Abstract
Essential tremor (ET) was the original indication for deep brain stimulation (DBS), with USA Food and Drug Administration approval since 1997. Despite the efficacy of DBS, it is associated with surgical complications that cause sub-optimal clinical outcomes. Given that ET is a progressive disease with increase in symptom severity with increasing age, this study evaluated the impact of increasing age on short-term complications following DBS surgery for ET. Thomson-Reuters MarketScan database was utilized (New York, NY, USA). Patients selected were over age 18 and underwent DBS for ET between the years 2000 and 2009. Multivariable logistic regression analysis was used to calculate complication odds ratios (OR) for a 5 year increase in age, after controlling for other covariates. Six hundred sixty-one patients were included in the analysis. The mean (standard deviation) age was 61.9 (14.3) years, with 17% of individuals aged ≥75 years. Overall 56.9% of patients were male, and 44.6% had a Charlson Comorbidity Score of ≥1. Additionally, 7.1% of patients experienced at least one complication within 90 days, including wound infections (3.0%), pneumonia (2.4%), hemorrhage or hematoma (1.5%), or pulmonary embolism (0.6%). Increasing age was not significantly associated with the overall 90 day complication rates (OR 0.89; 95% confidence interval [CI] 0.77–1.02; p = 0.102). The risk of the two most common procedure-related complications, hemorrhage and infection, did not significantly increase with age (hemorrhage: OR 1.02; 95%CI 0.77–1.37; p = 0.873; and infection: OR 0.88; 95%CI 0.72–1.07; p = 0.203). Our findings suggest that age should not be a primary exclusion factor for determining candidacy for DBS and also suggest a possible expansion of the traditional therapeutic window since postoperative complications remained relatively stable.
Keywords: Age, Deep brain stimulation, Complications, Essential tremor
1. Introduction
Essential tremor (ET) is one of the most common movement disorders in the USA with an estimated prevalence of up to 5%1-3. ET is characterized by postural and action tremor involving the upper extremities and less commonly the lower extremities, head and voice4. This causes significant functional disability in affected patients5,6. Due to the adverse effects and time-dependent loss of efficacy, medical management for ET has been associated with sub-optimal outcomes4,7,8. For ET patients with advanced and medically refractory symptoms or with complications to medical therapy, deep brain stimulation (DBS) of the ventral intermediate (VIM) nucleus of the thalamus has been shown to dramatically reduce tremor-associated disability9-11. The safety, efficacy, adjustability, reversibility of adverse effects and non-destructive nature of DBS has driven its widespread adoption, replacing brain lesioning, with efficacy comparable to thalamotomy12,13. However, as with any surgical intervention, DBS is associated with potential peri and post-operative complications such as infection, hemorrhage/hematoma and pneumonia14.
The existence of comorbidities such as hypertension and diabetes in elderly patients creates an increased baseline risk of surgical complications following DBS for ET. The increased prevalence of ET in patients over 60 years2,9 brings further importance to the impact of advancing age on DBS outcomes. With as many as 15–25% of ET patients being forced into early job retirement15, there is a need for novel insights into the role of DBS on outcomes in elderly patients seeking surgical management for this exceedingly disabling pathology. Currently, there is a paucity of data addressing this paradigm. Given that the prevalence of ET increases nearly 10-fold with age1-3, together with the aging USA population, we evaluated the step-wise impact of increasing age on short-term complications following DBS surgery. We hypothesized that increasing age would be associated with an increase in post-operative complications.
2. Methods
2.1. Data source
Data for the study were obtained using the Thomson-Reuters MarketScan database (New York, NY, USA). The MarketScan database contains claims records from employers, health plans, government, and public organizations for over 158 million patients in the USA since 1996. For this study, we used the Commercial Claims and Encounters, the Medicare Supplemental and Coordination of Benefits, and the Medicaid databases within MarketScan. In MarketScan, each patient is assigned a unique, encrypted enrollee identification number that can be used to link patient information across different tables. For this study, all patient data from the years 2000 to 2009 were examined including inpatient admissions, inpatient services, outpatient services, outpatient pharmaceuticals, and enrollment tables representing all available subsections of the MarketScan database.
2.2. Study sample
Diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) and procedure (CPT) codes were used to identify patients diagnosed with Essential Tremor (ICD-9-CM: 333.1) having undergone DBS (ICD-9-CM: 02.93; CPT-4: 61683, 61687 or 95961) for inclusion in the study. Only patients 18 years and older at the time of the index hospitalization were retained for the analysis.
2.3. Main outcome measures
For purposes of describing the population, patients were divided into 5 year epochs, ranging from <50 up to 90 years of age. Primary outcomes were the overall complication rate and also, specifically, pneumonia, post-operative infection, pulmonary embolism, and intracranial hemorrhage or hematoma within 90 days after surgery.
2.4. Statistical analysis
In statistical models, age was analyzed as a continuous variable. Mortality, lead removal or revision, and generator removal or revisions within 90 days from surgery were summarized but only descriptive statistical analysis was performed. Univariable and multivariable logistic regression models were constructed to evaluate the impact of age on 90 day post-operative complications. Multivariable models were adjusted for Charlson Comorbidity Index (dichotomized as 0 or ≥1), insurance type (Medicare, Medicaid, or commercial), and sex. Possible nonlinear effect of age was examined by including terms in the models, but tests for nonlinearity were not statistically significant. Statistical significance was defined by p < 0.05. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient cohort
A total of 661 patients met the inclusion criteria and underwent DBS for ET between 2000 and 2009. Demographic characteristics of the cohort are listed in Table 1. The mean (standard deviation) patient age was 61.9 (14.3) years, with 17.1% of individuals aged ≥75. Overall, 56.9% of patients were male, and 44.6% had a Charlson Comorbidity Score of ≥1. The majority of the patients had either commercial (43.9%) or Medicare (47.2%) insurance. Figure 1 shows the distribution of ET patients with or without DBS surgery across all the age groups. The majority of patients (119) were ≤50 years old, with an overall trend towards decreased DBS intervention with advancing age.
Table 1. Baseline demographics of essential tremor patients with essential tremor.
| Overall | |
|---|---|
|
| |
| Patients | 661 (100.0) |
| Age | |
| Mean (SD) | 61.9 (14.3) |
| Median (Q1, Q3) | 63.0 (55.0–72.0) |
| Age group | |
| ≤50 | 119 (18.0) |
| 50 to <55 | 57 (8.6) |
| 55 to <60 | 91 (13.8) |
| 60 to <65 | 97 (14.7) |
| 65 to <70 | 87 (13.2) |
| 70 to <75 | 97 (14.7) |
| 75 to <80 | 76 (11.5) |
| 80 to <85 | 31 (4.7) |
| 85 to <90 | 6 (0.9) |
| Sex | |
| Male | 376 (56.9) |
| Female | 285 (43.1) |
| Charlson Score | |
| 0 | 366 (55.4) |
| ≥1 | 295 (44.6) |
| Insurance type | |
| Commercial | 290 (43.9) |
| Medicaid | 59 (8.9) |
| Medicare | 312 (47.2) |
Data are presented as number (%) unless otherwise indicated. Q1 = quartile one, Q3 = quartile 3, SD = standard deviation.
Fig. 1.
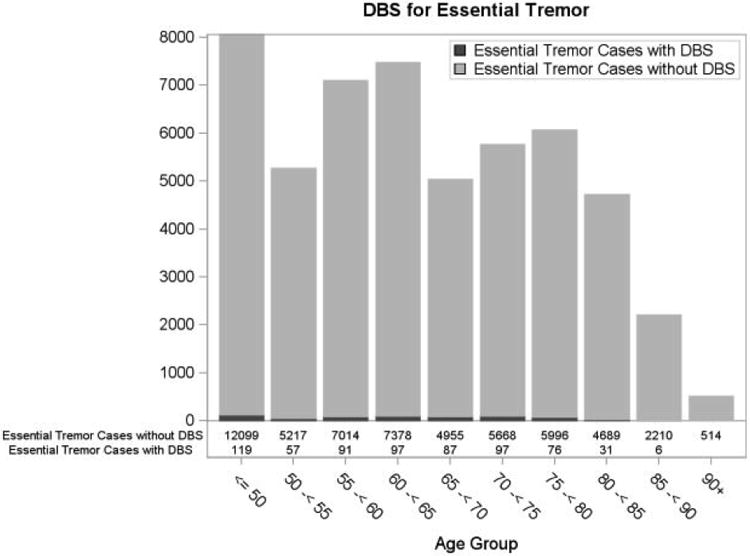
Distribution of essential tremor patients with (black) and without (grey) deep brain stimulation for essential tremor.
DBS = deep brain stimulation.
Overall, 7.1% of patients experienced at least one complication within 90 days of surgery. The most common complication observed within 90 days was wound infection (3.0%), followed by pneumonia (2.4%), hemorrhage or hematoma (1.5%), and pulmonary embolism (0.6%). Within 90 days, lead replacement or revision was performed for 0.3% of patients and generator removal or revision in 1.1% of patients. Additionally, 2.0% of patients had a length of stay following surgery greater than 1 day and only one (0.2%) patient died within 90 days of DBS. The distribution of 90 day post-operative complications is summarized in Table 2.
Table 2. Outcomes following deep brain stimulation for essential tremor stratified by age.
| Outcome within 90 days post-operatively | Overall | Age <75 years | Age ≥75 years |
|---|---|---|---|
| Total patients, n | 661 | 548 | 113 |
| No complication or revision | 608 (92.0) | 503 (91.8) | 105 (92.9) |
| Any complication | 47 (7.1) | 40 (7.3) | 7 (6.2) |
| Hemorrhagic complication | 10 (1.5) | 8 (1.5) | 2 (1.8) |
| Infection | 20 (3.0) | 18 (3.3) | 2 (1.8) |
| Pulmonary embolism | 4 (0.6) | 4 (0.7) | 0 (0.0) |
| Pneumonia | 16 (2.4) | 13 (2.4) | 3 (2.7) |
| Lead revision | 2 (0.3) | 2 (0.4) | 0 (0.0) |
| Generator revision | 7 (1.1) | 7 (1.3) | 0 (0.0) |
| Mortality | 1 (0.2) | 0 (0.0) | 1 (0.9) |
| Length of stay following surgery >1 day | 13 (2.0) | 13 (2.4) | 0 (0.0) |
Data are presented as number (%) unless otherwise indicated.
3.2. Multivariate analysis
After adjusting for covariates (sex, Charlson score and insurance type), increasing age ranging from <50 to 90 years was not significantly associated with overall 90 day complication rates (odds ratio [OR] 0.89 per 5 year increase; 95% confidence interval [CI] 0.77–1.02; p = 0.102). The two most common procedure-related complications, hemorrhage and infection, were not significantly associated with older age (hemorrhage: OR 1.02; 95%CI 0.77–1.37; p = 0.873; and infection: OR 0.88; 95%CI 0.72–1.07; p = 0.203). Similarly, there was no significant association between pulmonary embolism and age (OR 0.86; 95%CI 0.58–1.29; p = 0.470). However, there was a statistically significant correlation between increasing age and the risk of pneumonia (OR 0.77; 95% CI 0.60–0.98; p = 0.037). Table 3 shows the complete regression model results.
Table 3. Effect of 5 year increase in age on post-operative complications within 90 days of deep brain stimulation surgery.
| Predictor | Outcome | Odds ratio (95% CI) | p value |
|---|---|---|---|
| Age (5 year intervals) | All complications | 0.89 (0.77–1.02) | 0.102 |
| Hemorrhagic complications | 1.02 (0.77–1.37) | 0.873 | |
| Infectious complications | 0.88 (0.72–1.07) | 0.203 | |
| Pulmonary embolism complications | 0.86 (0.58–1.29) | 0.470 | |
| Pneumonia complications | 0.77 (0.60–0.98) | 0.037 |
CI = confidence interval.
4 Discussion
ET was the original indication for DBS, with USA Food and Drug Administration approval since 1997. For patients with advanced and medically refractory ET, DBS is an effective therapeutic modality based on randomized controlled studies16 with the VIM of the thalamus being the preferred target of stimulation. Not only is DBS safe and efficacious, it is very personalized and reversible. Studies have shown that patients undergoing DBS for ET have a robust reduction in tremor symptoms9-11. However, hemorrhagic complication, post-operative infection, pneumonia and death are rare, but often devastating, complications that have been associated with DBS14,17. The exact role of age on adverse events following DBS for ET is poorly defined and is of great relevance given that ET is a progressive disease with increasing symptom severity with age18. Older patients stand to suffer from post-operative complications due to the existence of multiple comorbidities such as hypertension, decreased cardio-pulmonary function and physical deconditioning due to sub-optimal physiologic reserve. Elderly patients interested in having DBS for disabling tremor symptoms should be informed of the surgical risks and benefits associated with DBS. However, the limited data investigating the association between increasing age and the incidence of peri and post-operative complication makes it very challenging for patients to make informed decisions pre-operatively. In the absence of such data, strict age cut-offs (i.e. >75 years) and the presence of medical comorbidities in elderly patients are often utilized when counseling patients as exclusion criteria for DBS surgery. Given the rapidly expanding elderly population in the USA, it is increasingly essential to have a deeper understanding of the age-associated risks of surgical intervention. The availability of large, secondary administrative databases provides an opportunity to determine mean complication rates in a wide variety of academic and community practices across the USA. In this study we retrospectively examined the Thomson-Reuters MarketScan Database from 2000 to 2009, examining the impact of increasing age on complications associated with DBS surgery for ET. We did not find an association between advanced age and the rates of complications including hemorrhage, infection and pulmonary embolism; however, the number of events in our study was low.
In our study, advancing age had no significant association with hemorrhagic complications within 90 days following surgery. This is in line with several other studies evaluating the impact of age on hemorrhagic complication following DBS for ET19-23. Binder et al.20 showed that age was not significantly associated with risk of hematoma in a study of 208 patients that underwent implantation of DBS electrodes for movement disorders. However, there are some studies that demonstrate an age-associated increase in incidence of hemorrhagic complications following DBS surgeries14,24-26. Given that the aforementioned studies have been single institutional studies (with the exception of Voges et al.14), our national level cohort study may reduce the discrepancies in evidence of the role of aging on complications following DBS surgeries. Despite the conflicting evidence in the literature, hemorrhagic complication should be viewed as a multifaceted phenomenon with several pre-operative contributing factors that increase the baseline surgical risk. In order to minimize or avoid intracranial hemorrhage, patients should be thoroughly screened for risk factors such as hypertension, coagulopathy, history of hemorrhage prior to surgery, blood vessel abnormality, medication usage (such as recent antiplatelet usage) and adequate intra-operative and peri-operative blood pressure control.
The occurrence of post-operative infection remains a major surgical impediment, with ramifications for the patient's quality of life and overall healthcare cost. Any implanted device carries a risk of infection and there is a variation in the reported rates of hardware related post-operative infection (4–12.2%)17,27-33. Despite the general incidence of infection, our study showed no association between advancing age and infection. Our findings are similar to a study by Falowski et al.27 evaluating patients who underwent DBS for movement disorder. They showed in a multivariable analysis that age had no effect on complications including infection. Nonetheless, infection remains a debilitating consequence that may compromise the integrity of DBS leads and generators, resulting in surgical debridement, lead revisions and re-operations. Extensive care and attention should therefore be implemented to minimize the incidence of such complications.
In our retrospective series, we found no association between increasing age and the incidence of pulmonary embolism. However, there was an association between advancing age and a lower risk of pneumonia, which contradicts several studies demonstrating that elderly patients are at risk for pulmonary complications34,35. It remains unclear if DBS lead implantation poses any added risk of respiratory or thromboembolic complication to patients. Age is a proxy for comorbidities19 such as decreased respiratory reserve and activity level, which puts a patient at increased risk for complications. Baseline pre-operative risk factors such as smoking and obesity are confounding variables, which influence the incidence of pneumonia and pulmonary embolism. Nonetheless, preventive measures, such as early ambulation and the use of incentive spirometry should be encouraged in the immediate post-operative period among elderly patients.
This study has evaluated a nationally selected cohort of patients, reducing the discrepancies and biases that result from selecting patients from single institutions or states. Additionally, as the MarketScan database is comprehensive and inclusive, it more accurately reflects the wide variety of practices across the USA. Despite these strengths, several limitations are present in this study. Firstly, various patient and clinical factors could not be discerned, including pre-operative symptom severity (such as long-term gait, falls), medication usage and requirement, and neuro-cognitive assessments in the elderly. Additionally, the efficacy of DBS in reducing tremor could not be determined. DBS efficacy may have had an influence on revision rates. The database did not capture pre-operative screening by neurology, neurosurgery and neuropsychology, which could influence outcomes after DBS surgery. Also, as patients and procedures were selected using diagnosis and procedure codes, miscoding may be present. We have attempted to reduce this inaccuracy by using both ICD-9-CM and CPT codes allowing for greater data accuracy. The number of events in the study was low, which may have impacted the statistical power to detect significant associations. Another limitation is that this study is retrospective and non-randomized. As a result, the study is susceptible to the effects of unmeasured confounding variables. In particular, there could be a procedure bias with healthier elderly patients being preferentially selected for DBS intervention. The presence of unmeasured confounders may explain our unexpected finding of a lower risk of pneumonia with advancing age. This was a broad-level study, examining the impact of advancing age on post-operative DBS complications longitudinally and across many different centers. While this is a retrospective review, carrying all of the inherent limitations, it helps identify on a large scale the role of aging on complications following DBS for ET.
5. Conclusion
Among older ET patients, 90 day complication risk and the risk of post-operative hemorrhage or infection remained relatively stable, despite increasing age. Physicians should not underestimate the effect that tremor has on patient's functionality and should be able to identify cases where medications are refractory and surgical intervention may offer improved quality of life. As DBS technology and imaging platforms continue to evolve, refinement of surgical techniques should continue to produce superior clinical outcomes while minimizing surgical complications. Our findings suggest that age should not be a primary exclusion factor for determining candidacy for DBS and support a possible expansion of the traditional therapeutic window. Individual risk assessment should be incorporated in pre-operative counseling of elderly patients.
Acknowledgments
The Duke CTSA team was supported by the USA National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Shivanand Lad, MD, PhD, has consulted for or received grant support from Medtronic Inc., Boston Scientific and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center which has received research funding from NIH KM1 CA 156687.
Footnotes
Conflicts of Interest: The remaining authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement disorders : official journal of the Movement Disorder Society. 2010;25(5):534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED. Clinical practice. Essential tremor. The New England journal of medicine. 2001;345(12):887–891. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32(3):208–214. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zappia M, Albanese A, Bruno E, et al. Treatment of essential tremor: a systematic review of evidence and recommendations from the Italian Movement Disorders Association. Journal of neurology. 2013;260(3):714–740. doi: 10.1007/s00415-012-6628-x. [DOI] [PubMed] [Google Scholar]
- 5.Koller W, Biary N, Cone S. Disability in essential tremor: effect of treatment. Neurology. 1986;36(7):1001–1004. doi: 10.1212/wnl.36.7.1001. [DOI] [PubMed] [Google Scholar]
- 6.Busenbark KL, Nash J, Nash S, Hubble JP, Koller WC. Is essential tremor benign? Neurology. 1991;41(12):1982–1983. doi: 10.1212/wnl.41.12.1982. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Koller WC. Introduction. Essential tremor. Neurology. 2000;54(11 Suppl 4):S1. [PubMed] [Google Scholar]
- 8.Lyons KE, Pahwa R. Deep brain stimulation and essential tremor. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2004;21(1):2–5. doi: 10.1097/00004691-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Lyons KE, Pahwa R, Comella CL, et al. Benefits and risks of pharmacological treatments for essential tremor. Drug safety : an international journal of medical toxicology and drug experience. 2003;26(7):461–481. doi: 10.2165/00002018-200326070-00003. [DOI] [PubMed] [Google Scholar]
- 10.Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O. Long-termefficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Movement disorders : official journal of the Movement Disorder Society. 2003;18(2):163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- 11.Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, Wharen RE. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology. 2000;54(12):2342–2344. doi: 10.1212/wnl.54.12.2342. [DOI] [PubMed] [Google Scholar]
- 12.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. The New England journal of medicine. 2000;342(7):461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 13.Pahwa R, Lyons KE, Wilkinson SB, et al. Comparison of thalamotomy to deep brain stimulation of the thalamus in essential tremor. Movement disorders : official journal of the Movement Disorder Society. 2001;16(1):140–143. doi: 10.1002/1531-8257(200101)16:1<140::aid-mds1025>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Voges J, Hilker R, Botzel K, et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Movement disorders : official journal of the Movement Disorder Society. 2007;22(10):1486–1489. doi: 10.1002/mds.21481. [DOI] [PubMed] [Google Scholar]
- 15.Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain : a journal of neurology. 1994;117(Pt 4):805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Klassen BT, Stead M. Surgery for movement disorders. Journal of neurosurgical sciences. 2011;55(4):305–317. [PubMed] [Google Scholar]
- 17.Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotactic and functional neurosurgery. 2001;77(1-4):73–78. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- 18.Blomstedt P, Sandvik U, Hariz MI, et al. Influence of age, gender and severity of tremor on outcome after thalamic and subthalamic DBS for essential tremor. Parkinsonism & related disorders. 2011;17(8):617–620. doi: 10.1016/j.parkreldis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Rughani AI, Hodaie M, Lozano AM. Acute complications of movement disorders surgery: effects of age and comorbidities. Movement disorders : official journal of the Movement Disorder Society. 2013;28(12):1661–1667. doi: 10.1002/mds.25610. [DOI] [PubMed] [Google Scholar]
- 20.Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56(4):722–732. doi: 10.1227/01.neu.0000156473.57196.7e. discussion 722-732. [DOI] [PubMed] [Google Scholar]
- 21.Elias WJ, Sansur CA, Frysinger RC. Sulcal and ventricular trajectories in stereotactic surgery. Journal of neurosurgery. 2009;110(2):201–207. doi: 10.3171/2008.7.17625. [DOI] [PubMed] [Google Scholar]
- 22.Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson's disease. Review of 272 procedures. Acta neurochirurgica. 2007;149(9):867–875. doi: 10.1007/s00701-007-1267-1. discussion 876. [DOI] [PubMed] [Google Scholar]
- 23.Xiaowu H, Xiufeng J, Xiaoping Z, et al. Risks of intracranial hemorrhage in patients with Parkinson's disease receiving deep brain stimulation and ablation. Parkinsonism & related disorders. 2010;16(2):96–100. doi: 10.1016/j.parkreldis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009;64(4):754–762. doi: 10.1227/01.NEU.0000339173.77240.34. discussion 762-753. [DOI] [PubMed] [Google Scholar]
- 25.Ory-Magne F, Brefel-Courbon C, Simonetta-Moreau M, et al. Does ageing influence deep brain stimulation outcomes in Parkinson's disease? Movement disorders : official journal of the Movement Disorder Society. 2007;22(10):1457–1463. doi: 10.1002/mds.21547. [DOI] [PubMed] [Google Scholar]
- 26.Sansur CA, Frysinger RC, Pouratian N, et al. Incidence of symptomatic hemorrhage after stereotactic electrode placement. Journal of neurosurgery. 2007;107(5):998–1003. doi: 10.3171/JNS-07/11/0998. [DOI] [PubMed] [Google Scholar]
- 27.Falowski S, Ooi YC, Smith A, Verhargen Metman L, Bakay RA. An evaluation of hardware and surgical complications with deep brain stimulation based on diagnosis and lead location. Stereotactic and functional neurosurgery. 2012;90(3):173–180. doi: 10.1159/000338254. [DOI] [PubMed] [Google Scholar]
- 28.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Videnovic A, Metman LV. Deep brain stimulation for Parkinson's disease: prevalence of adverse events and need for standardized reporting. Movement disorders : official journal of the Movement Disorder Society. 2008;23(3):343–349. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- 30.Voges J, Waerzeggers Y, Maarouf M, et al. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery--experiences from a single centre. Journal of neurology, neurosurgery, and psychiatry. 2006;77(7):868–872. doi: 10.1136/jnnp.2005.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariz MI. Complications of deep brain stimulation surgery. Movement disorders : official journal of the Movement Disorder Society. 2002;17(Suppl 3):S162–166. doi: 10.1002/mds.10159. [DOI] [PubMed] [Google Scholar]
- 32.Joint C, Nandi D, Parkin S, Gregory R, Aziz T. Hardware-related problems of deep brain stimulation. Movement disorders : official journal of the Movement Disorder Society. 2002;17(Suppl 3):S175–180. doi: 10.1002/mds.10161. [DOI] [PubMed] [Google Scholar]
- 33.Kondziolka D, Whiting D, Germanwala A, Oh M. Hardware-related complications after placement of thalamic deep brain stimulator systems. Stereotactic and functional neurosurgery. 2002;79(3-4):228–233. doi: 10.1159/000070836. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto K, Ichiyama S, Shimokata K, Nakashima N. Postoperative pneumonia in elderly patients: incidence and mortality in comparison with younger patients. Internal medicine. 1993;32(4):274–277. doi: 10.2169/internalmedicine.32.274. [DOI] [PubMed] [Google Scholar]
- 35.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. Journal of the American Medical Directors Association. 2011;12(5):344–354. doi: 10.1016/j.jamda.2010.12.099. [DOI] [PubMed] [Google Scholar]


