex vivo. The results showed that insulin release from PKO islets was significantly reduced at 11.2 and 16.7 mmol/L glucose, as well as in response to the membrane-depolarizing agent KCl (Fig. 4E). Consistent with these findings, serum insulin levels in the refed state fell by nearly 40%, accompanied by a 20-fold rise in proinsulin levels (Fig. 4F). Pancreatic insulin concentration rose two- and threefold in 3- and 8-month-old PKO mice, respectively, as did glucagon concentration (Fig. 4G and Supplementary Fig. 4A). However, when we normalized insulin concentration by β-cell mass, we found a 40% decrease in PKO mice (Fig. 4H), which might explain the reduced response to KCl. In contrast, IKO β-cell function appeared indistinguishable from controls at these ages (Supplementary Figure 4B–D). The findings are suggestive of defects in insulin production, processing, and secretion in PKO mice.
Foxo1-Deficient Endocrine Progenitors Yield Defective β-Cells
The enlarged β-cell mass in PKO was accompanied by a 50% increase of pancreas weight in adult mice (Supplementary Fig. 1G), raising the question of whether it was simply a result of increased pancreas size. Thus, we wanted to determine whether the effect of Foxo1 ablation was exerted in pancreatic or endocrine progenitors. To analyze this point, we compared endocrine progenitor–specific Foxo1 knockouts (denoted NKO) with WT and PKO mice. To rule out the potential mosaicism of Neurog3-Cre transgene expression (23), we introduced a Rosa26-GFP allele into NKO mice to verify the cell types in which recombination had occurred. Immunohistochemistry with GFP and either insulin or a cocktail of Pp, glucagon, and somatostatin antibodies showed 98% colocalization. Assuming that Rosa26-GFP is a faithful surrogate of Foxo1 deletion, NKO mice should be considered a model of endocrine cell–specific Foxo1 knockout (Supplementary Fig. 5A).
The prediction was that if increased islet size and impaired β-cell function in PKO mice were due to an effect of Foxo1 in pancreatic progenitors, these phenotypes should not appear when inactivating Foxo1 at the endocrine progenitor stage (Fig. 1A), whereas if they were due to effects in endocrine progenitors, NKO mice should phenocopy PKO mice. In islets isolated from 3-month-old NKO mice, Foxo1 expression decreased by 85%, confirming efficient deletion of Foxo1. Similar to PKO and IKO mice, Foxo3 and Foxo4 levels rose (Supplementary Fig. 5B).
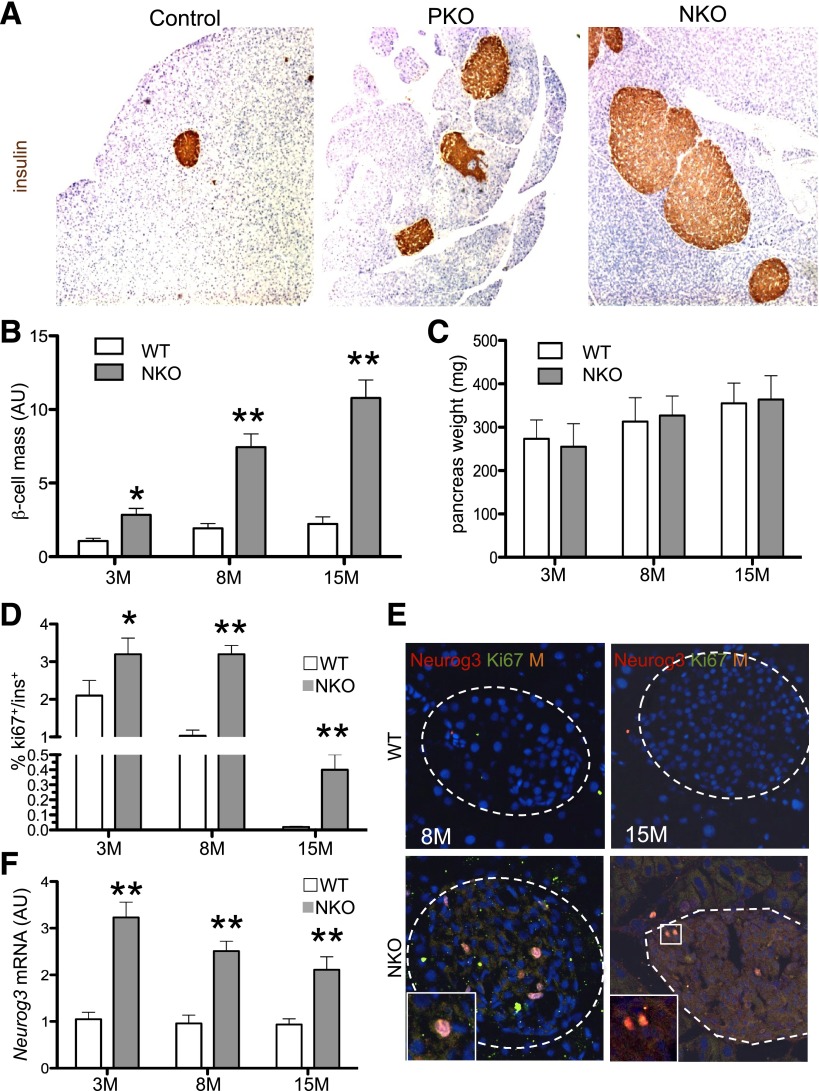
β-Cell mass increased as a function of age in NKO mice, peaking at approximately fivefold of controls at 15 months (Fig. 5A and B), and was ∼18% larger than in age-matched PKO mice (Fig. 1), whereas NKO pancreas weight was identical to WT (Fig. 5C). Next, we examined β-cell replication by flow cytometry. We detected a threefold increase of Ki67+/insulin+ cells in islets from 8-month-old NKO mice (Fig. 5D). Moreover, ∼0.5% of insulin+ cells were still replicating in 12-month-old NKO mice, compared with none in WT. In addition, we observed Ki67+/Neurog3+ cells in 8- and 15-month-old NKO mice (Fig. 5E). Neurog3 mRNA levels were consistently two- to threefold higher in NKO mice compared with WT, regardless of age (Fig. 5F). Moreover, quantitative measurements of mRNAs encoding cell cycle genes showed evidence of increased cell replication, with higher transcripts of cyclin G1, cyclin H, CDK5, p18, and p21 (Supplementary Fig. 5C).
Figure 5.
β-Cell mass and endocrine progenitor proliferation in NKO mice. A: Representative insulin immunohistochemistry (brown) of pancreatic sections from 15-month-old mice. Original magnification ×40. B: Age-dependent increase in β-cell mass, measured as percent insulin-immunoreactive area normalized by pancreas weight (n = 6 each genotype). β-Cell mass in WT littermates at 3 months (3M) was normalized to 1; the others are compared with WT, which is considered = 1. C: Pancreas weight in mice of the indicated ages and genotypes (n = 6 each genotype). D: Quantification of Ki67+/insulin+ cells normalized by total insulin+ cells per pancreas (n = 4–6 each genotype). E: Immunofluorescence with anti-Neurog3 (red) and anti-Ki67 antibodies (green) in pancreas sections from 8- and 15-month-old mice (n = 4 each genotype). Insets show Neurog3+/Ki67+ cells. A dashed line is used to outline the islet. F: qPCR measurements of Neurog3 mRNA from collagenase-purified islets (n = 6 each genotype). WT = 1 for fold change. *P < 0.05; **P < 0.01. AU, arbitrary units.
We evaluated whether Neurog3+ progenitors were present in adult NKO pancreas, using NKO mice bearing a Neurog3-GFP reporter. Similar to PKO pancreas, GFP staining was detectable within and outside the islet, consistent with increased Neurog3 mRNA (Fig. 5F and Supplementary Fig. 5D). This result indicates that cell-autonomous changes in endocrine progenitors caused by Foxo1 ablation during embryonic development allow them to persist in adult mice. These data support a model in which the effects of Foxo1 on adult β-cell mass and function are exerted in endocrine progenitors, rather than (or in addition to) pancreatic precursors.
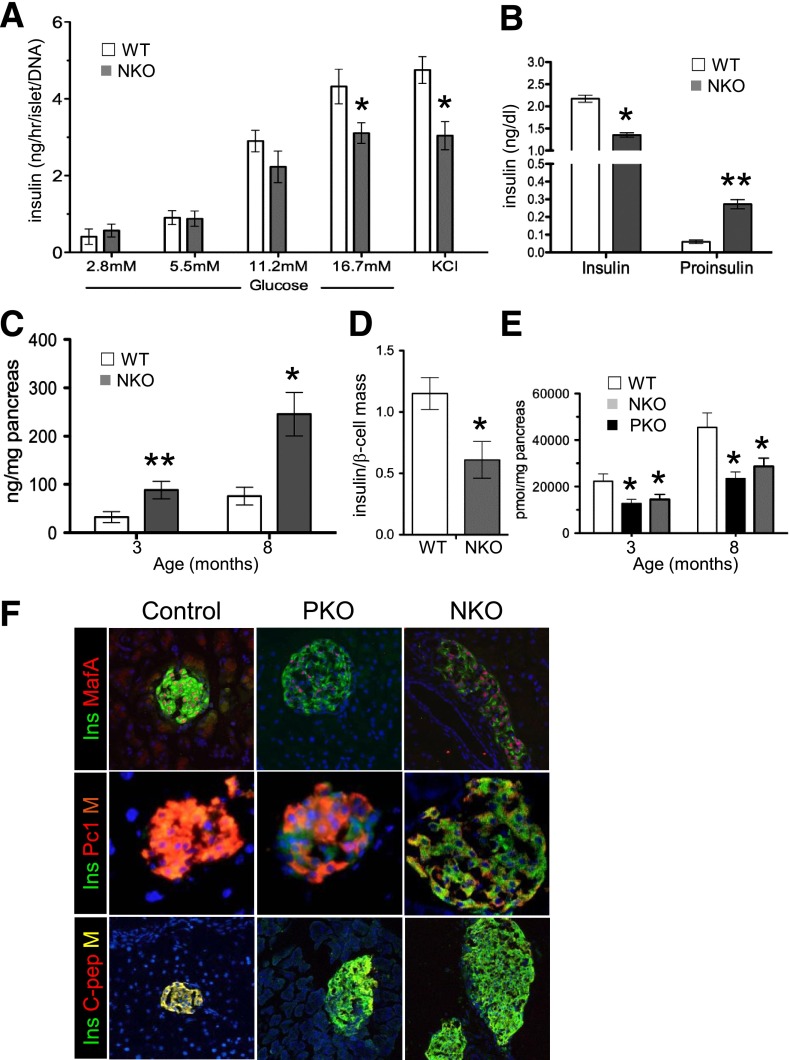
Like PKO, NKO mice exhibited decreased insulin release in response to glucose and KCl (Fig. 6A), ∼30% reduction of refed insulin levels, and 11-fold increase of proinsulin levels, compared with WT littermates (Fig. 6B). Whereas total insulin (Fig. 6C) and glucagon concentration (Supplementary Fig. 6A) were increased in NKO islets, insulin and C-peptide concentration per β-cell declined by 50% (Fig. 6D and E). The latter was similarly reduced in PKO mice (Fig. 6E). Thus, Foxo1-deficient endocrine progenitors recapitulate features of PKO mice, including increased β-cell mass, altered gene expression, and reduced in vivo function.
Figure 6.
β-Cell characterization. A: Insulin release from static incubations of islets isolated from 3-month-old mice normalized by islet DNA concentration (n = 5 each genotype). Three independent experiments were performed. B: Serum insulin and proinsulin in random-fed 8-month-old mice (n = 8 each genotype). C: Pancreatic insulin concentration. D: Ratio of insulin concentration to β-cell mass in control and NKO pancreas (n = 6 each genotype). E: C-peptide 2 concentration in acid-ethanol extracts from 3- and 8-month-old mice. F: Immunofluorescence with anti-insulin (green), anti-MafA (red), anti-Pc1/3 (red), and anti–C-peptide 2 in pancreatic sections from 8-month-old mice (n = 4 each genotype). Data show means ± SEM. *P < 0.05; **P < 0.01.
β-Cell Markers in PKO and NKO
We compared β-cell markers in 3-month-old PKO and NKO mice by immunohistochemistry. Expression of MafA, Pc1/3, and C-peptide was reduced (Fig. 6F). qRT-PCR of islet mRNA from 3-month-old PKO and NKO mice showed that Pdx1 and Neurog3 mRNA were three- to fivefold higher, whereas MafA was reduced by ∼60–70% in both PKO and NKO (Supplementary Fig. 6B), consistent with findings that Foxo1 suppresses Pdx1 (6) and Neurog3 (9) but activates MafA (21). Since Foxo1 ablation impairs Notch signaling (15,34,35), and the latter is required to activate Neurog3 (36), we measured mRNA transcripts of genes required for Notch signaling and observed a 30% reduction of Hes1 in PKO and NKO islets, consistent with the increase in Neurog3 (Supplementary Fig. 6B and C). We also saw increased expression of Nkx6.1 and Nkx2.2 and reduced expression of Ins2 and PC1/3 (Supplementary Fig. 6D–F).
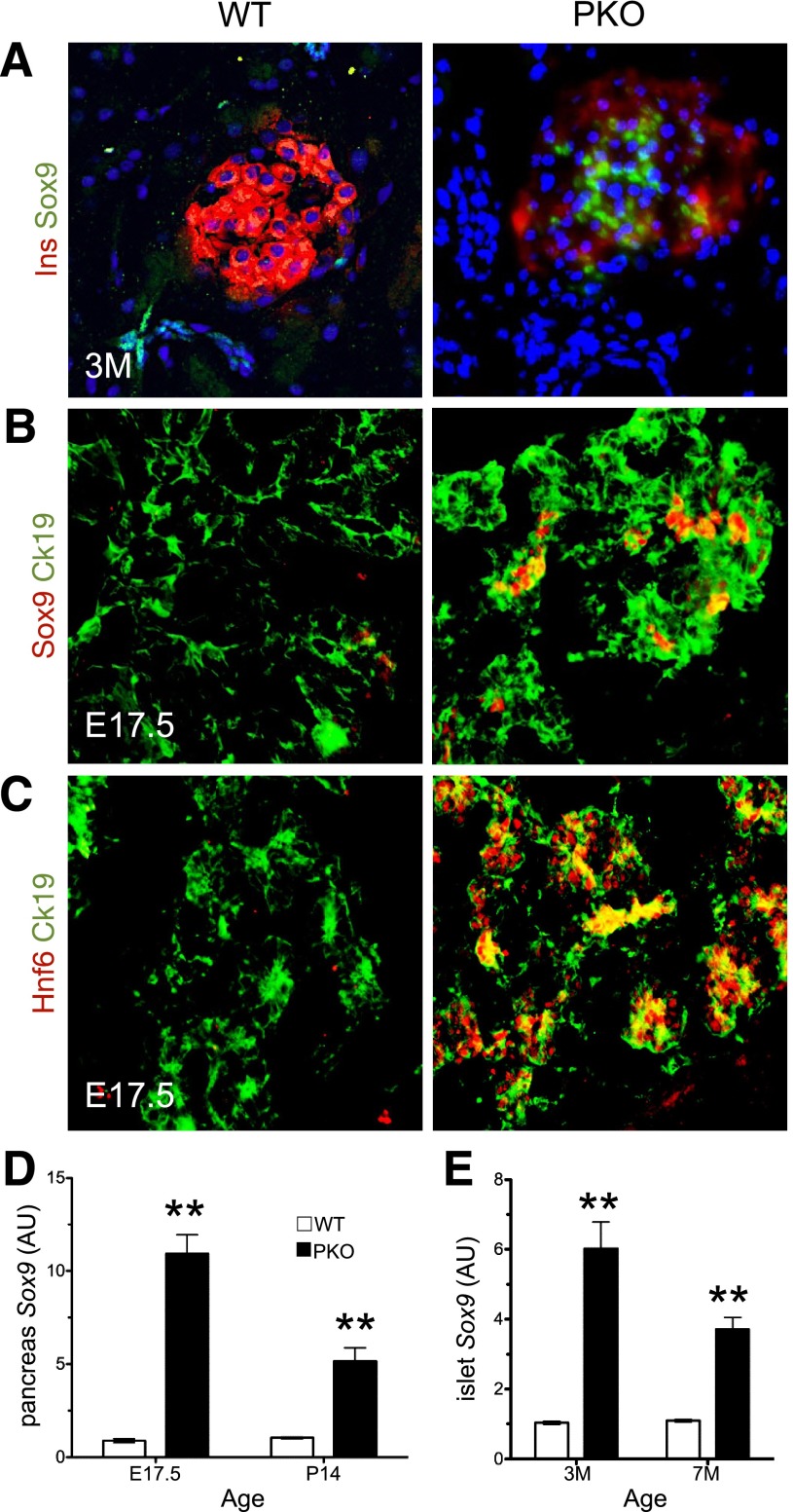
Transcription factor Sox9 is expressed in pancreatic progenitors but not in adult islets (37) and becomes reactivated in human diabetic islets (38) and in diabetic mice harboring Vhl mutations (39). Given the similarities between Sox9 gain of function and PKO mice, we asked whether Sox9 expression was altered. Indeed, immunohistochemistry showed similar numbers of Sox9+ progenitors in PKO and WT mice at E15.5 (Supplementary Fig. 2B) but a 10-fold increase at E17.5 (Fig. 7A and B), a time when Sox9+ cells become restricted to a subset of ductal cells in normal mice (Supplementary Fig. 7A) (37). These findings were associated with persistent expression of Hnf6 (a marker of early pancreatic progenitors, whose suppression is required for endocrine differentiation [40]) and elevated transcripts of Hnf6 at E17.5 and postnatal day 14 (Fig. 7C and Supplementary Fig. 7B). Moreover, we detected Sox9+/insulinlow cells in 3-month-old PKO but not WT mice, as well as increased Sox9 mRNA in 3- and 7-month-old PKO mice (Fig. 7A and D).
Figure 7.
Altered Sox9 expression in PKO pancreas. A: Immunohistochemistry with anti-Sox9 (green) and anti-insulin antibodies (red) in pancreata of 3-month-old (3M) mice (n = 4 each genotype). B and C: Immunohistochemistry with anti-Sox9 (red), anti-Hnf6 (red), or anti-Ck19 antibodies (green) in sections from E17.5 embryos (n = 4 each genotype). qPCR analysis of RNA extracted from whole pancreas (D) or collagenase-purified islets (E) of mice of different ages (n = 6–8 for each genotype). WT = 1 for fold change in D and E. **P < 0.01. AU, arbitrary units; P, postnatal day.
If elevated Sox9 was responsible for the phenotype of PKO mice, we would expect a similar increase in NKO mice, but Sox9 protein and mRNA levels were neither detected in islets nor increased in whole pancreas (Supplementary Fig. 7C and D). These data indicate that the temporal and spatial regulation of Sox9 expression during pancreatic development is altered by Foxo1 ablation in pancreatic, but not in endocrine, progenitors, raising the possibility that the exocrine effect of PKO can be accounted for by Sox9 activation, whereas regulation of endocrine mass and function is dependent on Foxo1 action in endocrine progenitors.
Discussion
The key conclusion of this work is that altered Foxo1 function during pancreas development causes a legacy effect on adult organ plasticity and maturation, predisposing to β-cell dysfunction. Given the role of Foxo1 as a sensor of the nutritional and proliferative status of the cell (5), it could be envisioned that a nutrient-rich, hyperinsulinemic environment, by causing Foxo1 nuclear exclusion, will mimic aspects of Foxo1 ablation, potentially providing an explanation for why offspring of hyperinsulinemic or insulin-resistant mothers tend to have larger pancreata and islets (25) and progress to diabetes as adults nonetheless (4).
Another novel finding of our study pinpoints the expanded β-cell mass of PKO mice as a result of Foxo1 action in endocrine progenitors, rather than β-cells proper. Thus, it appears that during pancreatogenesis, Foxo1 inhibits differentiation and expansion of pancreatic and endocrine progenitors, allowing them to mature (7). The delayed differentiation of Foxo1-deficient pancreatic precursors allows the pancreas to retain cells with progenitor-like features and maintain its developmental plasticity into adulthood. PKO pancreata show sustained and ectopic Sox9 expression, consistent with the role of insulin receptor (41) and Akt (42) in the generation of Sox9+ progenitors. We should emphasize that Foxo1 regulation of progenitor cell number is both Sox9 dependent and independent. Ablation of early Sox9+ precursors results in pancreatic hypoplasia, and early Sox9+ cells give rise to Neurog3+ progenitors (43). However, while increasing the number of Foxo1-deficient Sox9+ progenitors increased exocrine mass, reduced numbers of Sox9+ cells fail to affect pancreas size (37).
Foxo1 activation sets timing of endocrine progenitor cell formation and determines their number. FOXO1 knockdown by siRNA increased NEUROG3+ cell number in human fetal pancreatic epithelium (44). The results in PKO mice do not allow us to establish whether Foxo1 controls Neurog3+ endocrine progenitors directly or indirectly (28). The expansion of Neurog3+ progenitors into adulthood in Foxo1 knockouts, independent of exocrine or ductal plasticity, phenocopies the developmental stage-specific effects of Notch inhibition during endocrine differentiation (36). These data indicate that there is a specific temporal window during which these signaling pathways play a role in lineage determination.
Transgenic gain of function of Foxo1 in pancreatic progenitors causes exocrine hypoplasia (7). Consistent with these data, we show that Foxo1 ablation increases exocrine mass. In the transition from pancreatic progenitor replication to differentiation, other temporal regulators include Wnt and Notch. Wnt is required to expand pancreatic epithelial progenitors: gain of β-catenin function induces exocrine hyperplasia, without affecting endocrine cell formation (45,46). Notch activation allows pancreatic and endocrine progenitors to survive, but Notch inhibition blocks both exocrine and endocrine differentiation (36). Foxo1 inhibition in endocrine progenitors gives rise to cells arrested at a precursor-like stage (Pdx1hi, Neurog3hi, MafAlow, and Inslow) in adult life, indicating that changes in hormone sensitivity during the latter half of fetal development change the proliferative and functional capacity of adult β-cells.
Based on extensive data (44), we can integrate the findings into a general theory of Foxo function in the endocrine pancreas. The three isoforms play overlapping and redundant roles (8). During embryogenesis, Foxos suppress differentiation, similar to their roles in other cell types. In terminally differentiated β-cells, Foxos act as transcriptional sensors of nutrient and hormone signaling; Foxos are to β-cell transcription what glucokinase is to insulin secretion. In response to an altered metabolic environment, Foxos translocate to the nucleus and activate the Hnf4/Hnf1α networks, while suppressing nuclear receptors Pparα and γ (8). If the metabolic stress persists, Foxos are degraded via deacetylation, possibly caused by altered NAD-to-NADH ratios (21). The loss of Foxo function impairs the β-cell’s ability to sense glucose and activates lipid oxidation, resulting in impaired mitochondrial function and reduced insulin secretion. β-Cells lose their terminally differentiated features and revert to a “progenitor-like” state, possibly as an escape mechanism to prevent death. Thus, Foxos belong to a core network of β-cell transcription factors whose function is necessary to maintain “functional” β-cell mass (47,48). Our report highlights that β-cell mass per se is not sufficient to preserve physiologic insulin secretion and prevent the development of diabetes.
Article Information
Acknowledgments. The authors thank members of the Accili laboratory for discussion of the data.
Funding. This work was supported by National Institutes of Health grants DK-64819 and DK-63608 (Columbia University Diabetes Research Center) and a grant from the JPB Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.C.T. designed, performed, and analyzed experiments and wrote the manuscript. D.A. oversaw data acquisition and analysis and wrote the manuscript. D.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1696/-/DC1.
References
- 1.Vaag AA, Grunnet LG, Arora GP, Brøns C. The thrifty phenotype hypothesis revisited. Diabetologia 2012;55:2085–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation Diabetes Atlas. Brussels, Belgium, International Diabetes Federation, 2007 [Google Scholar]
- 3.Gatford KL, Simmons RA. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin Obstet Gynecol 2013;56:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holder T, Giannini C, Santoro N, et al. A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014;57:2413–2420 [DOI] [PubMed] [Google Scholar]
- 5.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004;117:421–426 [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 2002;110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Kitamura YI, Kobayashi M, et al. Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol Cell Biol 2009;29:4417–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab 2014;20:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M, Kikuchi O, Sasaki T, et al. FoxO1 as a double-edged sword in the pancreas: analysis of pancreas- and β-cell-specific FoxO1 knockout mice. Am J Physiol Endocrinol Metab 2012;302:E603–E613 [DOI] [PubMed] [Google Scholar]
- 11.Kaneto H, Miyatsuka T, Shiraiwa T, et al. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem 2007;14:1745–1752 [DOI] [PubMed] [Google Scholar]
- 12.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smelt MJ, Faas MM, de Haan BJ, de Vos P. Pancreatic beta-cell purification by altering FAD and NAD(P)H metabolism. Exp Diabetes Res 2008;2008:165360 [DOI] [PMC free article] [PubMed]
- 15.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet 2012;44:406–412, S1 [DOI] [PMC free article] [PubMed]
- 16.Xuan S, Kitamura T, Nakae J, et al. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 2002;110:1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kido Y, Burks DJ, Withers D, et al. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest 2000;105:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matoba S, Hiramatsu R, Kanai-Azuma M, et al. Establishment of testis-specific SOX9 activation requires high-glucose metabolism in mouse sex differentiation. Dev Biol 2008;324:76–87 [DOI] [PubMed] [Google Scholar]
- 19.Pierreux CE, Poll AV, Kemp CR, et al. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 2006;130:532–541 [DOI] [PubMed] [Google Scholar]
- 20.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab 2005;2:153–163 [DOI] [PubMed] [Google Scholar]
- 21.Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J Clin Invest 2006;116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–450 [DOI] [PubMed] [Google Scholar]
- 23.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 2004;270:443–454 [DOI] [PubMed] [Google Scholar]
- 24.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab 2013;18:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab 2010;21:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007;445:886–891 [DOI] [PubMed] [Google Scholar]
- 27.Johansson KA, Dursun U, Jordan N, et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465 [DOI] [PubMed] [Google Scholar]
- 28.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000;127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 29.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 30.Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol 2000;20:3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Jensen JN, Seymour PA, et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A 2009;106:9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev 2002;16:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatrai S, Elghazi L, Balcazar N, et al. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006;55:318–325 [DOI] [PubMed] [Google Scholar]
- 34.Kitamura T, Kitamura YI, Funahashi Y, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 2007;117:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pajvani UB, Shawber CJ, Samuel VT, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med 2011;17:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 2003;100:14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A 2007;104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marselli L, Thorne J, Dahiya S, et al. Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS ONE 2010;5:e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev 2013;27:2563–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tweedie E, Artner I, Crawford L, et al. Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of beta-cells. Diabetes 2006;55:3264–3270 [DOI] [PubMed] [Google Scholar]
- 41.Nef S, Verma-Kurvari S, Merenmies J, et al. Testis determination requires insulin receptor family function in mice. Nature 2003;426:291–295 [DOI] [PubMed] [Google Scholar]
- 42.Elghazi L, Weiss AJ, Barker DJ, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology 2009;136:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A 2007;104:10500–10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Masri M, Krishnamurthy M, Li J, et al. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia 2010;53:699–711 [DOI] [PubMed] [Google Scholar]
- 45.Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development 2005;132:4663–4674 [DOI] [PubMed] [Google Scholar]
- 46.Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development 2006;133:2023–2032 [DOI] [PubMed] [Google Scholar]
- 47.Mondal P, Song WJ, Li Y, Yang KS, Hussain MA. Increasing β-cell mass requires additional stimulation for adaptation to secretory demand. Mol Endocrinol 2015;29:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]