Abstract
Bladder exstrophy (BE) is a complex congenital anomaly characterized by a defect in the closure of the lower abdominal wall and bladder. We aimed to provide an overview of the literature and conduct an epidemiologic study to describe the prevalence, and maternal and case characteristics of BE. We used data from 22 participating member programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). All cases were reviewed and classified as isolated, syndrome, and multiple congenital anomalies. We estimated the total prevalence of BE and calculated the frequency and odds ratios for various maternal and case characteristics. A total of 546 cases with BE were identified among 26,355,094 births. The total prevalence of BE was 2.07 per 100,000 births (95% CI: 1.90–2.25) and varied between 0.52 and 4.63 among surveillance programs participating in the study. BE was nearly twice as common among male as among female cases. The proportion of isolated cases was 71%. Prevalence appeared to increase with increasing categories of maternal age, particularly among isolated cases. The total prevalence of BE showed some variations by geographical region, which is most likely attributable to differences in registration of cases. The higher total prevalence among male cases and older mothers, especially among isolated cases, warrants further attention.
Keywords: bladder exstrophy, prevalence, sex ratio, maternal age
INTRODUCTION
Bladder exstrophy (BE) is a rare, complex, and severe congenital anomaly. It is characterized by a defect in the closure of the lower abdominal wall and bladder [ICBDSR, 2009]. The bladder and related structures (bladder mucosa, ureteral orifices, posterior bladder neck, and urethra) are everted through the ventral wall of the abdomen between the umbilicus and symphysis pubis. BE is often associated with structural anomalies of the pubic bones. Although BE can be diagnosed with a prenatal ultrasound [Evangelidis et al., 2004], the diagnosis is usually made at the time of birth.
In male cases, BE is associated with epispadias. The phallus is short and broad with a dorsal chordee. The glans lies open and flat and the dorsal component of the foreskin is absent. The urethral plate extends the length of the phallus without a roof. The bladder plate and urethral plate are in continuity with the verumontanum and the ejaculatory ducts are visible within the prostatic urethral plate. The anus is anteriorly displaced with a normal sphincter mechanism. The testicles may be undescended.
In female cases the clitoris is uniformly bifid with divergent labia superiorly. The open urethral plate is in continuity with the bladder plate. The vagina and anus are anteriorly displaced.
In both male and female cases the pubic symphysis is widely separated. Divergent rectus muscles remain attached to the pubis [Gearhart, 2002; Stevenson and Hall, 2006; Ebert et al., 2009].
In this report we (1) provide an overview of historical aspects, embryology, etiology, clinical characteristics and genetics, epidemiology, prognosis, and treatment of BE, and (2) describe the current epidemiology of BE using a large dataset from the International Clearing-house for Birth Defects Surveillance and Research (ICBDSR).
BACKGROUND
Historical Aspects
In ancient texts, such as in the cuneiform tablets of Chaldea dating from 2000 BC, congenital anomalies were recorded, including genital malformations. Description of BE in the cuneiform tables was suggested [Ballantyne, 1894] but a definitive description was not corroborated [Gearhart, 2002]. Several case reports of BE published in the English literature dating from the 1800s have been reviewed [Ballantyne, 1904]. It has been suggested that BE was first described in 1595, or perhaps as early as 1583, by the German physician Johann Schenck von Grafenberg, and later by John Wood in 1869 [Ludwig et al., 2009]. The Museum Vrolik collection at the University of Amsterdam in The Netherlands founded by Gerardus Vrolik (1775–1859) and his son Willem Vrolik (1801–1863) includes specimens with midline anomalies [Oostra et al., 1998]. Among the specimens there is a dried pelvis with a marked diastasis of the pubic rami of a 6-year-old boy, with an ectopic bladder visible through an infraumbilical defect of the abdominal wall, a phenotype characteristic of classic BE [Oostra et al., 1998; Ludwig et al., 2009]. The term “exstrophy” is derived from the Greek word for inside out, ekstriphein, and was first used by Chaussier in 1780 [Gearhart, 2002; Rösch and Ebert, 2007].
Embryology
Despite progress in the understanding of developmental mechanisms, the pathogenesis of BE remains unclear. During normal embryological development, separation of the primitive cloaca into the urogenital sinus and hindgut occurs at the same time as maturation of the anterior abdominal wall [Marshall and Muecke, 1962]. By the end of the sixth to seventh week of development, the infraumbilical mesenchyme migrates between the ectodermal and endodermal layers of the cloacal membrane, which is located at the caudal end of the embryo [Sadler, 2006]. It has been suggested that failure of mesenchyme to migrate fully leads to instability of the cloacal membrane [Mildenberger et al., 1988; Gearhart, 2002; Stevenson and Hall, 2006]. Premature rupture of the membrane before its caudal translocation leads to a complex of anomalies: the posterior wall of the bladder is exposed, as well as other structures derived from the infraumbilical mesenchyme. Rupture of the cloacal membrane after complete separation of the genitourinary and gastrointestinal tracts results in classical BE [Jones, 2006]. In recent articles, authors have suggested that BE is a milder manifestation of a later event in embryogenesis, in contrast to the more severe consequences of cloacal exstrophy resulting from an earlier event [Martínez-Frías et al., 2001; Gearhart, 2002]. A more detailed description of the normal development and possible pathogenetic mechanisms of bladder and cloacal exstrophy is given by Feldkamp et al. [2011] in this issue.
Etiology, Clinical Characteristics, and Clinical Genetics
BE is the most commonly identified congenital anomaly in the so-called epispadias–exstrophy complex. The etiology of BE is not well understood and there have been suggestions that BE is part of the spectrum with cloacal exstrophy [Hendren, 1998; Gearhart, 2002], while others argue that BE is a distinct defect [Carey, 2001], or a different expression of a primary developmental field defect [Martínez-Frías et al., 2001]. Outside the genitourinary system, association with other anomalies is relatively uncommon for BE, but can include omphalocele, anal defects, neural tube defects, and skeletal defects [Cadeddu et al., 1997; Martínez-Frías et al., 2001; Ebert et al., 2009]. BE is also part of the Omphalocele-bladder Exstrophy-Imperforate anus-Spinal defects (OEIS) complex [Carey et al., 1978; Källén et al., 2000; Carey, 2001]. The rare variant forms of BE include pseudoexstrophy, duplicate exstrophy, closed exstrophy, superior vesical fistula or fissure, inferior vesicle, penopubic epispadias, and balanic penile epispadias [Marshall and Muecke, 1962]. In cases with exstrophy variants, low lying umbilicus and umbilical and ventral hernias are frequently described.
Prenatal diagnosis can be made by ultrasound examinations and the following findings could suggest BE: absence of bladder filling, a low lying umbilicus, widening of the pubic rami, diminutive genitalia, and a lower abdominal mass [Gearhart, 2002; Evangelidis et al., 2004]. Maternal serum AFP is elevated due to the exposure of the bladder mucosa to the amniotic fluid [Stevenson and Hall, 2006]. Following prenatal diagnosis, appropriate genetic counseling and psychological support are important parts of pregnancy management [Gearhart, 2002; Ebert et al., 2009].
Whether environmental factors play a role in the etiology of BE is unknown. Suggested risk factors [Ludwig et al., 2009] include maternal smoking [Gambhir et al., 2008]; alcohol consumption [Pinette et al., 1996; Robin et al., 1996]; exposure to drugs and medications during pregnancy such as misoprostol, heparin, valproic acid, diazepam [Lizcano-Gil et al., 1995; Orioli and Castilla, 2000; Wakefield et al., 2002; Keppler-Noreuil et al., 2007]; rubella infection [Jordan et al., 1968]; and in vitro fertilization [Wood et al., 2007]. However, a Hungarian study did not find an association between the use of very large doses of diazepam by pregnant women for suicide attempt and congenital anomalies [Gidai et al., 2008].
Most cases of BE are sporadic. However, there is some evidence to suggest that genetic factors may play a role. The risk of recurrence of BE in a given family is approximately 1% [Ives et al., 1980; Jones, 2006] with recurrence of some variant forms ranging from 0.3% to 2.3% [Shapiro et al., 1984; Messelink et al., 1994; Reutter et al., 2003; Boyadjiev et al., 2004]. It has been suggested that in a few families the exstrophy–epispadias complex may follow Mendelian inheritance [Reutter et al., 2003; Ludwig et al., 2009]. Twin studies have indicated a much higher rate of the exstrophy–epispadias complex among monozygotic twins compared with dizygotic twins [Reutter et al., 2007b]. One study reported a one in 70 chance that a parent with BE will have a child with the same malformation [Shapiro et al., 1984]. There have been a few cytogenetic and molecular genetic studies which indicated that chromosome structural anomalies [Boyadjiev et al., 2004; Thauvin-Robinet et al., 2004; Ludwig et al., 2009] and some mutations may be more common in patients with exstrophy–epispadias complex [Nye et al., 2000; Reutter et al., 2006, 2007b], but no specific gene has been identified to date. Based on previous studies, it has been suggested that BE may have a polygenic multifactorial mode of inheritance [Reutter et al., 2007a,b], and developmental errors such as somatic mutations play a role in the formation of the anomaly [Boyadjiev et al., 2004].
Descriptive Epidemiology From Published Literature
BE occurs in approximately 1:30,000–50,000 live births [Stevenson and Hall, 2006], and is more likely to occur in males (Table I). There appears to be geographical variation in the prevalence of BE. Rickham [1961] reviewed hospital data in the Liverpool Region in the United Kingdom, and found a prevalence of 2.5 and 10.0 per 100,000 live births for years 1941–1953 and 1954–1960, respectively. In an international report, the total prevalence was 3.3 per 100,000 births, and ranged from 2.1 per 100,000 births in France to 4.7 per 100,000 births in Denmark [ICBDMS, 1987]. In a study from Spain the prevalence of BE was 2.8 per 100,000 births for years 1976–1999 [Martínez-Frías et al., 2001]. Based on the most recent data available on the EUROCAT website, the total prevalence of BE and/or epispadias among European member registries is 5.5 per 100,000 births for years 2000–2009 [EUROCAT, 2011], with a wide range of variation in prevalence from 0.0 to 25.6. These differences could be attributable to variations in sample size, registration, and inclusion of epispadias in this category. In the United States, one study found higher prevalence among newborns in the Northeast, South, and Midwest region (2.15–2.47 per 100,000) compared with the Western part of the country (1.37 per 100,000), with an overall prevalence of 2.15 per 100,000 [Nelson et al., 2005]. A study of national rates of congenital anomalies among hospitalized newborns in the United States reported a prevalence of 3.2 per 100,000 births using hospital discharge databases with approximately 4 million live births [Bird et al., 2006]. Using data from the New York State Congenital Malformation Registry, Caton et al. [2007] described a downward trend by year between 1983 and 1999, with an overall prevalence of 2.1 per 100,000 live births. Among Native Americans a very high prevalence, 8.0 per 100,000 births has been reported [James et al., 1994].
TABLE I.
Prevalence and Sex Ratio of Cases With Bladder Exstrophy in Published Studies
| Refs. | Study period | Number of cases | Population | Prevalence per 100,000 births | Male-to-female ratio |
|---|---|---|---|---|---|
| Rickham [1961] | 1941–1953 1954–1960 |
16 28 |
Live births | 2.5 10.0 |
Not reported |
| Caton et al. [2007] | 1983–1999 | 95 | Live births | 2.1 | 1.75:1a |
| ICBDMS [1987] | 1967–1985 | 208 | All births | 3.3 | 1.5:1 |
| Martínez-Frías et al. [2001] | 1976–1999 | 45 | All births | 2.8 | 1.32:1 |
| Nelson et al. [2005] | 1988–2000 | 205b | Live births | 2.15 | 1:1 |
| Bird et al. [2006] | 1997–2001 | Not reported | Live births | 3.2 | Not reported |
Among isolated/sequence BE cases.
Cloacal exstrophy cases may be included.
The male-to-female ratio was 1.5:1 in an international study [ICBDMS, 1987], but much higher male-to-female ratios of 2.3:1 to 6.0:1 have been reported by other investigators [Higgins, 1962; Lattimer and Smith, 1966; Ives et al., 1980; Shapiro et al., 1984; Grady et al., 1999]. However, in two other studies no significant male preponderance was found [Yang et al., 1994; Nelson et al., 2005]. An earlier study reported that the variant forms of BE are more common among females than males in contrast to the higher prevalence among males seen in classical BE [Marshall and Muecke, 1962].
In a recent study using data from the New York State Congenital Malformations Registry, investigators suggested summer conception, non-Hispanic white race/ethnicity, and male infant sex as possible risk factors for BE [Caton et al., 2007]. Nelson et al. [2005] found some significant associations with other factors: mothers of Black, Hispanic, and other race/ethnicity had lower risk of having a child with BE compared with White mothers; the risk was higher among mothers with government or private insurance coverage versus self pay; and the risk was higher among mothers with high versus low socioeconomic status. Other associations/potential risk factors that have been suggested include young maternal age and high parity [ICBDMS, 1987]. A lower birth weight distribution for infants with BE and multiple congenital anomalies (MCA) compared with infants with isolated BE was also described in the same study [ICBDMS, 1987]. Although, some studies examined the occurrence of associated defects among cases with BE [ICBDMS, 1987; Martínez-Frías et al., 2001; Nelson et al., 2005], there has been no report to date on demographic or clinical characteristics among BE cases by isolated and MCA status.
Prognosis, Treatment, Survival, Long-Term Health, and Quality of Life
The prognosis for BE has significantly improved in recent decades. BE is a life-threatening condition; therefore, surgical intervention is required. The complexity of the reconstructive surgery depends on the extent of the malformation. The best results with BE have been achieved with staged reconstruction, a series of surgeries that take place over a number of years [Duffy, 1996; Baird et al., 2007]. Bladder and pelvic closure are carried out in the newborn period. Epispadias repair in the male occurs during the first years of life, and an operation to correct urine flow and improve continence is carried out between ages 3 and 6. Some infants will be candidates for newborn closure with epispadias repair at the same time. Vesicoureteric reflux is very common among infants after bladder closure [Jeffs, 1987]. With appropriate management from the newborn period, the child has a much greater likelihood of having a functional urinary tract, a much improved psychosocial development, and an excellent quality of life [Ben-Chaim et al., 1996a b; Ebert et al., 2005, 2008, 2010a b; Catti et al., 2006]. There have been some reports of an increased risk for malignant tumors of the urinary tract (bladder and kidney) among patients with BE between their third and fifth decades [Kandzari et al., 1974; Smeulders and Woodhouse, 2001], suggesting the need for a careful long-term follow-up of these patients.
METHODS FOR CURRENT ANALYSIS
We used data from 22 participating member programs of the ICBDSR. Surveillance programs were asked to provide anonymous case data following a common protocol, with information on phenotype, genetic testing, and selected demographic and prenatal information. The collected data were reviewed by three authors (CS, MF, PM), and often required consultation with participating program directors to clarify cases for inclusion in the study. We reviewed diagnosis codes and clinical descriptions (when available) to classify BE cases as isolated/sequence, multiple, or syndrome. In some cases additional information was requested from member programs of ICBDSR to clarify the diagnosis. BE cases without an additional major defect, or with only related urogenital malformations were classified as isolated/sequence. Cases with recognized syndromes and chromosomal syndromes were classified as syndromes. The remaining cases were classified as MCA. Cases with BE, for which the defect was part of OEIS complex, were considered cloacal exstrophy cases, and therefore, excluded from this analysis.
We calculated the prevalence for each birth defects surveillance program (live births +stillbirths +elective terminations of pregnancy for fetal anomalies (ETOPFA) cases / live births + stillbirths) and 95% confidence intervals (CI) according to the Poisson distribution. The total prevalence was computed by summing up all the cases in each surveillance program and dividing these for all the births of the participating surveillance programs. We also calculated the frequency of various maternal and case characteristics (sex, outcome, birth weight, gestational age, parity, previous spontaneous abortions, plurality, maternal age, and maternal education) among isolated and MCA cases. We estimated the crude prevalence and prevalence ratios (PR) for maternal age groups overall, and stratified by isolated and MCA status. We used the chi-square test for trend to analyze temporal trends. To compare the selected clinical and demographic characteristics of BE cases with MCA with those of isolated BE cases, we calculated crude and adjusted odds ratios (OR) and the 95% CIs. Adjustments were made for tertiles of percentage of MCA cases to account for possible differences in the proportion of MCA cases. Statistical analyses were done with Stata software, version 10.0 [StataCorp, 2007]. A more detailed description of the data collection method and variables for cases and denominator data, as well as statistical analysis and adjustments is provided in the introductory paper [Castilla and Mastroiacovo, 2011].
RESULTS
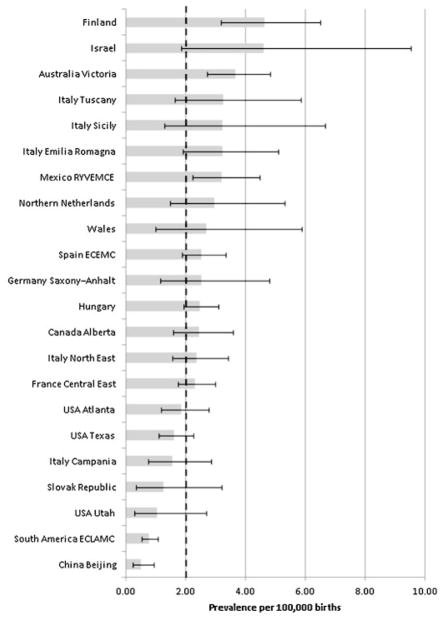
Among 26,355,094 births we identified 546 cases with BE. Five surveillance programs (Australia Victoria, France Central East, Hungary, South America ECLAMC, and Spain ECEMC) contributed approximately 50% of the cases. The estimated total prevalence of BE was 2.07 per 100,000 births (95% CI: 1.90–2.25) and varied between 0.52 and 4.63 among surveillance programs participating in the study (Table II). China Beijing and South America ECLAMC had the lowest prevalence, while Australia Victoria, Finland, and Mexico had the highest prevalence with 95% CIs that did not include the overall prevalence (2.07 per 100,000 births) for all surveillance programs (Table II and Fig. 1). Among cases with BE, the overall percentage of ETOPFA was 4.2, or 5.5% (n = 23/415) when restricting only to the 18 surveillance programs that registered ETOPFA cases.
TABLE II.
Total Prevalence of Bladder Exstrophy in 22 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| Surveillance program | Period | Births | Total number of cases | % of total cases that were ETOPFA | Prevalence per 100,000 births | 95% CI |
|---|---|---|---|---|---|---|
| Canada Alberta | 1980–2005 | 1,062,483 | 26 | 0 | 2.45 | 1.60–3.59 |
| USA Utah | 1997–2004 | 380,706 | 4 | 0 | 1.05 | 0.29–2.69 |
| USA Atlanta | 1968–2004 | 1,283,999 | 24 | 0 | 1.87 | 1.20–2.78 |
| USA Texas | 1996–2002 | 2,054,788 | 33 | 0 | 1.61 | 1.11–2.26 |
| Mexico RYVEMCE | 1978–2005 | 1,058,885 | 34 | NP | 3.21 | 2.22–4.49 |
| South America ECLAMC | 1982–2006 | 4,556,173 | 35 | NP | 0.77 | 0.54–1.07 |
| Finland | 1993–2004 | 713,494 | 33 | 6.1 | 4.63 | 3.18–6.50 |
| Wales | 1998–2004 | 222,309 | 6 | 0 | 2.70 | 0.99–5.87 |
| Northern Netherlands | 1981–2003 | 369,658 | 11 | 0 | 2.98 | 1.49–5.32 |
| Germany Saxony–Anhalt | 1980–2004 | 355,184 | 9 | 33.3 | 2.53 | 1.16–4.81 |
| Slovak Republic | 2000–2005 | 318,257 | 4 | 0 | 1.26 | 0.34–3.22 |
| Hungary | 1980–2005 | 3,022,194 | 75 | 0 | 2.48 | 1.95–3.11 |
| France Central East | 1979–2004 | 2,500,214 | 58 | 20.7 | 2.32 | 1.76–3.00 |
| Italy North East | 1981–2004 | 1,186,497 | 28 | 3.6 | 2.36 | 1.57–3.41 |
| Italy Emilia Romagna | 1982–2004 | 558,176 | 18 | 0 | 3.22 | 1.91–5.10 |
| Italy Tuscany | 1992–2004 | 336,744 | 11 | 18.2 | 3.27 | 1.63–5.84 |
| Italy Campania | 1992–2004 | 643,962 | 10 | 0 | 1.55 | 0.74–2.86 |
| Italy Sicily | 1991–2002 | 216,257 | 7 | 0 | 3.24 | 1.30–6.67 |
| Spain ECEMC | 1980–2004 | 2,045,751 | 52 | NR | 2.54 | 1.90–3.33 |
| Israel | 1975–2005 | 151,562 | 7 | 14.3 | 4.62 | 1.86–9.52 |
| China Beijing | 1992–2005 | 1,927,622 | 10 | NR | 0.52 | 0.25–0.95 |
| Australia Victoria | 1983–2004 | 1,390,179 | 51 | 3.9 | 3.67 | 2.73–4.82 |
| Total | 26,355,094 | 546 | 4.2a | 2.07 | 1.90–2.25 |
ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino Americano de Malformaciones Congénitas; RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas; ETOPFA, elective termination of pregnancy for fetal anomaly; CI, confidence interval; NP, not permitted; NR, not reported.
The percentage computed on the 18 surveillance programs registering ETOPFA is 5.5% (n = 23/415).
Figure 1.
Total prevalence of bladder exstrophy per 100,000 births (bar) and 95% confidence interval (line) by surveillance program, and overall prevalence (dotted line), in 22 surveillance programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
After excluding nine syndromic cases (three Edwards syndrome, two Klinefelter syndrome, two CHARGE syndrome, and two pentalogy of Cantrell), Table III shows the distribution of nonsyndromic cases (n = 537) by maternal and case characteristics and by clinical phenotype (i.e., isolated and MCA cases). The majority of cases (71%) had no additional major defects. Among cases with MCA (n = 156) the most frequent major unrelated malformations we identified (data not shown) were the following: 53 omphalocele (34%), 33 anal defects (21%), 28 neural tube defects (18%), 26 renal defects (17%), and 23 cardiac defects (15%).
TABLE III.
Characteristics of Nonsyndromic Cases With Bladder Exstrophy (BE) Reported by 22 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| All cases (n = 537)
|
Cases with isolated BE (n = 381)
|
Cases with BE and MCA (n = 156)
|
||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Sex | ||||||
| Male | 320 | 59.6 | 257 | 67.5 | 63 | 40.4 |
| Female | 173 | 32.2 | 123 | 32.3 | 50 | 32.1 |
| Indeterminate | 41 | 7.6 | 0 | 0.0 | 41 | 26.3 |
| Missing data | 3 | 0.6 | 1 | 0.3 | 2 | 1.3 |
| Outcome | ||||||
| Live births | 494 | 92.0 | 371 | 97.4 | 123 | 78.8 |
| Stillbirths | 20 | 3.7 | 4 | 1.0 | 16 | 10.3 |
| ETOPFA | 21 | 3.9 | 4 | 1.0 | 17 | 10.9 |
| Missing data | 2 | 0.4 | 2 | 0.5 | 0 | 0.0 |
| Birth weight among live births (g) | ||||||
| <1,500 | 9 | 1.8 | 2 | 0.5 | 7 | 5.7 |
| 1,500–2,499 | 59 | 11.9 | 28 | 7.5 | 31 | 25.2 |
| >2,500 | 412 | 83.4 | 330 | 88.9 | 82 | 66.7 |
| Missing data | 14 | 2.8 | 11 | 3.0 | 3 | 2.4 |
| Gestational age among live births (weeks) | ||||||
| <32 | 12 | 2.4 | 4 | 1.1 | 8 | 6.5 |
| 32–36 | 57 | 11.5 | 26 | 7.0 | 31 | 25.2 |
| ≥37 | 385 | 77.9 | 304 | 81.9 | 81 | 65.9 |
| Missing data | 40 | 8.1 | 37 | 10.0 | 3 | 2.4 |
| Parity | ||||||
| 0 | 102 | 19.0 | 76 | 19.9 | 26 | 16.7 |
| 1 | 141 | 26.3 | 93 | 24.4 | 48 | 30.8 |
| ≥2 | 104 | 19.4 | 67 | 17.6 | 37 | 23.7 |
| Missing data | 190 | 35.4 | 145 | 38.1 | 45 | 28.8 |
| Previous spontaneous abortions | ||||||
| 0 | 184 | 34.3 | 130 | 34.1 | 54 | 34.6 |
| ≥1 | 35 | 6.5 | 20 | 5.2 | 15 | 9.6 |
| Missing data | 318 | 59.2 | 231 | 60.6 | 87 | 55.8 |
| Plurality | ||||||
| Single | 489 | 91.1 | 347 | 91.1 | 142 | 91.0 |
| Twin | 16 | 3.0 | 8 | 2.1 | 8 | 5.1 |
| Triplet | 1 | 0.2 | 1 | 0.3 | 0 | 0.0 |
| Missing data | 31 | 5.8 | 25 | 6.6 | 6 | 3.8 |
| Maternal education (years) | ||||||
| <9 | 57 | 10.6 | 32 | 8.4 | 25 | 16.0 |
| ≥9 | 103 | 19.2 | 77 | 20.2 | 26 | 16.7 |
| Missing data | 377 | 70.2 | 272 | 71.4 | 105 | 67.3 |
ETOPFA, elective termination of pregnancy for fetal anomaly.
Syndromic cases (n = 9) were excluded from analysis.
Among all nonsyndromic cases with BE, 41 cases had indeterminate sex, while for 3 cases the sex was not reported. The male-to-female sex ratio among cases with known sex was 1.85:1 (P <0.01). The ratio was higher (2.09:1) among isolated cases compared with cases with MCA (1.26:1), and this difference was statistically significant (P = 0.02). The majority of nonsyndromic cases were live births (92.0%), had normal birth weight (83.4% of live births), and were born at term (77.9% of live births).
The analysis of the association of the main maternal and case characteristics of MCA cases compared with isolated BE cases (Table IV) suggests that female cases were more frequent among MCA cases (aOR = 1.56; 95% CI: 1.00–2.44). Among MCA cases, the frequency of stillbirths (aOR = 24.63; 95% CI: 5.22–116.20) and ETOPFA (aOR = 18.24; 95% CI: 5.74–57.93) was significantly higher than among isolated cases. Compared with isolated BE cases, cases with MCA were more likely to be of low birth weight (<1,500 g: aOR = 7.03; 95% CI: 1.37–36.09; 1,500–2,499 g: aOR = 4.34; 95% CI: 2.37–7.95), preterm (<32 weeks: aOR = 4.36; 95% CI: 1.20–15.80; 32–36 weeks: aOR = 4.00; 95% CI: 2.16–7.42), and have a mother with less than 9 years of education (aOR = 2.85; 95% CI: 1.12–7.29).
TABLE IV.
Crude and Adjusted Odds Ratios (OR) With 95% Confidence Intervals (95% CI) for the Association of Various Characteristics Among Multiple Congenital Anomalies Cases (Cases) Versus Isolated Cases (Controls) of Bladder Exstrophy Reported by 22 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| Crude OR | 95% CI | Adjusteda OR | 95% CI | |||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.66 | 1.08 | 2.55 | 1.56 | 1.00 | 2.44 |
| Outcomeb | ||||||
| Live births | 1.00 | 1.00 | ||||
| Stillbirths | 23.21 | 5.09 | 105.92 | 24.63 | 5.22 | 116.20 |
| ETOPFA | 16.44 | 5.37 | 50.29 | 18.24 | 5.74 | 57.93 |
| Birth weight among live births (g) | ||||||
| <1,500 | 14.08 | 2.87 | 69.07 | 7.03 | 1.37 | 36.09 |
| 1,500–2,499 | 4.45 | 2.53 | 7.84 | 4.34 | 2.37 | 7.95 |
| ≥2,500 | 1.00 | 1.00 | ||||
| Gestational age among live births (weeks) | ||||||
| <32 | 7.51 | 2.20 | 25.55 | 4.36 | 1.20 | 15.80 |
| 32–36 | 4.47 | 2.52 | 7.96 | 4.00 | 2.16 | 7.42 |
| ≥37 | 1.00 | 1.00 | ||||
| Parity | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.75 | 0.94 | 3.27 | 1.30 | 0.66 | 2.54 |
| ≥2 | 1.78 | 0.93 | 3.41 | 1.61 | 0.80 | 3.25 |
| Previous spontaneous abortions | ||||||
| 0 | 1.00 | 1.00 | ||||
| ≥1 | 1.65 | 0.78 | 3.51 | 1.83 | 0.80 | 4.20 |
| Plurality | ||||||
| Single | 1.00 | 1.00 | ||||
| Twin | 2.44 | 0.90 | 6.64 | 2.35 | 0.81 | 6.84 |
| Maternal education (years) | ||||||
| <9 | 2.46 | 1.06 | 5.74 | 2.85 | 1.12 | 7.29 |
| ≥9 | 1.00 | 1.00 | ||||
ETOPFA, elective termination of pregnancy for fetal anomalies.
Surveillance programs with more than 20% missing data were excluded from the analysis.
Adjustments were made for tertiles of percentage of MCA cases in each program.
OR computed only for the 18 programs reporting ETOPFA.
The prevalence of BE increased with maternal age, from 1.52 per 100,000 births in age group <20 years to 2.69 per 100,000 births in age group ≥40 years (data not shown). Figure 2 shows the PR for various maternal age groups relative to the reference age group of <20 years. PRs showed a significant (P <0.01) increase in prevalence by maternal age group, with the highest prevalence rates in the age groups of 35–39 years (PR = 1.76; 95% CI: 1.16–2.67) and ≥40 years (PR = 1.76; 95% CI: 0.92–3.39). When PRs were analyzed by presence of MCA, the increase in PRs remained significant among isolated cases [with the highest prevalence in the maternal age group of 35–39 years (PR = 2.85; 95% CI: 1.60–5.08)], but not among MCA cases (Fig. 3).
Figure 2.
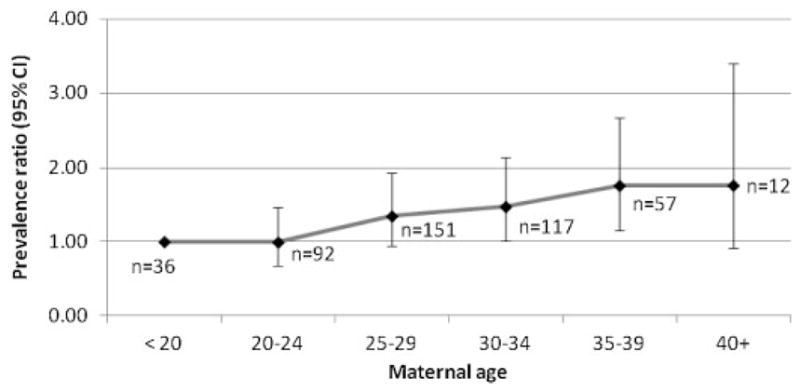
Prevalence ratios for maternal age groups relative to the reference age of <20 years with corresponding 95% CIs for bladder exstrophy in 22 surveillance programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Chi square for trend = 16.23; P <0.01.
Figure 3.
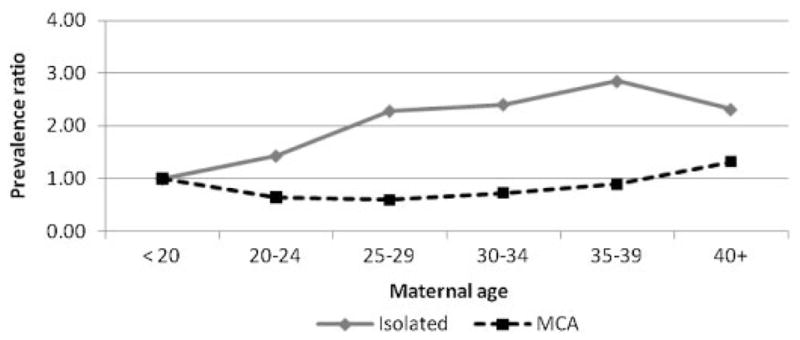
Prevalence ratios for maternal age groups by isolated and multiple congenital anomalies (MCA) cases relative to the reference age of <20 years with corresponding 95% CIs for bladder exstrophy in 22 surveillance program members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Isolated cases: Chi-square for trend = 20.77; P <0.01. MCA cases: Chi-square for trend = 0.18; P = 0.67.
DISCUSSION
Using the largest international dataset of BE assembled to date, the total prevalence is estimated to be 2.07 per 100,000 births (95% CI: 1.90–2.25). This is in good agreement with prevalence estimates previously reported (Table I); however, those earlier figures were based on smaller numbers of cases, and most of them included live births only. The variation in total prevalence by surveillance program in our study could reflect differences in sample size, reporting, and registration. The lower prevalence of BE in some of the surveillance programs of ICBDSR (e.g., China Beijing) may be attributable to ETOPFA cases not being reported in such programs. It is interesting to note that several surveillance programs had no BE cases in the ETOPFA group. This could be explained by differences in clinical practice, prenatal diagnosis, and pregnancy management, or differences in the frequency of associated major defects by surveillance program. The percentage of ETOPFA may also be affected by the gestational age limit specified in the abortion law of the specific country. In a series of cases with exstrophy–epispadias complex obtained from the Malformations Surveillance Program at Brigham and Women’s Hospital in Boston, USA, 25% of pregnancies suspected to have this defect were electively terminated following prenatal diagnosis [Cromie et al., 2001]. In a recent study in the United Kingdom, investigators found that among women with antenatal suspicion of bladder or cloacal exstrophy, 31% of them opted for pregnancy termination [Goyal et al., 2011]. It can be hypothesized that cases with other associated major defects are probably terminated more often than isolated cases with BE. This latter possibility is consistent with our findings; the proportion of ETOPFA was much higher (10.9%) among MCA cases than among isolated cases (1.0%).
As suggested by several earlier studies [Higgins, 1962; Lattimer and Smith, 1966; Ives et al., 1980; Shapiro et al., 1984; ICBDMS, 1987; Grady et al., 1999], we also found a higher male-to-female ratio (1.85:1), with this ratio being higher (2.01:1) among isolated cases with BE. It is important to point out that not all studies found male preponderance for BE [Yang et al., 1994; Nelson et al., 2005], which could reflect differences in the classification of cases with BE between studies. We can conclude that the predominance of male cases, especially among isolated cases with BE, is most likely a real biological phenomenon, suggesting perhaps an underlying genetic mechanism.
Besides the expected higher rate of associated genital and renal anomalies among MCA cases with BE, we observed a higher rate of omphalocele (34%), anal defects (21%), neural tube defects (18%), and cardiac defects (15%), which is consistent with similar findings by previous investigators [Cadeddu et al., 1997; Martínez-Frías et al., 2001; Ebert et al., 2009]. It is worth noting that aneuploidy of sex chromosomes has been reported in association with BE [Ludwig et al., 2009]. We also found two cases with Klinefelter syndrome (47,XXY) in our study.
Compared with isolated cases, MCA cases had a marginally higher prevalence of females, which may be due to the increased proportion of indeterminate sex reflecting some misclassification of males as having indeterminate sex among MCA cases. Low birth weight and prematurity are frequently present among cases of BE with MCA; such cases are also are more likely to result in stillbirths and pregnancy terminations than among isolated cases [Stoll et al., 2002; Bourke et al., 2005; Fretts, 2005; Garne et al., 2005; Lewis et al., 2010]. Our findings of higher odds of stillbirths, ETOPFA, low birth weight, and preterm birth among MCA cases are consistent with these earlier observations. The higher prevalence of BE among infants with MCA whose mother had less than 9 years of education is a new finding and may simply reflect a wide variation in cultures and populations, or possibly suggests that factors associated with education and socioeconomic status could be explored as potential risk factors for BE. Only one previous study on BE conducted in the US examined maternal education as a possible risk factor, but found no association [Caton et al., 2007]. In the study by Nelson et al. [2005], higher socioeconomic status (based on median ZIP code income) was associated with a higher prevalence of BE.
Our analysis by maternal age group revealed a significantly higher prevalence with increasing maternal age. The stratified analysis by presence of MCA suggests that this higher prevalence is observable only among isolated cases with BE. In a previous study, investigators did not find an association with maternal age [Caton et al., 2007]. The reference group for maternal age used in that study was 20–34 years; the PR was lower in the age group <20 years, and higher in the age group ≥35 years, both in the crude and adjusted analyses but did not reach a level of statistical significance. In another study [ICBDMS, 1987], investigators found an excess of cases among young mothers (<20 years). In both of these studies, the sample size was much smaller and may explain the lack of association with maternal age. The higher prevalence among older mothers in our study suggest that some genetic and/or environmental factors (i.e., higher in vitro fertilization rate among older women) may play a role in the formation of isolated BE [Wood et al., 2007] and warrants further investigation.
In a previous international study, investigators found an increased risk for mothers with high parity [ICBDMS, 1987]. In our study we could not confirm this finding. When we compared MCA cases with isolated cases with BE, the adjusted OR was higher among multiparous compared with nulliparous mothers, but the CI included 1.0. This nonsignificant association with parity could reflect a small sample size and/or bias because of the high proportion of missing data (~35%) for this variable.
The strengths of our study include the largest sample of cases of BE examined to date in one study, a diverse population from 22 different surveillance programs representing several countries in the world, and analysis of prevalence by various maternal and case characteristics. A further strength of the study is the centralized and standardized classification of cases with associated anomalies by experts in the field. It is important to recognize the need for careful review and assessment of cases with BE in future studies instead of analyzing the data based only on congenital anomaly codes, which may include cases with cloacal exstrophy, and may consequently yield higher prevalence estimates. One of the limitations of the present study is the large proportion of missing data for some of the variables such as previous spontaneous abortion and maternal education. In addition, we were not able to analyze some of the factors that have been suggested to be associated with BE in previous studies such as race/ethnicity, season of birth, socioeconomic status, and presence and type of medical insurance.
CONCLUSIONS
BE is a very rare congenital anomaly with an occurrence of approximately 1 in 50,000 births. Much of the geographical variation in prevalence is most likely attributable to differences in registration of cases. The higher prevalence among male cases and older mothers, especially among isolated cases are important factors to note for clinicians when assessing risk, and to include in future epidemiologic studies.
Acknowledgments
The authors are grateful to each surveillance program’s staff and members for their work in collecting case data and submission to the ICBDSR Centre. This work was in part supported by Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain, and the Fundación 1000 sobre Defectos Congénitos, of Spain. CIBERER is an initiative of ISCIII. Components of ECEMC’s Peripheral Group are gratefully acknowledged. The work conducted at the ICBDSR Centre was supported by the Centers for Disease Control and Prevention (1U50DD000524-02). Grant sponsor for South America ECLAMC: MCT/CNPq, Brazil; Grant numbers: 573993/2008-4, 476978/2008-4, 554755/2009-2; 306750/2009-0; 402045/2010-6. The Tuscany Registry of Birth Defects is funded by the “Direzione Generale Diritti di cittadinanza e Coesione sociale—Regione Toscana.”
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Ballantyne JW. The teratological records of Chaldea. Teratologia. 1894;1:132. [Google Scholar]
- Ballantyne JW. The embryo. Edinburgh: William Green & Sons; 1904. Manual of antenatal pathology and hygiene. [Google Scholar]
- Baird AD, Nelson CP, Gearhart JP. Modern staged repair of bladder exstrophy: A contemporary series. J Pediatr Urol. 2007;3:311–315. doi: 10.1016/j.jpurol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Ben-Chaim J, Docimo SG, Jeffs RD, Gearhart JP. Bladder exstrophy from childhood into adult life. J R Soc Med. 1996a;89:39–46. doi: 10.1177/014107689608900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chaim J, Jeffs RD, Reiner WG, Gearhart JP. The outcome of patients with classic bladder exstrophy in adult life. J Urol. 1996b;155:1251–1252. [PubMed] [Google Scholar]
- Bird TM, Hobbs CA, Cleves MA, Tilford JM, Robbins JM. National rates of birth defects among hospitalized newborns. Birth Defects Res A Clin Mol Teratol. 2006;76(11):762–769. doi: 10.1002/bdra.20323. [DOI] [PubMed] [Google Scholar]
- Bourke J, Bower C, Blair E, Charles A, Knuiman M. The effect of terminations of pregnancy for fetal abnormalities on trends in mortality to one year of age in Western Australia. Paediatr Perinat Epidemiol. 2005;19(4):284–293. doi: 10.1111/j.1365-3016.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Dodson JL, Radford CL, Ashrafi GH, Beaty TH, Mathews RI, Broman KW, Gearhart JP. Clinical and molecular characterization of the bladder exstrophy-epispadias complex: Analysis of 232 families. BJU Int. 2004;94(9):1337–1343. doi: 10.1111/j.1464-410X.2004.05170.x. [DOI] [PubMed] [Google Scholar]
- Catti M, Paccalin C, Rudigoz RC, Mouricand P. Quality of life for adult women born with bladder and cloacal exstrophy: A long-term follow up. J Pediatr Urol. 2006;2:16–22. doi: 10.1016/j.jpurol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Cadeddu JA, Benson JE, Silver RI, Lakshmanan Y, Jeffs RD, Gearhart JP. Spinal abnormalities in classic bladder exstrophy. Br J Urol. 1997;79(6):975–978. doi: 10.1046/j.1464-410x.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- Carey JC, Greenbaum B, Hall BD. The OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) Birth Defects Orig Artic Ser XIV. 1978:253–268. [PubMed] [Google Scholar]
- Carey JC. Exstrophy of the cloaca and the OEIS complex: One and the same. Am J Med Genet. 2001;99(4):270. doi: 10.1002/ajmg.1211. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo P. Very rare defects: What can we learn? Am J Med Genet Part C. 2011;XXXC doi: 10.1002/ajmg.c.30315. [DOI] [PubMed] [Google Scholar]
- Caton AR, Bloom A, Druschel CM, Kirby RS. Epidemiology of bladder and cloacal exstrophies in New York State, 1983–1999. Birth Defects Res A Clin Mol Teratol. 2007;79(11):781–787. doi: 10.1002/bdra.20402. [DOI] [PubMed] [Google Scholar]
- Cromie WJ, Lee K, Houde K, Holmes L. Implications of prenatal ultrasound screening in the incidence of major genitourinary malformations. J Urol. 2001;165(5):1677–1680. [PubMed] [Google Scholar]
- Duffy PG. Bladder exstrophy. Semin Pediatr Surg. 1996;5(2):129–132. [PubMed] [Google Scholar]
- Ebert A, Scheuering S, Schott G, Roesch WH. Psychosocial and psychosexual development in childhood and adolescence within the exstrophy-epispadias complex. J Urol. 2005;174(3):1094–1098. doi: 10.1097/01.ju.0000169171.97538.ed. [DOI] [PubMed] [Google Scholar]
- Ebert AK, Bals-Pratsch M, Seifert B, Reutter H, Rösch WH. Genital and reproductive function in males after functional reconstruction of the exstrophy-epispadias complex—Long-term results. Urology. 2008;72(3):566–569. doi: 10.1016/j.urology.2007.11.166. discussion 569–570. [DOI] [PubMed] [Google Scholar]
- Ebert AK, Reutter H, Ludwig M, Rösch WH. The exstrophy-epispadias complex. Orphanet J Rare Dis. 2009;4:23. doi: 10.1186/1750-1172-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AK, Falkert A, Brandl R, Hirschfelder H, Koller M, Rösch WH. Pelvic-floor imaging using three-dimensional ultrasonography and magnetic resonance imaging in the long term follow-up of the bladder-exstrophy-epispadias complex. BJU Int. 2010a;105(2):248–253. doi: 10.1111/j.1464-410X.2009.08736.x. [DOI] [PubMed] [Google Scholar]
- Ebert AK, Schott G, Bals-Pratsch M, Seifert B, Rösch WH. Long-term follow-up of male patients after reconstruction of the bladder-exstrophy-epispadias complex: Psychosocial status, continence, renal and genital function. J Pediatr Urol. 2010b;6(1):6–10. doi: 10.1016/j.jpurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- EUROCAT. [Accessed on June 28, 2011];EUROCAT Website Database. 2011 http://www.eurocat-network.eu/ACCESSPREVALENCEDATA/Prevalence-Tables (data uploaded 12/04/2011)
- Evangelidis A, Murphy JP, Gatti JM. Prenatal diagnosis of bladder exstrophy by 3-dimensional ultrasound. J Urol. 2004;172:1111. doi: 10.1097/01.ju.0000135595.83972.6a. [DOI] [PubMed] [Google Scholar]
- Feldkamp ML, Botto LD, Krikov S, Amar E, Bakker MK, Bianca S, Canfield MA, Castilla EE, Clementi M, Siffel C, Csaky-Szunyogh M, Merlob P, Leoncini E, Li Z, Lowry RB, Bermejo-Sánchez E, Mastroiacovo P, Morgan M, Mutchinick OM, Rissmann A, Ritvanen A, Carey JC. Cloacal exstrophy: An epidemiologic study from the international clearinghouse of birth defects surveillance and research. Am J Med Genet Part C. 2011;XXXC doi: 10.1002/ajmg.c.30317. [DOI] [PubMed] [Google Scholar]
- Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193(6):1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- Gambhir L, Höller T, Müller M, Schott G, Vogt H, Detlefsen B, Ebert AK, Fisch M, Beaudoin S, Stein R, Boyadjiev SA, Gearhart JP, Rösch W, Utsch B, Boemers TM, Reutter H, Ludwig M. Epidemiological survey of 214 families with bladder exstrophy-epispadias complex. J Urol. 2008;179(4):1539–1543. doi: 10.1016/j.juro.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garne E, Loane M, Dolk H, De Vigan C, Scarano G, Tucker D, Stoll C, Gener B, Pierini A, Nelen V, Rösch C, Gillerot Y, Feijoo M, Tincheva R, Queisser-Luft A, Addor MC, Mosquera C, Gatt M, Barisic I. Prenatal diagnosis of severe structural congenital malformations in Europe. Ultrasound Obstet Gynecol. 2005;25(1):6–11. doi: 10.1002/uog.1784. [DOI] [PubMed] [Google Scholar]
- Gearhart JP. Exstrophy, epispadias, and other bladder anomalies. In: Walsh PC, Wein AJ, Vaughan ED, Retik AB, editors. Campbell’s urology. 8. Philadelphia: Saunders; 2002. pp. 2136–2196. [Google Scholar]
- Gidai J, Acs N, Bánhidy F, Czeizel AE. No association found between use of very large doses of diazepam by 112 pregnant women for a suicide attempt and congenital abnormalities in their offspring. Toxicol Ind Health. 2008;24(1–2):29–39. doi: 10.1177/0748233708089019. [DOI] [PubMed] [Google Scholar]
- Goyal A, Fishwick J, Hurrell R, Cervellione RM, Dickson AP. Antenatal diagnosis of bladder/cloacal exstrophy: Challenges and possible solutions. J Pediatr Urol. 2011 Jun 3; doi: 10.1016/j.jpurol.2011.05.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Grady RW, Carr MC, Mitchell ME. Complete primary closure of bladder exstrophy. Epispadias and bladder exstrophy repair. Urol Clin North Am. 1999;26:95–109. viii. doi: 10.1016/s0094-0143(99)80009-3. [DOI] [PubMed] [Google Scholar]
- Hendren WH. Cloaca, the most severe degree of imperforate anus: Experience with 195 cases. Ann Surg. 1998;228:331–346. doi: 10.1097/00000658-199809000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CC. Exstrophy of the bladder: Report of 158 cases. Am Surg. 1962;28:99. [PubMed] [Google Scholar]
- ICBDMS. Epidemiology of bladder exstrophy and epispadias: A communication from the International Clearinghouse for Birth Defects Monitoring Systems. Teratology. 1987;36(2):221–227. doi: 10.1002/tera.1420360210. [DOI] [PubMed] [Google Scholar]
- ICBDSR. Annual Report 2009 with data for 2007. The Centre of the International Clearinghouse for Birth Defects Surveillance and Research; Rome, Italy. 2009. [Google Scholar]
- Ives E, Coffey R, Carter CO. A family study of bladder exstrophy. J Med Genet. 1980;17(2):139–141. doi: 10.1136/jmg.17.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Erickson JD, McClearn AB. Prevalence of birth defects. In: Wilcox LS, Marks JS, editors. From data to action—CDC’s Public Health Surveillance for Women, Infants, and Children. Atlanta: Centers for Disease Control and Prevention; 1994. pp. 203–216. [Google Scholar]
- Jeffs RD. Exstrophy, epispadias, and cloacal and urogenital sinus abnormalities. Pediatr Clin North Am. 1987;34(5):1233–1257. doi: 10.1016/s0031-3955(16)36328-3. [DOI] [PubMed] [Google Scholar]
- Jones KL. Smith’s recognizable patterns of human malformations. Philadelphia: Elsevier Saunders; 2006. p. 626. [Google Scholar]
- Jordan M, Poole CA, Fogel BJ. Exstrophy of the bladder associated with congenital rubella syndrome. J Fla Med Assoc. 1968;55:98–99. [PubMed] [Google Scholar]
- Källén K, Castilla EE, Robert E, Mastroiacovo P, Källén B. OEIS complex—A population study. Am J Med Genet. 2000;92(1):62–68. doi: 10.1002/(sici)1096-8628(20000501)92:1<62::aid-ajmg11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kandzari SJ, Majid A, Orteza AM, Milam DF. Exstrophy of urinary bladder complicated by adenocarcinoma. Urology. 1974;3(4):496–498. doi: 10.1016/s0090-4295(74)80174-3. [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil K, Gorton S, Foo F, Yankowitz J, Keegan C. Prenatal ascertainment of OEIS complex/cloacal exstrophy—15 new cases and literature review. Am J Med Genet Part A. 2007;143A(18):2122–2128. doi: 10.1002/ajmg.a.31897. [DOI] [PubMed] [Google Scholar]
- Lattimer JK, Smith MJV. Exstrophy closure: A followup on 70 cases. J Urol. 1966;95:356. doi: 10.1016/S0022-5347(17)63460-8. [DOI] [PubMed] [Google Scholar]
- Lewis S, McGillivray G, Rowlands S, Halliday J. Perinatal outcome following suspected fetal abnormality when managed through a fetal management unit. Prenat Diagn. 2010;30(2):149–155. doi: 10.1002/pd.2431. [DOI] [PubMed] [Google Scholar]
- Lizcano-Gil LA, Garcia-Cruz D, Sanchez-Corona J. Omphalocele-exstrophy-imperforate-anus-spina bifida (OEIS) complex in a male prenatally exposed to diazepam. Arch Med Res. 1995;26:95–96. [PubMed] [Google Scholar]
- Ludwig M, Ching B, Reutter H, Boyadjiev SA. The bladder exstrophy-epispadias complex. Birth Defects Res Part A Clin Mol Teratol. 2009;85(6):509–522. doi: 10.1002/bdra.20557. [DOI] [PubMed] [Google Scholar]
- Marshall VF, Muecke EC. Variations in exstrophy of the bladder. J Urol. 1962;88:766. [Google Scholar]
- Martínez-Frías ML, Bermejo E, Rodriguez-Pinilla E, Frías JL. Exstrophy of the cloaca and exstrophy of the bladder: Two different expressions of a primary developmental field defect. Am J Med Genet. 2001;99(4):261–269. doi: 10.1002/ajmg.1210. [DOI] [PubMed] [Google Scholar]
- Messelink EJ, Aronson DC, Knuist M, Heij HA, Vos A. Four cases of bladder exstrophy in two families. J Med Genet. 1994;31(6):490–492. doi: 10.1136/jmg.31.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildenberger H, Kluth D, Dziuba M. Embryology of bladder exstrophy. J Pediatr Surg. 1988;23(2):166–170. doi: 10.1016/s0022-3468(88)80150-7. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Dunn RL, Wei JT. Contemporary epidemiology of bladder exstrophy in the United States. J Urol. 2005;173(5):1728–1731. doi: 10.1097/01.ju.0000154821.21521.9b. [DOI] [PubMed] [Google Scholar]
- Nye JS, Hayes EA, Amendola M, Vaughn D, Charrow J, McLone DG, Speer MC, Nance WE, Pandya A. Myelocystocelecloacal exstrophy in a pedigree with a mitochondrial 12S rRNA mutation, amino-glycoside induced deafness, pigmentary disturbances, and spinal anomalies. Teratology. 2000;61:165–171. doi: 10.1002/(SICI)1096-9926(200003)61:3<165::AID-TERA3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Oostra RJ, Baljet B, Verbeeten BW, Hennekam RC. Congenital anomalies in the teratological collection of Museum Vrolik in Amsterdam, The Netherlands. III: Primary field defects, sequences, and other complex anomalies. Am J Med Genet. 1998;80(1):46–59. doi: 10.1002/(sici)1096-8628(19981102)80:1<46::aid-ajmg8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Orioli IM, Castilla EE. Epidemiological assessment of misoprostol teratogenicity. BJOG. 2000;107(4):519–523. doi: 10.1111/j.1471-0528.2000.tb13272.x. [DOI] [PubMed] [Google Scholar]
- Pinette MG, Pan YQ, Pinette SG, Stubblefield PG, Blackstone J. Prenatal diagnosis of fetal bladder and cloacal exstrophy by ultrasound. A report of three cases. J Reprod Med. 1996;41(2):132–134. [PubMed] [Google Scholar]
- Reutter H, Shapiro E, Gruen JR. Seven new cases of familial isolated bladder exstrophy and epispadias complex (BEEC) and review of the literature. Am J Med Genet Part A. 2003;120A(2):215–221. doi: 10.1002/ajmg.a.20057. [DOI] [PubMed] [Google Scholar]
- Reutter H, Thauvin-Robinet C, Boemers TM, Rösch WH, Ludwig M. Bladder exstrophy-epispadias complex: Investigation of suppressor of variegation, enhancer of zeste and Thrithorax (SET) as a candidate gene in a large cohort of patients. Scand J Urol Nephrol. 2006;40:221–224. doi: 10.1080/00365590600621204. [DOI] [PubMed] [Google Scholar]
- Reutter H, Hoischen A, Ludwig M, Stein R, Radlwimmer B, Engels H, Wolffenbuttel KP, Weber RG. Genome-wide analysis for micro-aberrations in familial exstrophy of the bladder using array-based comparative genome hybridization. BJU Int. 2007a;100:646–650. doi: 10.1111/j.1464-410X.2007.07086.x. [DOI] [PubMed] [Google Scholar]
- Reutter H, Qi L, Gearhart JP, Boemers T, Ebert AK, Rösch WH, Ludwig M, Boyadjiev SA. Concordance analyses of twins with bladder exstrophy-epispadias complex suggest genetic etiology. Am J Med Genet Part A. 2007b;143A(22):2751–2756. doi: 10.1002/ajmg.a.31975. [DOI] [PubMed] [Google Scholar]
- Rickham PP. The incidence and treatment of ectopia vesicae. Proc R Soc Med. 1961;54:389–392. doi: 10.1177/003591576105400510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin NH, Neidich JA, Bason LD, Whitaker LA, McDonald-McGinn D, Hunter J, Snyder HM, III, Zackai EH. Frontonasal malformation and cloacal exstrophy: A previously unreported association. Am J Med Genet. 1996;61(1):75–78. doi: 10.1002/(SICI)1096-8628(19960102)61:1<75::AID-AJMG15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rösch WH, Ebert AK. Development of treatment for exstrophy-epispadias in Germany. Urologe A. 2007;46(12):1691–1696. doi: 10.1007/s00120-007-1573-5. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman’s medical embryology. 10. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- Shapiro E, Lepor H, Jeffs RD. The inheritance of the exstrophy-epispadias complex. J Urol. 1984;32(2):308–310. doi: 10.1016/s0022-5347(17)49605-4. [DOI] [PubMed] [Google Scholar]
- Smeulders N, Woodhouse CR. Neoplasia in adult exstrophy patients. BJU Int. 2001;87(7):623–628. doi: 10.1046/j.1464-410x.2001.02136.x. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Stevenson RE, Hall JG. Human malformations and related anomalies. 2. New York: Oxford University Press; 2006. pp. 1232–1234. [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. Impact of prenatal diagnosis on livebirth prevalence of children with congenital anomalies. Ann Genet. 2002;45(3):115–121. doi: 10.1016/s0003-3995(02)01130-9. [DOI] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Faivre L, Cusin V, Khau Van Kien P, Callier P, Parker KL, Fellous M, Borgnon J, Gounot E, Huet F, Sapin E, Mugneret F. Cloacal exstrophy in an infant with 9q34.1-qter deletion resulting from a de novo unbalanced translocation between chromosome 9q and Yq. Am J Med Genet Part A. 2004;126A:303–307. doi: 10.1002/ajmg.a.20596. [DOI] [PubMed] [Google Scholar]
- Wakefield MR, Steinbecker KM, Krambeck AE, Teague JL. Primary surgical repair of combined gastroschisis and bladder exstrophy. J Pediatr Surg. 2002;37(11):1634–1636. doi: 10.1053/jpsu.2002.36201. [DOI] [PubMed] [Google Scholar]
- Wood HM, Babineau D, Gearhart JP. In vitro fertilization and the cloacal/bladder exstrophy-epispadias complex: A continuing association. J Pediatr Urol. 2007;3(4):305–310. doi: 10.1016/j.jpurol.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Yang P, Khoury MJ, Stewart WF, Beaty TH, Chee E, Beatty JC, Diamond EL, Gordis L. Comparative epidemiology of selected midline congenital abnormalities. Genet Epidemiol. 1994;11:141–154. doi: 10.1002/gepi.1370110205. [DOI] [PubMed] [Google Scholar]