Abstract
Purpose
AZD2014 is a novel, oral, m-TORC 1/2 inhibitor which has shown in-vitro and in-vivo efficacy across a range of preclinical human cancer models.
Experimental Design
A rolling six dose escalation was performed to define a maximal tolerated dose (MTD) (Part A) and at MTD a further cohort of patients was treated to further characterize toxicities and perform pre- and post-treatment biopsies (Part B). AZD2014 was administered orally twice a day (BD) continuously. Flow cytometry, ELISA and immunohistochemistry were used to quantify pharmacodynamic biomarkers. Pharmacokinetic analysis was carried out by mass spectrometry.
Results
A total of 56 patients were treated across a dose range of 25 -100 mg. The MTD was 50 mg BD. The dose limiting toxicities were fatigue and mucositis. At the MTD the most common AEs were fatigue (78%), nausea (51%) and mucositis (49%) but these were equal to or greater than grade 3 in only 5% of patients. Drug levels achieved at the MTD (AUCss 6686 ng.hr/mL, Cmaxss 1664 ng/mL) were consistent with activity in pre-clinical models. A reduction in p-S6 levels and Ki67 staining was observed in 8/8 and 5/9 evaluable paired biopsy samples. Partial responses were seen in a patient with pancreatic cancer and a patient with breast cancer who were found to have a PDGFR and ERBB2 mutation, respectively.
Conclusions
The recommended phase II dose for further evaluation of AZD2014 is 50 mg BD and at this dose it has been possible to demonstrate pharmacologically relevant plasma concentrations, target inhibition in tumor and clinical responses.
Keywords: m-TORC1 inhibitor, m-TORC2 inhibitor, pharmacokinetics, pharmacodynamics, Phase I
INTRODUCTION
The PI3K pathway is deregulated in over 50% of all cancers. Mechanisms of deregulation include activating mutations in PIK3CA and AKT and loss of function of tumor suppressor genes such as PTEN.(1) Mammalian target of rapamycin (m-TOR) consists of two essential complexes, TORC1 and TORC2 and is a crucial node in the PI3K signaling network (2). Inhibition of TORC1 could lead to inhibition of cell growth and metabolism via inactivation of downstream targets such as p-S6, p-4EBP1 and p-GSK3B (3). In addition, m-TORC2 is critical to AKT signaling(4).
Drugs such as sirolimus, temsirolimus and everolimus are allosteric inhibitors of m-TOR. Sirolimus is licensed for use in transplant medicine (5) and other allosteric m-TOR inhibitors such as temsirolimus and everolimus have been licensed for use in a variety of cancers (6-10).
Pre-clinical studies have suggested that allosteric inhibition of m-TOR by a rapalog inhibits only m-TORC1 and leads to an increase in phosphorylation of AKT due to feedback loops (11) and continued activity of m-TORC2(12). This has been confirmed clinically in post-treatment biopsy samples in patients treated with rapalogs (13, 14). The activation of AKT is hypothesized to be a mechanism of resistance to these agents.
AZD2014 is a rationally designed ATP competitive m-TORC1/2 inhibitor with an IC50 of 2.81 nM and has shown pre-clinical activity across a range of in-vitro and in-vivo pre-clinical models (15). Pre-clinical experiments showed a 95% protein binding in human plasma and bioavailability of 29%. [14C]-AZD2014 was extensively metabolised following a single dose in rats with N-hydroxymethyl and desmethyl components accounting for 25 and 10% of parent area under the curve in plasma, respectively. Urinary and faecal excretion in this model accounted for approximately 2 and 85% of dosed radioactivity respectively. In human hepatocytes and heterologously expressed CYP isozymes, AZD2014 was primarily metabolised by CYP 3A5 and 2C8 with some contribution from 3A4.
The primary aim of this first-in-human study was to investigate the safety and tolerability of AZD2014. The secondary aims included determining the pharmacokinetic (PK) profile, confirming target engagement by studying changes in pharmacodynamic (PD), proof-of-mechanism (POM) and proof-of-concept (POC) biomarkers in addition to documenting preliminary clinical activity.
The highest severely non-toxic dose (HSNTD) in dogs was not established. The highest dose administered to dogs in a one month toxicology study was 5 mg/kg/day. The human equivalent dose of 1/6th this dose level in a 60 kg human was 27 mg/day. However, the International Conference on Harmonization (ICH) S9 guideline recommended that the starting dose should be “a pharmacologically active dose that is reasonably safe to use”. Using physiologically based pharmacokinetic modelling, the IC50 demonstrated in the U87-MG mouse xenograft model, and allowing for differences in plasma protein binding in man and mouse, suggests that a single dose of 50 mg would give plasma concentrations close to the IC50 at maximum concentration (Cmax) in patients. Further exposures in patients following a 50 mg starting dose were expected to be consistent with the exposures that caused monitorable and reversible effects in the toxicology studies, with the exception of the testicular changes and partial recovery in bone marrow. Finally, AZD2014 was predicted to have a modest half-life of 4 hours, it was not anticipated that the subsequent repeat dosing phase at 50 mg twice daily (100 mg total daily dose) would show significant accumulation. Thus, the proposed starting dose was 50 mg twice daily after the washout period.
TORC1/2 inhibitors that are currently under phase I evaluation or have completed phase I evaluation include AZD2014(15), INK-128/MLN-128(16, 17), DS-3078a(18), OSI-027(19, 20), and AZD8055 (21, 22).
MATERIALS AND METHODS
This study used a rolling six dose escalation design during the dose escalation phase. At the recommended phase II dose, an expansion cohort to further characterize tolerability, PK and PD profiles including pre- and post-treatment biopsies in a subset of patients was instituted. Patients were recruited at the Royal Marsden and Christie Hospital NHS Foundation Trust in the UK following ethics committee approvals. Patients with advanced solid tumors who had already received standard-of-care treatment and had adequate organ function were eligible for the study. There was no difference between the eligibility criteria between dose escalation and expansion cohorts of the study. Following informed, written consent, patients received a single run-in dose of AZD2014 in a liquid (solution) formulation. During the clinical trial, AZD2014 was administered orally, with patients fasting (except for water) for 2 hours prior to dosing and one hour after dosing. Dose-limiting toxicities (DLTs) were evaluated during the run-in dose and the first 21 days of continuous dosing. CTC grade 4 hematological toxicity or any grade 3 or grade 4 non hematological toxicity were considered DLTs, with the exception of alopecia, inadequately treated grade 3 or 4 nausea and vomiting or isolated laboratory change without any clinical significance. Concomitant exposure to potent and moderate inhibitors and inducers of CYP3A4/5 and CYP2C8 were not permitted. Details of drugs and washout periods are mentioned in the Supplementary Data. Blood was drawn for PK and PD analysis over 24 hrs. The PD samples for the run-in single dose were drawn at pre dose, 2 hrs, 6-8 hrs and 24 hrs post-dose. The patient then commenced continuous dosing of AZD2014 twice a day (BD), 3-7 days later. The length of a cycle was 28 days. Adverse events were recorded using National Cancer Institute Common Toxicity Criteria 3.1. On day 15, 21 and 28, blood was collected over 12 hrs to assess steady state PK profiles. Tumor biopsies were carried out between days 8-15. Patients were seen every week to assess safety. CT scans were carried out at baseline and every 8 weeks to assess disease response using RECIST 1.1. The drug concentrations in plasma were assessed using mass spectrometry (see supplementary data for details). PK analysis was carried out using Phoenix™-WinNonLin® v6 for NCA. The pharmacodynamic assays included p-4EBP1 in peripheral blood mononuclear cells (PBMNCs) using flow cytometry, p-AKT in platelet-rich plasma (PRP) which was performed using an immunoassay from Meso Scale Discovery. p-S6, p-AKT, p-4EBP1 and Ki67 in pre- and post-treatment biopsies were assessed using immunohistochemistry. A biopsy pair was considered evaluable for determination of inhibition of phosphorylation on therapy if two or more of the triplicate sections for each pre-treatment sample had an H-score above 10 (see supplementary data for details of methods for PD analysis). Tumors of all patients entered into the study were not sequenced. The Drug Development Unit at The Institute of Cancer Research and The Royal Marsden runs a generic molecular characterization protocol (CCR 3171) which allows sequencing of patients’ archival tumor blocks. Targeted exome sequencing of pre-treatment tumor samples from two patients who had a partial response using the TruSeq panel on the MiSeq platform was carried out at The Institute of Cancer Research. FDG PET scans were done at baseline and on day 8 ± 2 days of continuous dosing. The interval between the administration of PET tracer and scan was standardized to 1hr.
RESULTS
A total of 56 patients across a dose range of 25 mg BD – 100 mg BD were treated on this phase I study (n= 23 in Part A and n= 33 in Part B). Nineteen male and 37 female patients were treated and the median age was 59 (range 33 - 76). The most common tumor type recruited to the study was breast cancer (15/56). Details of the demographic profile, ECOG performance status and tumor types treated are listed in Supplementary Table 1.
Dose escalation
The starting dose was 50 mg BD and, as no DLTs were seen in 6 patients, the dose was doubled to 100 mg BD. As there were 4/4 dose limiting toxicities, an intermediate dose of 70 mg BD was explored. A total of 5 patients were dosed in this cohort, but one patient withdrew consent before becoming evaluable for the dose decision. A further 2/4 evaluable patients had a DLT at 70 mg BD and thus the remaining two patients who had consented to this cohort received 50 mg BD. Thus, 50 mg BD was considered the maximally tolerated dose (MTD). Fifty mg BD was both the starting dose and the MTD and this dose showed pharmacodynamic activity in normal tissue. It is possible that future combination studies would use lower doses of AZD2014 and thus a further cohort of 6 patients were treated at 25 mg BD to further characterize PK and PD profiles over a range of doses of AZD2014 which could be used in the future. Subsequently, 50 mg BD was declared the recommended phase II dose (RP2D) and a further 33 patients were treated to further evaluate toxicity, PK and PD.
Dose limiting toxicities
At the dose of 100 mg BD, DLTs were seen in 4/4 patients. In all instances these were CTC grade 2 or 3 fatigue, occurring within the first week of treatment. The fatigue was reversible on discontinuation of the drug. At a dose of 70 mg BD, DLTs were seen in 2/4 evaluable patients treated, with grade 3 fatigue and grade 3 mucositis in one patient and grade 3 fatigue in another. All events were reversible on cessation of AZD2014.
Adverse events
The adverse events that occurred in >15% of patients at the RP2D of 50 mg BD are listed in Table 1. All AEs reported in this paragraph pertain to patients treated at 50 mg BD on Part A and Part B (Table 1). Fatigue was the most commonly seen adverse event in 32/41 (78%) patients, however, only 2/41 (5%) were grade 3 or higher and this was reversible on interruption of dosing. Gastrointestinal symptoms such as nausea, mucositis, diarrhea and vomiting were seen in 21/41 (51%), 20/41 (49%) 17/41 (42%), and 12/41 (29%), respectively, however, less than 5% of these adverse events were grade 3 or 4. Nausea and vomiting were well controlled by antiemetics, such as domperidone, if necessary, and the grade 1 - 2 diarrhea did not consistently require treatment. Mucositis was grade 1 - 2 in most instances and patients responded to mouth-washes and did not require dose interruptions due to it. A predominantly maculopapular rash was observed in 17/41 (42%) of patients and less than 5% of these were grade 3 or higher. Lower respiratory tract infections were seen in 7/41 (17%) and it was grade 3 in only 1/41 (2%). Interestingly, no patients were diagnosed with pneumonitis. The most common laboratory abnormality was anemia, with 7/41 (17%) having recorded grade 1-2 anemia; given the advanced cancer and degrees of co-morbidities in these patients, it was difficult to attribute this specifically to AZD2014. Hyperglycemia was seen in 5/41 (12%), 0/5 (0%), and 2/4 (50%) of patients treated at 50 mg BD, 70 mg BD and 100 mg BD, respectively. At the RP2D of 50 mg BD, no grade 3 or 4 hyperglycemia was seen. Further, no grade 3 or above hypercholesterolemia or triglyceridemia was seen in this cohort. ECG monitoring revealed an increase in QT corrected Bazett’s formula (QTCB) and a reduction in QTCB by 30 – 60 msec in 3/41 (7%) and 5/41 (12%), respectively, thus showing no defined trend for increase or decrease in QTCB. Due to the modest number of patients treated across different dose levels and the standard timing of ECG done, a formally powered testing of the relationship of plasma concentrations to QTCB has not been done. At the RP2D of 50 mg BD, 10/41 (24.4%) had an interruption and 1/41 (2.4%) had a dose reduction due to an adverse event.
Table 1.
Toxicities seen in ≥ 15% of patients on the phase I clinical trial
| Number (%) of patients |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Part A 25mg BD solution (N=6) | All 50mg BD solution (N=41) | Part A 70mg BD solution (N=5) | Part A 100mg BD solution (N=4) | total (N=56) | |||||||||||
|
| |||||||||||||||
| MedDRA Prefered Term | CTCAE grade 1-2 | CTCAE grade >=3 | Any grade | CTCAE grade 1-2 | CTCAE grade >=3 | Any grade | CTCAE grade 1-2 | CTCAE grade >=3 | Any grade | CTCAE grade 1-2 | CTCAE grade >=3 | Any grade | CTCAE grade 1-2 | CTCAE grade >=3 | Any grade |
| Fatigue | 2 (33.3) | 1 (16.7) | 3 (50.0) | 30 (73.2) | 2 (4.9) | 32 (78.0) | 2 (40.0) | 1 (20.0) | 3 (60.0) | 2 (50.0) | 2 (50.0) | 4(100.0) | 36 (64.3) | 6 (10.7) | 42 (75.0) |
|
| |||||||||||||||
| Nausea | 2 (33.3) | 0 | 2 (33.3) | 19 (46.3) | 2 (4.9) | 21 (51.2) | 3 (60.0) | 0 | 3 (60.0) | 4 (100.0) | 0 | 4 (100.0) | 28 (50.0) | 2 (3.6) | 30 (53.6) |
|
| |||||||||||||||
| Mucositis | 2 (33.3) | 0 | 2 (33.3) | 19 (46.3) | 1 (2.4) | 20 (48.8) | 3 (60.0) | 2 (40.0) | 5 (100.0) | 3 (75.0) | 0 | 3 (75.0) | 27 (48.2) | 3 (5.4) | 30 (53.6) |
|
| |||||||||||||||
| Decreased appetite | 3 (50.0) | 0 | 3 (50.0) | 17 (41.5) | 0 | 17 (41.5) | 2 (40.0) | 0 | 2 (40.0) | 4 (100.0) | 0 | 4 (100.0) | 26 (46.4) | 0 | 26 (46.4) |
|
| |||||||||||||||
| Diarrhea | 2 (33.3) | 0 | 2 (33.3) | 17 (41.5) | 0 | 17 (41.5) | 3 (60.0) | 0 | 3 (60.0) | 1 (25.0) | 0 | 1 (25.0) | 23 (41.1) | 0 | 23 (41.1) |
|
| |||||||||||||||
| Rash | 1(16.7) | 0 | 1(16.7) | 15 (36.6) | 2 (4.9) | 17 (41.5) | 0 | 1 (20.0) | 1 (20.0) | 0 | 0 | 0 | 16 (28.6) | 3 (5.4) | 19 33.9) |
|
| |||||||||||||||
| Constipation | 2 (33.3) | 0 | 2 (33.3) | 13 (31.7) | 0 | 13 (31.7) | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (25.0) | 16 (28.6) | 0 | 16 (28.6) |
|
| |||||||||||||||
| Vomiting | 0 | 0 | 0 | 11 (26.8) | 1 (2.4) | 12 (29.3) | 0 | 0 | 0 | 4 (100.0) | 0 | 4 (100.0) | 15 (26.8) | 1 (1.8) | 16 (28.6) |
|
| |||||||||||||||
| Pruritus | 0 | 0 | 0 | 13 (31.7) | 0 | 13 (31.7) | 0 | 0 | 0 | 0 | 0 | 0 | 13 (23.2) | 0 | 13 (23.2) |
|
| |||||||||||||||
| Cough | 3 (50.0) | 0 | 3 (50.0) | 8 (19.5) | 0 | 8 (19.5) | 1 (20.0) | 0 | 1 (20.0) | 0 | 0 | 0 | 12 (21.4) | 0 | 12 (21.4) |
|
| |||||||||||||||
| Dyspnea | 1 (16.7) | 0 | 1 (16.7) | 5 (12.2) | 2 (4.9) | 7 (17.1) | 1 (20.0) | 0 | 1 (20.0) | 2 (50.0) | 0 | 2 (50.0) | 9 (16.1) | 2 (3.6) | 11 (19.6) |
|
| |||||||||||||||
| Dry mouth | 1 16.7) | 0 | 1 16.7) | 8 (19.5) | 0 | 8 (19.5) | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (25.0) | 10 (17.9) | 0 | 10 (17.9) |
|
| |||||||||||||||
| Anemia | 0 | 0 | 0 | 7 (17.1) | 0 | 7 (17.1) | 0 | 1 (20.0) | 1 (20.0) | 0 | 1 (25.0) | 1 (25.0) | 7 (12.5) | 2 (3.6) | 9 (16.1) |
|
| |||||||||||||||
| Back pain | 0 | 0 | 0 | 6 (14.6) | 1 (2.4) | 7 (17.1) | 0 | 0 | 0 | 2 (50.0) | 0 | 2 (50.0) | 8 (14.3) | 1 (1.8) | 9 (16.1) |
|
| |||||||||||||||
| Hypokalemia | 2 (33.3) | 0 | 2 (33.3) | 5 (12.2) | 1 (2.4) | 6 (14.6) | 1 (20.0) | 0 | 1 (20.0) | 0 | 0 | 0 | 8 (14.3) | 1 (1.8) | 9 (16.1) |
|
| |||||||||||||||
| Lower respiratory tract infection | 0 | 1 (16.7) | 1 (16.7) | 6 (14.6) | 1 (2.4) | 7 (17.1) | 1 (20.0) | 0 | 1 (20.0) | 0 | 0 | 0 | 7 (12.5) | 2 (3.6) | 9 (16.1) |
Pharmacokinetics
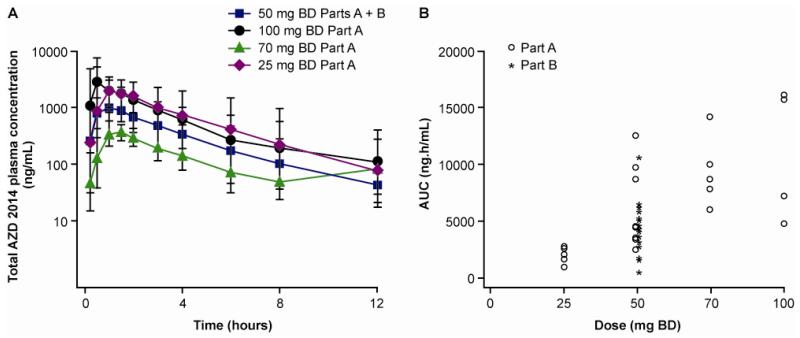
Following oral administration, AZD2014 was rapidly absorbed, with median time to peak following a single dose between 0.5 and 1 hr across the 25 to 100 mg dose range. Terminal elimination half-life was approximately 3 hrs. Geomean exposure (AUC and Cmax) increased greater than proportionally with increasing dose and inter-patient variability was seen with exposures overlapping across the dose range (Figure 1A, 1B and Table 2). At dose of 50 mg BD n=27, the Gmean Cmin SS was 169.5 ng/mL, with a CV of 163.6% and a range of 0 - 2120 ng/mL
Figure 1.
Pharmacokinetic profile of AZD2014.
A) Concentration of drug over time across a dose range of 25 - 100mg following a single dose. In the range of doses studied, there was considerable overlap of plasma concentrations across different cohorts. B) The relationship of AUC to the dose across the dose range studied.
Table 2.
Pharmacokinetic profile of AZD2014
The pharmacokinetic parameters across the dose range of 25-100 mg BD are shown.
| Parameter | Statistic | 25 mg | 50 mg | 70 mg | 100 mg |
|---|---|---|---|---|---|
| Single dose | N=6 | N=27 | N=5 | N=4 | |
|
| |||||
| AUC (ng.h/mL) | Gmean | 1640 | 4015 | 8967 | 9671 |
| CV (%) | 52 | 78 | 33 | 66 | |
| Range | 883–2730 | 453–12600 | 6010–14200 | 4770–16100 | |
|
| |||||
| Cmax (ng/mL) | Gmean | 435 | 1151 | 2382 | 2787 |
| CV (%) | 42 | 57 | 39 | 72 | |
| Range | 221–681 | 234–2840 | 1440–3690 | 1410–5050 | |
|
| |||||
| t½ (h) | Mean | 2.96 | 2.48 | 3.11 | 3.23 |
| SD | 2.13 | 1.42 | 1.99 | 2.47 | |
| Range | 0.88–6.71 | 0.90–8.04 | 1.35–5.98 | 0.82–6.49 | |
|
| |||||
| Steady state | N=6 | N=37 | N=2 | N=0 | |
|
| |||||
| AUCss (ng.h/mL) | Gmean | 2984 | 6686 | NC | - |
| CV (%) | 79 | 79 | NC | ||
| Range | 1000–6560 | 1280–47800 | 7500–22500 | ||
|
| |||||
| Cmax ss (ng/mL) | Gmean | 747 | 1664 | NC | - |
| CV (%) | 72 | 48 | NC | ||
| Range | 227–1450 | 657–6410 | 1500–3870 | ||
|
| |||||
| t½, ss (h) | Mean | 2.89 | 3.01 | NC | - |
| SD | 1.02 | 0.96 | NC | ||
| Range | 1.34–4.06 | 1.18–4.80 | 3.63–4.23 | ||
AUC- area under curve, Cmax – maximal concentration, t1/2, half-life, a subscript of ss indicates steady state.
Pharmacodynamics
In samples of patients treated at 50 mg, phosphorylation of 4E-BP1 was reduced in PBMNCs to approximately −45% and −41% of baseline at 2 and 6 - 8 hrs following a single dose of AZD2014, respectively. Phosphorylation of AKT (S473) in PRP was reduced to −62% and −37% of baseline at 2 and 6 - 8 hrs, respectively. These data indicate inhibition of m-TORC1 and m-TORC2 (Figure 2A and 2B). Changes in phosphorylation of 4EBP-1 and AKT were seen at dose levels of 50 - 70 mg and, importantly, changes in phosphorylation of AKT were seen at doses below the MTD (25 mg cohort), (Figure 2C and 2D). POM PD biomarkers such as p-S6, p-4EBP1 (m-TORC1) and p-AKT (m-TORC2) and POC biomarkers (Ki67) were assessed in pre- and post-treatment biopsies and showed evidence of target inhibition (Figure 3A - 3E). Of note, 11 patients in Part B (50 mg BD) had paired FDG PET scans, 8/11 patients showed a reduction of SUVmax, with 3/11 attaining a partial response (30% reduction in SUVmax) (Figure 3F).
Figure 2.
Pharmacodynamic profile of AZD2014 in normal tissue
A) The reduction of p-4EBP1 levels in monocytes after a single dose of AZD2014 at the RP2D of 50 mg BD. The mean % change in p-4EBP1 after a single 50 mg BD dose of AZD2014 in Part B was −45 % (n =14 CI −60%:−30%) at 2 hours and −41% (n=14, CI −52%:−29%) at 6-8 hours. At 24 hours (12 hours after the 2nd BD dose) the mean change from baseline was −21% (n=13, CI −39%:−3%). B) The reduction of p-AKT levels in PRP after a single dose of AZD2014 at the RP2D of 50mg BD. The mean % change was −62% (n=32, CI −70%:−54%) at 2 hours and −37% (n=32, CI −47%:−27%) at 6-8 hours. At 24 hours (12 hours after the 2nd BD dose) the mean change from baseline was −2% (n=32, CI −26%:+22%). C) Percentage change from baseline in p-4EBP1 in PBMNCs across dose levels 50-100 mg BD at 2 hours post-dose. The columns on the waterfall plot represent individual patients. D) Percentage change from baseline in the reduction of p-AKT across dose levels 25-100 mg BD in PRP at 2 hours post-dose.
Figure 3.
Pharmacodynamic profile of AZD2014 in tumor tissue
A) Percentage reduction in mean H score of p-S6 seen in post-treatment samples. 8/8 samples showed a reduction of p-S6 levels. This is a POM PD biomarker of m-TORC1 inhibition. B) Percentage reduction in mean H score of p-4EBP1 seen in post-treatment samples. 3/7 samples showed a reduction of p-4EBP1 levels. This is a POM PD biomarker of m-TORC1 inhibition. C) Percentage reduction in mean H score of p-AKT seen in post treatment samples. 3/4 samples showed a reduction of p-AKT levels. This is a POM PD biomarker of m-TORC2 inhibition. D) Percentage reduction in Ki67 seen in post treatment samples. 5/9 samples showed reduction in Ki67 expression. This is a POC distal biomarker which reflects proliferation. E) Representative data from a patient who had a partial response showing reduction in p-S6, p-AKT levels and % of cells stained for Ki67. F) Maximal reduction in SUVmax in FDG PET scans in 11 patients treated at 50mg BD who had evaluable paired pre and post treatment FDG PET scans. 8/11 patients showed a reduction in SUVmax. This is a distal POC biomarker which reflects metabolism.
Efficacy
There were two partial responses in the study. The first was a patient with acinar pancreatic cancer who had previously responded to two lines of gemcitabine-based treatment. The patient had a maintained partial response and was on treatment for six cycles. The patient was found to have KRAS, PDGFRA, APC, ERB4, KIT and FBXW7 mutation. The second patient to respond was a patient with estrogen receptor positive breast cancer who had six prior lines of chemotherapy and one line of hormonal treatment for her metastatic breast cancer. She received AZD2014 treatment for 4 cycles. Her tumor had a mutation in HRAS, NRAS, TP53 and ERBB2 (Figure 4). In addition, two patients, one each with ovarian and endometrial cancer, had prolonged stable disease and remained on treatment for more than one year.
Figure 4.
Patients on study who achieved confirmed partial responses.
A) A patient with acinar pancreatic cancer who was previously treated with a Whipple’s operation and two lines of gemcitabine-based chemotherapy for metastatic breast cancer. He received 6 cycles of treatment. Arrows denote a mediastinal metastasis. B) A patient with oestrogen receptor positive metastatic breast cancer who had 6 lines of chemotherapy and one line of hormonal therapy for metastatic breast cancer prior to entry in the clinical trial. She received 4 cycles of treatment. The arrow denotes hepatic metastasis.
DISCUSSION
The toxicity profile of AZD2014 had similarities with other TORC 1/2 inhibitors and more generally with allosteric m-TOR inhibitors and these were rash, mucositis, and fatigue (17, 18, 20, 22-25). At 50 mg BD continuous dosing AZD2014 was well tolerated. Interestingly, in this present study there were no instances of grade 3-4 hyperglycemia that had been seen in clinical trials of m-TOR inhibitors (23-25). Patients with diabetes were excluded and all patients had to have a fasting glucose of less than 126 mg/dL (7 mmol/L). Given previous experience of hyperglycemia seen with m-TOR inhibitors, it is not known how AZD2014 would affect glycemic control of patients with type I or type II diabetes. Renal, (20) hepatic (22) and left ventricular dysfunction (20) seen with other m-TORC 1/2 inhibitors were not seen in patients treated with AZD2014. Of note, there were no instances of pneumonitis, seen more generally across m-TOR inhibitors (7, 9, 26) in this study. The tolerability of once a day AZD2014 given continuously and twice a day dosing given intermittently (two days every week) has been subsequently studied and the results will be presented separately when the studies are complete.
The pharmacokinetic profile of AZD2014 showed rapid absorption. Whilst the elimination half-life of AZD2014 was approximately 3 hrs and is shorter than allosteric m-TOR inhibitors such as everolimus, temsirolimus or ridaforolimus, which have half-lives of approximately 24 hrs or longer (23-25), it allows twice-daily dosing and the possibility of more flexible intermittent dosing when used in combination with other anticancer drugs such as cytotoxic chemotherapy or other targeted agents. There are multiple reasons that could give rise to the inter-individual pharmacokinetic variability. Preliminary analysis of some of the potential reasons have been investigated and include differences in % drug bound to protein between patients but a preliminary investigation using measured AZD2014 free plasma concentrations instead of the usual way of using total plasma concentrations to determine PK parameters found that they were equally variable and the relationships with PD no better. It is also not likely to be formulation performance since the drug is a solution and therefore does not require a dissolution step before being absorbed. It could be hypothesised that differences in CYP metabolising enzymes and/or transporter enzymes may affect ability to clear and/or distribute the drug in and out of tissue and affect PK variability. The data in this publication relate to the liquid formulation of AZD2014. Further studies have been conducted to evaluate the PK of the tablet formulation and the effect of food on exposure to AZD2014 and will be submitted for publication once complete. AZD2014 produces >70% total growth inhibition (TGI) at 4500 nM.h free weekly AUC, and ≥100% TGI at 17000 nM.h in MCF7 xenograft models and human PK achieved in this trial was in the range that would be efficacious in this model (on average the 50mg BD solution dose achieved a free weekly AUC of 11050 nM.h, with the vast majority of patients achieving more than 4500 nM.h). In order to provide the comparison of the clinical exposure with the preclinical data the measured clinical exposure of AZD2014 reported in ng.h/mL of total (bound and unbound) drug was converted to nM.h of free (unbound) drug using the molecular weight of AZD2014 (462.56 g/mol) and the measured human plasma protein binding of 5.47% free (unbound) drug.
The PD profile of AZD2014 showed target engagement in normal tissue. POM biomarkers of m-TORC1 and m-TORC2 inhibition, i.e. reduction in levels of p-4EBP1 in PBMNCs and p-AKT in PRP, respectively, were seen at 2 - 8 hrs but recovered at 24 hrs following a single dose of AZD2014, supporting a twice a day schedule. Importantly, reduction in p-AKT was seen at the 25 mg cohort suggesting PD activity at this dose if dose reductions are necessary in future trials. Substantial inter-individual variability was seen in the PK profile and this is likely to influence the pharmacodynamic profile seen in this study. Further studies evaluating PK, PD and PK-PD profiles of the tablet formulation which will be taken forward into later phase clinical trials are warranted. Crucially, it was possible to demonstrate reduction of p-S6 (m-TORC1 inhibition) in all evaluable post-treatment biopsies. Reduction in p-AKT levels was seen in 3/4 assessable post-treatment biopsies and no instances of induction of phosphorylation of AKT were seen in these samples. This is in contrast to increase in phosphorylation of AKT due to a feedback loop via IRS1 which is a possible mechanism of resistance seen when tumor tissue is exposed to allosteric m-TOR inhibitors(13, 14). In addition to the POM PD biomarkers discussed above, more distal, POC biomarkers such as reduction of proliferation (Ki67) and reduction in metabolism (FDG-PET scans) also support evidence of target inhibition in tumor. Overall PD biomarkers have been informative to the trial; normal tissue has shown a PD engagement at 25 - 100 mg BD, which will be taken into consideration while designing intermittent schedules and combination studies with dosing a higher or lower doses of AZD2014. At the MTD of 50 mg BD pre- and post-biopsies and FDG PET scans have confirmed target engagement in tumor tissue.
Single agent efficacy was seen in a population of heavily pre-treated solid tumors. Interestingly, both patients who responded did not have a PIK3CA or AKT mutation, however, the tumors did have activating mutations upstream of m-TORC1/2.
The recommended phase II dose of AZD2014 took into consideration toxicity, PK and PD findings. At this dose, the drug was well tolerated, exhibited a favorable PK profile, showed robust evidence of target engagement and showed evidence of clinical efficacy as a single agent. PD studies indicated target engagement in normal tissue in the range 25 - 100 mg, thus these doses could be explored in future intermittent schedules or taken into consideration if dose reductions are necessary. The clinical efficacy of AZD2014 as a single agent and in combination with other anticancer agents should be explored in clinical trials.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The mammalian target of rapamycin (m-TOR) consists of two essential complexes, m-TORC1 and m-TORC2. Conventional, allosteric m-TOR inhibitors, inhibit m-TORC1 but not m-TORC2 function. This can lead to feedback loops activating PI3K via IRS1 and continued phosphorylation of AKT at Ser473 by uninhibited m-TORC2. AZD2014 is a dual m-TORC1 and m-TORC2 kinase inhibitor. This study identified the maximally tolerated dose and schedule for AZD2014 as 50 mg BID. It was possible to demonstrate inhibition of m-TORC1 inhibition in normal tissue and tumor reduction in the phosphorylation of 4EBP1 and S6, respectively) and, importantly, m-TORC2 inhibition (reduction in p-AKT) normal tissue and tumor. Further, there was evidence of reduced metabolism as evidenced by reduction in SUVmax in FDG PET scans. Other key findings included reduction in proliferation (Ki67 reduction in post treatment biopsies and 2 partial responses). The dual m-TORC1/2 inhibitor, AZD2014, should be investigated further.
Acknowledgments
Financial support:
This study was funded by AstraZeneca. Infrastructure support was provided by a Cancer Research UK Joint Phase I Clinical Core Grant [grant number C51/A6883] to The Institute of Cancer Research and The Royal Marsden. The recruiting sites acknowledge infrastructural funding from the Experimental Cancer Medicine Centres and NIHR Biomedical Research Centre initiatives.
Footnotes
Declaration of interests
WMB, EAH, MC, GB, CW, SG and EO are employees of AstraZeneca and receive AstraZeneca shares; JdB and MR have had consulting or advisory roles with AstraZeneca; UB has received institutional research funding from AstraZeneca. BB, MO, ED, MP, and AG have declared no conflict of interest.
Previous presentation of data: This work has been presented in part at the 2012 ASCO Annual Meeting, Chicago (Banerji U et al, J Clin Oncol 30, 2012 (suppl; abstr 3004).
REFERENCES
- 1.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao D, Arojo O, et al. Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat Cell Biol. 2013;15:1340–50. doi: 10.1038/ncb2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goral S, Helderman JH. The evolution and future of immunosuppression in renal transplantation. Semin Nephrol. 1997;17:364–72. [PubMed] [Google Scholar]
- 6.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 7.Albiges L, Chamming’s F, Duclos B, Stern M, Motzer RJ, Ravaud A, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:1943–53. doi: 10.1093/annonc/mds115. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 12.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pike KG, Malagu K, Hummersone MG, Menear KA, Duggan HM, Gomez S, et al. Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett. 2013;23:1212–6. doi: 10.1016/j.bmcl.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Janes MR, Vu C, Mallya S, Shieh MP, Limon JJ, Li LS, et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia. 2013;27:586–94. doi: 10.1038/leu.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabernero J, Cervantes A, Gordon M, Chiorean E, Burris H, Macarulla T, et al. A phase I, open label, dose escalation study of oral mammalian target of rapamycin inhibitor INK128 administered by intermittent dosing regimens in patients with advanced malignancies. Cancer Res. 2012;72 Abstract CT-02. [Google Scholar]
- 18.Capelan M, Kumar P, Tolcher A, Zivi A, Desai M, Papadopoulos K, et al. A first-in-human Phase I study of DS-3078a, an oral TORC1/2 inhibitor, in patients with advanced solid tumors: Preliminary results. Mol Cancer Ther. 2013;12 Abstract C173. [Google Scholar]
- 19.Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10:1394–406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 20.Tan D, Dumez H, Olmos D, Sandhu S, Hoeben A, Stephens A, et al. First-in-human phase I study exploring three schedules of OSI-027, a novel small molecule TORC1/TORC2 inhibitor, in patients with advanced solid tumors and lymphoma. J Clin Oncol. 2010;28 Abstract 3006. [Google Scholar]
- 21.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 22.Naing A, Aghajanian C, Raymond E, Olmos D, Schwartz G, Oelmann E, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer. 2012;107:1093–9. doi: 10.1038/bjc.2012.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo M, Buckner JC, Erlichman C, Pollack MS, Boni JP, Dukart G, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–63. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 25.Hartford CM, Desai AA, Janisch L, Karrison T, Rivera VM, Berk L, et al. A phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin Cancer Res. 2009;15:1428–34. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demetri GD, Chawla SP, Ray-Coquard I, Le Cesne A, Staddon AP, Milhem MM, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31:2485–92. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.