Abstract
The mammalian superior colliculus (SC) is a laminar midbrain structure that translates visual signals into commands to shift the focus of attention and gaze. The SC plays an integral role in selecting targets and ultimately generating rapid eye movements to those targets. In all mammals studied to date, neurons in the SC are arranged topographically such that the location of visual stimuli and the endpoints of orienting movements form organized maps in superficial and deeper layers, respectively. The organization of these maps is thought to underlie attentional priority by assessing which regions of the visual field contain behaviorally relevant information. Using voltage imaging and patch-clamp recordings in parasagittal SC slices from the rat, we found the synaptic circuitry of the visuosensory map in the SC imposes a strong bias. Voltage imaging of responses to electrical stimulation revealed more spread in the caudal direction than the rostral direction. Pharmacological experiments demonstrated that this asymmetry arises from GABAA receptor activation rostral to the site of stimulation. Patch-clamp recordings confirmed this rostrally directed inhibitory circuit and showed that it is contained within the visuosensory layers of the SC. Stimulation of two sites showed that initial stimulation of a caudal site can take priority over subsequent stimulation of a rostral site. Taken together, our data indicate that the circuitry of the visuosensory SC is hard-wired to give higher priority to more peripheral targets, and this property is conferred by a uniquely structured, dedicated inhibitory circuit.
Keywords: voltage imaging, electrophysiology, eye movements, visual spatial attention, vision, superficial layers, motor layers, rodent brain slice, rat
visual attention has been hypothesized to involve two interdependent mechanisms, “bottom-up” reflexive responses and “top-down” volitional attention shifts (Bisley and Goldberg 2010; Itti and Koch 2001). Top-down control of attention depends on the nature of a task and is significantly slower as it involves higher cognitive centers (Baluch and Itti 2011; Gilbert and Sigman 2007; Hopfinger et al. 2000). By contrast, bottom-up attention shifts are rapid reflexes depending only on lower-processing centers. Attention is hypothesized to rely on salience maps, representations of visual space in which salient objects compete in a winner-takes-all manner (Fecteau and Munoz 2006; Itti and Koch 2001; Koch and Ullman 1985). Integration of top-down and bottom-up inputs form priority maps that guide the focus of attention (Baluch and Itti 2011; Fecteau and Munoz 2006; Serences and Yantis 2006).
The neuronal architecture that creates saliency maps is poorly understood, particularly in regions of association cortex like the lateral intraparietal region of the cerebral cortex where there is no obvious retino- or spatiotopic map (Bisley 2011; Falkner et al. 2013). The mammalian superior colliculus (SC), in contrast, contains a well-organized and evolutionarily conserved map of visual and movement space in its superficial and motor layers, respectively (Drager and Hubel 1975; McHaffie and Stein 1982; Robinson 1972; Schiller and Koerner 1971; Sparks 1986; Tiao and Blakemore 1976; Vanegas 1984; Wurtz and Goldberg 1971). In this case, it is easy to imagine how local inhibition between neighboring regions of the map would produce a winner-takes-all or saliency map across the surface of the SC by contributing to the detection of visual stimuli that influence saccade choice (Basso and Wurtz 1998; Kim and Basso 2008; McPeek and Keller 2004; Munoz and Istvan 1998). However, the role of inhibition in the SC maps, particularly the motor map that controls saccades, remains controversial (Lee et al. 2007; Meredith and Ramoa 1998).
Inhibition plays critical roles in the processing of sensory information and the extraction of meaningful details from the environment (Isaacson and Scanziani 2011). In addition to local mechanisms, there must also be long-range mechanisms that mediate competition between a currently fixated object located centrally and a second object, usually located peripherally, which attracts attention. Whether hard-wired intrinsic circuits contribute to such functions is unknown. Because the vertebrate SC is known to mediate orienting behaviors and to play a role in target selection (Carello and Krauzlis 2004; Horwitz and Newsome 2001; Kardamakis et al. 2015; Krauzlis et al. 2013), we explored the network activity along the amplitude-encoding axis of the SC of the rat. SC slices cut in the parasagittal plane isolated this circuitry, and voltage imaging (Cohen and Salzberg 1978) revealed a surprising and very strong bias in the spread of responses to electrical stimulation, in some instances extending >3-fold further in the caudal direction than in the rostral direction. Patch-clamp recording revealed this asymmetry to be shaped by a GABAergic inhibitory circuit contained within the sensory layers. This asymmetric inhibition introduces a bias to priority maps, emphasizing sensory inputs from more peripheral locations at the expense of sensory inputs from more central locations. These findings suggest that within the SC, attention shifts may be influenced by hard-wired intrinsic circuitry.
MATERIALS AND METHODS
Slice preparation.
Sprague-Dawley rats of either sex (3–6 wk old) were decapitated under a state of narcosis induced by CO2 inhalation in accordance with the recommendations of the Panel on Euthanasia of the Veterinary Medical Association and the United States Public Health Service policy on the humane care and use of laboratory animals. All protocols were approved by the animal care and use committee at the University of Wisconsin, Madison. Brains were quickly removed and immersed in ice-cold cutting solution (composition in mM: 124 NaCl, 3.2 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 CaCl2, 6 MgSO4, and 10 glucose) bubbled with 95% O2-5% CO2. Brains were sectioned at 400 μm in a parasagittal plane using a Leica VT1200S tissue slicer. Slices were transferred to a recovery chamber containing bubbled artificial cerebrospinal fluid (aCSF; cutting solution with 2.5 mM CaCl2 and 1.2 mM MgSO4) at room temperature (21–24°C) and were allowed to recover for ∼60 min.
Voltage imaging.
Following recovery, slices were stained in oxygenated aCSF containing the absorbance voltage-sensitive dye RH482 (0.05 mg/ml; Chang and Jackson 2006; Konnerth et al. 1987; Vokoun et al. 2010) for ∼45 min. Experiments were performed at room temperature in a submerged recording chamber continuously perfused with oxygenated aCSF. Slices were visualized on a Reichert-Jung Diastar upright microscope (AMETEK, Buffalo, NY) using a ×10 Olympus UPlanApo objective [numerical aperture (NA) = 0.4; Center Valley, PA] and illuminated by a 100-W tungsten-halogen bulb powered by a Kepco ATE 36-30 DM power supply (Flushing, NY) passed through a 700- ± 25-nm band-pass filter (Andover, Salem, NH). High-resolution images were captured using a Hamamatsu C2400 CCD camera (Middlesex, NJ) connected to a Windows-based personal computer (PC) with a frame grabber (Data Translation, Marlboro, MA). Optical responses presented as changes in the amount of transmitted light were captured using a 464-channel fiber optic photodiode system (Chang and Jackson 2006; Wu and Cohen 1993). Signals were amplified to 5 V/nA photocurrent, low-pass filtered at 500 Hz using a 4-pole Bessel filter, and digitized at a frame rate of 5 kHz with a DAP 5200 data acquisition board (Microstar Laboratories, Bellevue, WA). Imaging data were acquired using an in-house computer program, PhotoZ (Chang 2006), which was also used for signal processing and initial analyses (see below). Images were acquired for 800 ms; a shutter opened 200 ms before recording and closed immediately after to reduce light exposure. Acquisition parameters, stimulus protocol, and light exposure were adjusted in PhotoZ. Electrical stimulation evoked responses that were visualized as change in transmitted light intensity divided by the resting light intensity (ΔI/I).
We applied stimulation through aCSF-filled electrodes fabricated from borosilicate glass capillaries (King Precision Glass, Claremont, CA) with 3- to 10-μm tips. Single pulses (200 μs, 100 μA) were applied 200 ms following the start of data acquisition using a Grass S88 wave pulse generator and a Grass photoelectric stimulus isolation unit (Grass Technologies, Warwick, RI) generating a pulse from the negative pole.
Patch-clamp recordings.
After recovery, slices were placed in a submerged recording chamber continuously perfused with oxygenated aCSF. Patch electrodes fabricated from borosilicate glass had resistances of 3–5 MΩ when filled with intracellular solution (composition in mM: 130 K-gluconate, 10 HEPES, 7 KCl, 1 EGTA, 2 Na2ATP, 2 MgATP) containing 2–5 mg/ml Lucifer yellow for post hoc staining of neuronal morphology. Slices were visualized using a Zeiss Axioskop upright microscope with IR-DIC optics, a ×63 water-immersion objective (Carl Zeiss Microscopy, Thornwood, NY), and a Hamamatsu C2400 CCD camera. After establishing whole cell recordings, current or voltage were recorded using an Axopatch 200B amplifier, digitized with a Digidata 1400A interface, and connected to a Windows-based PC running pCLAMP 10.0 (Molecular Devices, Sunnyvale, CA). Iontophoresis electrodes filled with 1 M l-glutamate (pH 8.0) had resistances of 28–32 MΩ. Iontophoretic stimulation pulses (1 s, 0.1–0.5 μA) were set with the pCLAMP software and applied through an A385 stimulus isolator (World Precision Instruments, Sarasota, FL).
Morphological processing.
Following whole cell patch-clamp recordings, neurons were visualized under epifluorescence to ascertain whether Lucifer yellow had filled the dendritic arbor. Slices were fixed for 15–30 min in paraformaldehyde (4% in PBS), washed several times with PBS, placed in PBS for up to 14 days at 4°C, mounted on gelatin-coated microscope slides (LabScientific, Highlands, NJ), and dehydrated in 2- × 15-min sequential incubations in 70, 90, and 100% ethanol. Following dehydration, slices were cleared with methyl salicylate, coverslipped, and viewed on an Ultima two-photon microscope (Bruker, Middleton, WI) illuminated by a Chameleon Ti:Sapphire laser (Coherent, Santa Clara, CA) tuned to 920 nm using a ×20 Olympus XLUMPlanFLN water-immersion objective (NA = 1.0). Emitted light was filtered at 525 ± 35 nm (Chroma Technology, Bellows Falls, VT).
Data processing and analysis.
Voltage imaging data acquisition, signal processing, and some analyses were performed in PhotoZ. Traces were filtered temporally using a three-point binomial filter, and baseline drift was corrected using a third-order polynomial. Peak ΔI/I values from each photodiode were used to create color maps of activity spread.
Distances from stimulating electrodes were measured by drawing a line perpendicular to the dorsal surface of the SC slice through the tip of the stimulating electrode, thus delineating rostral and a caudal side in relation to the stimulus. Distances to individual photodiodes were measured from either the tip of the stimulating electrode (for visuosensory layer stimulation) or the stratum griseum superficiale (SGS)-stratum opticum (SO) border intercept with the perpendicular line (for motor layer stimulation) using Adobe Photoshop CS6 Extended image analysis toolkit (Adobe Systems, San Jose, CA).
Areas of spread were measured by thresholding peak ΔI/I maps at 50% and measuring the areas on each side of the line perpendicular to the dorsal slice surface. Long axes of signal propagation were measured from the same thresholded areas.
Maximal ΔI/I of the initial spike (in the 0- to 10-ms window following stimulation) and afterdepolarization (ADP; 10- to 200-ms window following stimulation; Vokoun et al. 2010) were plotted vs. the distance from the stimulating electrode (for visuosensory layer stimulation) or the intercept of the SGS and SO border with the perpendicular line (for motor layer stimulation). A LOWESS spline nonlinear curve, which was approximated using a Gaussian function (r2 > 0.9), was fitted to the data.
Patch-clamp recordings were analyzed in Clampfit 10, and unprocessed traces were exported as Axon text files.
Final processing of voltage imaging data, patch-clamp recordings, and two-photon z-stacks was performed in Volumetry v.G6a (provided by Grant W. Hennig). Individual traces were smoothed using an unweighted sliding-average algorithm (m = 3). Spatiotemporal maps representing the spread of activity were constructed using rectangular regions of interest and averaging the absorbance signal perpendicular to the long axis. Maximal projections from two-photon microscope z-stacks were constructed and a green-fire-blue lookup table applied to emphasize the individual neuronal structure.
Statistical analysis.
Statistical analyses were performed in Prism 5.0 (GraphPad Software, La Jolla, CA). Results are expressed as means ± SE and statistical significance assessed using Student's unpaired t-test or one-way ANOVA, where appropriate, with P < 0.05 as the cutoff. Uppercase N represents the number of animals used, and lowercase n represents the number of neurons from which patch-clamp recordings were made.
Pharmacology.
Drugs were bath-applied through the solution bathing the slices. Gabazine hydrobromide (SR-95531) was used at 5 μM (Sigma-Aldrich, St. Louis, MO). CGP 52432 ((3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl)phosphinic acid) was used at 3 μM (Tocris, Bristol, United Kingdom).
RESULTS
Rostrocaudal asymmetry.
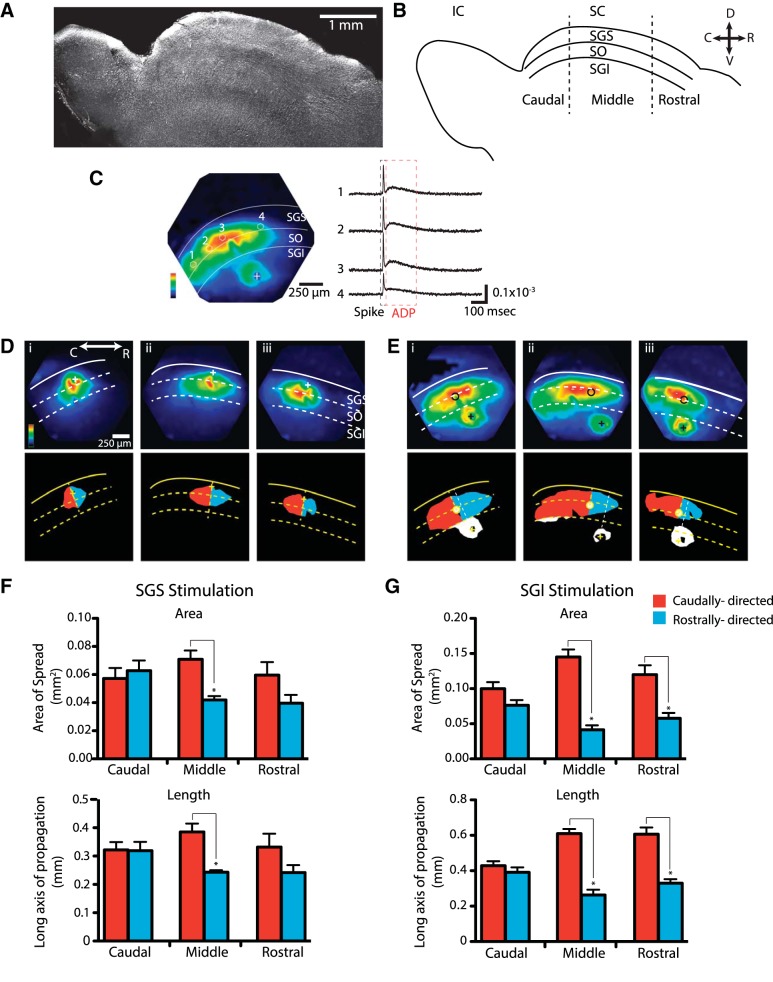
The rat SC extends ∼4 mm along the rostrocaudal axis (Fig. 1, A and B). The SGS and SO indicated in Fig. 1B are the major visuosensory layers and are referred to here simply as visuosensory layers. The stratum griseum intermediale (SGI) is referred to as the motor layer because the neuron projecting to the brain stem for the initiation of orienting movements resides in this layer. In these parasagittal slices, voltage imaging revealed responses to electrical stimulation (100 μA, 200 μs) that resemble those reported previously in coronal slices (Vokoun et al. 2010) with two distinct temporal components, an initial spike that rises and falls rapidly in the 1st ∼10 ms and an ADP that rises slowly immediately after the initial spike and lasts >200 ms (Fig. 1C, right). The use of the absorbance dye RH482 (Konnerth et al. 1987) in these experiments was particularly suitable as this dye preferentially stains neurons over glia (Kojima et al. 1999). Consistent with this, neither the initial spike nor the ADP as measured with RH482 is sensitive to glial glutamate transporter antagonism (Vokoun et al. 2010). The initial spike reflects rapid synaptic transmission along with antidromic action potentials (Vokoun et al. 2010). Reverberative excitatory circuit activity (Saito and Isa 2003) may contribute to the ADP.
Fig. 1.
Asymmetric spread of responses in parasagittal superior colliculus (SC) slices. A: low-magnification gradient-contrast micrograph of a rat parasagittal slice. B: schematic of a parasagittal slice shows the caudal (C), middle, and rostral (R) subdivisions of the SC highlighted here, separated by dashed lines. Layers of the SC are demarcated and labeled as: SGS, stratum griseum superficiale; SO, stratum opticum; SGI, stratum griseum intermediale; and IC, inferior colliculus. The SO and SGS constitute the visuosensory layers. D, dorsal; V, ventral. C, left: maximal amplitude map of voltage imaging responses evoked by electrical stimulation in the SGI. Response amplitude was normalized to the maximum in the field of view and scaled to 1 according to the color bar in the lower left corner. “+” Indicates the site of stimulation. Right: traces from individual photodiodes, as labeled on the map, reveal the 2-component response, an initial spike (black dashed box) and a subsequent afterdepolarization (ADP; red dashed box). D: maps from voltage imaging of responses evoked by electrical stimulation of the SGS at caudal (i), middle (ii), and rostral (iii) locations (see B). Maps were normalized as described in C. Outline images below were created using a threshold of 50%. Yellow dashed lines perpendicular to the dorsal surface through the site of stimulation (indicated with a +) subdivided the slice as rostral and caudal. Red fills show the spread in the caudal direction from the normal line; blue fills show the rostral spread. E: response maps as in D but to electrical stimulation in the SGI in caudal (i), middle (ii), and rostral (iii) regions. The site in the SGS with shortest latency responses (SGI stimulation) are indicated by a circle. Stimulus 100 μA, 200 μs. The yellow dashed line perpendicular to the dorsal surface runs through the site of stimulation, as in D, and the white dashed line perpendicular to the dorsal surface runs through the site of onset of responses in the sensory layers. F: using the line normal to the slice surface through the site of stimulation, areas and distances to the left (caudally directed; red) and right (rostrally directed; blue) were calculated for the threshold maps in D for stimulation in the SGS. Areas (above) and distances (below) illustrate the symmetry of response spread in the caudal region and asymmetry in the middle region. The differences between rostrally directed and caudally directed spread in the rostral region were not statistically significant. G: as in F but with SGI stimulation. Responses spread symmetrically in the caudal region, but in the middle and rostral regions responses showed a clear and statistically significant asymmetry. *P < 0.05.
Voltage imaging revealed a spatial spread of responses in parasagittal slices that in some respects resembled that seen in coronal slices (Vokoun et al. 2010). In addition to the similar temporal sequence of initial spike and ADP, stimulation in either the SGS or SGI evoked responses in the sensory layers that were more robust than responses in the motor layer (Fig. 1C, left). However, voltage imaging also revealed a striking difference in the spatial pattern of spread of responses in parasagittal SC slices compared with coronal slices. In coronal slices, responses spread equally in both medial and lateral directions. In parasagittal slices, responses spread asymmetrically, propagating much further in the caudal direction than the rostral direction. Within the 1.2-mm field of view of our imaging system, we selected different locations along the rostral-caudal axis in the caudal, middle, and rostral regions of the SC (Fig. 1B). Each of these regions comprised slightly less than one-third of the ∼4-mm rostral-caudal SC length. Distinct patterns of spread were observed within the different regions. Stimulation in the caudal third, either in the visuosensory (Fig. 1Di) or motor (Fig. 1Ei) layers, resulted in symmetric spread through the visuosensory layer in the rostral and caudal directions, similar to the mediolateral spread seen in coronal slices (Vokoun et al. 2010). However, stimulation within the middle and rostral thirds of either the visuosensory (Fig. 1, Dii and Diii) or motor (Fig. 1, Eii and Eiii) layer elicited responses in the visuosensory layers with a distinct skew in the caudal direction. These differences can be visualized in maps of peak response amplitude encoded as color (Fig. 1, D and E, top) or in maps thresholded at 50% of the maximal response (Fig. 1, D and E, bottom). In these maps, we highlighted the spread away from the site of stimulation in the caudal and rostral directions to facilitate the comparison. The caudally directed spread of activity within the visuosensory layers was more apparent with motor layer stimulation than visuosensory layer stimulation (Fig. 1, D and E). This likely reflected the significantly larger area of the visuosensory layers activated by stimulating the motor layers (73.7 ± 11.1% larger than with sensory layer stimulation) due to activation of an ascending excitatory pathway (Ghitani et al. 2014) making the skew easier to distinguish.
We quantified the asymmetry of spread in 50% threshold images (Fig. 1, D and E, bottom) using dividing lines perpendicular to the dorsal surface through the site of stimulation. In experiments with visuosensory layer stimulation, the site of stimulation coincided with the site of onset. In the caudal region, motor layer stimulation did not produce responses that were significantly different from the normal line drawn through the stimulus site. However, in the middle and rostral regions, motor layer stimulation produced responses in visuosensory layers that initiated slightly caudal to the dividing line (Fig. 1E) at distances of 184.8 ± 24 μm (N = 14) in the middle region and 87.5 ± 27 μm (N = 8) in the rostral region. This skew may reflect the trajectory of the ascending excitatory pathway from motor to visuosensory layers (Ghitani et al. 2014). For both visuosensory and motor layer stimulation, we quantified asymmetry with respect to the site of onset in the visuosensory layer. On each side of the dividing line, we calculated both the area within the >50% response region and the distance along the axis of propagation. These quantities show strong and statistically significant differences for both the areas and distances of caudal vs. rostral spread in the middle third of the SC with either visuosensory (Fig. 1F) or motor layer (Fig. 1G) stimulation. By contrast, none of these indices of spread show statistically significant differences in the caudal third of the SC for stimulation at either site (Fig. 1, F and G). Responses in the rostral third of the SC exhibited asymmetry, which was statistically significant for motor layer stimulation (Fig. 1G) but not for visuosensory layer stimulation (Fig. 1F). This analysis provides quantitative support for the asymmetric spread seen in response maps (Fig. 1, D and E).
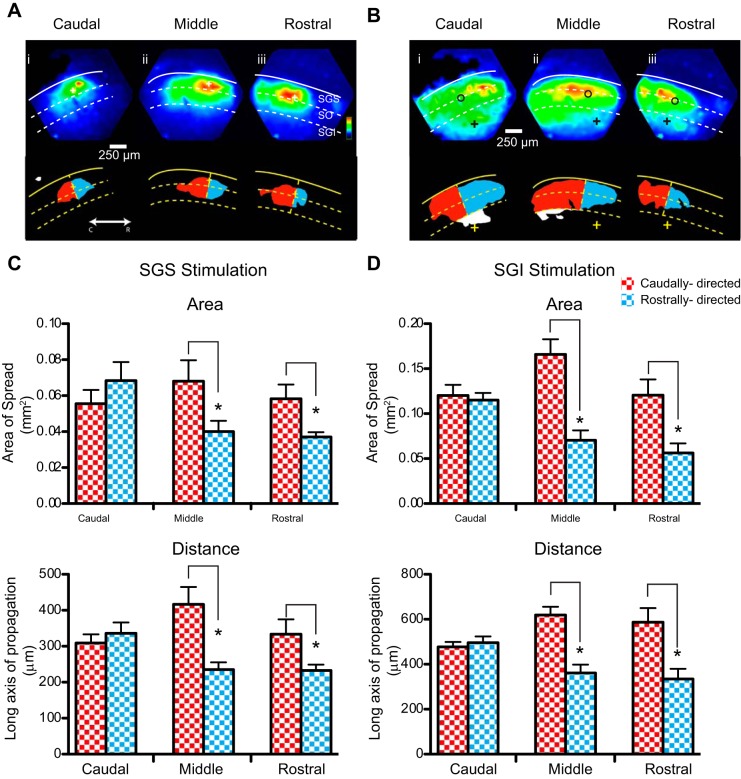
Color-encoded maps of response amplitude provide an overall view of how activity spreads through a slice, but the initial spike has a higher amplitude than the ADP (Fig. 1C), exceeding the amplitude of the ADP by two- to fourfold (P < 0.0001). As a result, the maps of maximal amplitude represent the spread of the initial spike (Fig. 1, D and E). Simply taking the maximum response renders a map of the spatial distribution of the initial spike. To ascertain whether this asymmetry is also present in the ADP component of the response, we measured the maximal amplitude of the response from 10 to 200 ms following the stimulus, thus omitting the initial spike from the analysis. Maximal projections made in this fashion showed that the ADP had the same spatial asymmetry as the initial spike in the middle and rostral regions of the SC slices (Fig. 2, A and B). Plotting the >50% response region for the ADP component to both visuosensory (Fig. 2C) and motor (Fig. 2D) layer stimulation revealed a caudal bias in the spread of excitation for both the area (top) and the long axis of propagation (bottom) in the middle and rostral regions.
Fig. 2.
Asymmetric spread of ADP excitatory component in parasagittal SC slices. A: maximal projection maps of voltage imaging data obtained 10–200 ms following superficial layer stimulus in the caudal (i), middle (ii), and rostral (iii) SC regions. Corresponding 50% thresholded images are shown below the maximal projections and indicate caudal (red) and rostral (blue) spread from the origin of the stimulus (+). B: maximal projections of voltage imaging data of the ADP following motor layer stimulation in the caudal (i), middle (ii), and rostral (iii) regions. Fifty percent thresholded images are shown below and indicate caudal (red) and rostral (blue) spread from the site of initial response in the superficial layers (open circle). C: areas (top) and distance of long axis of propagation (bottom) of the ADP in the caudal (red checker) and rostral (blue checker) direction in response to superficial layer stimulation in the caudal, middle, and rostral regions. D: spread of ADP, as in C, in response to motor layer stimulation. *P < 0.05.
GABAA receptor shaping of response asymmetry.
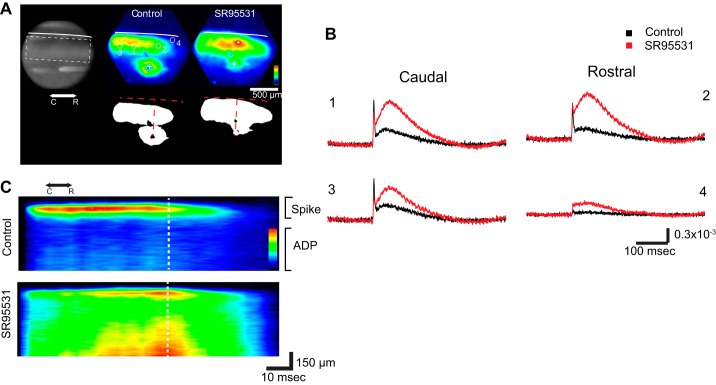
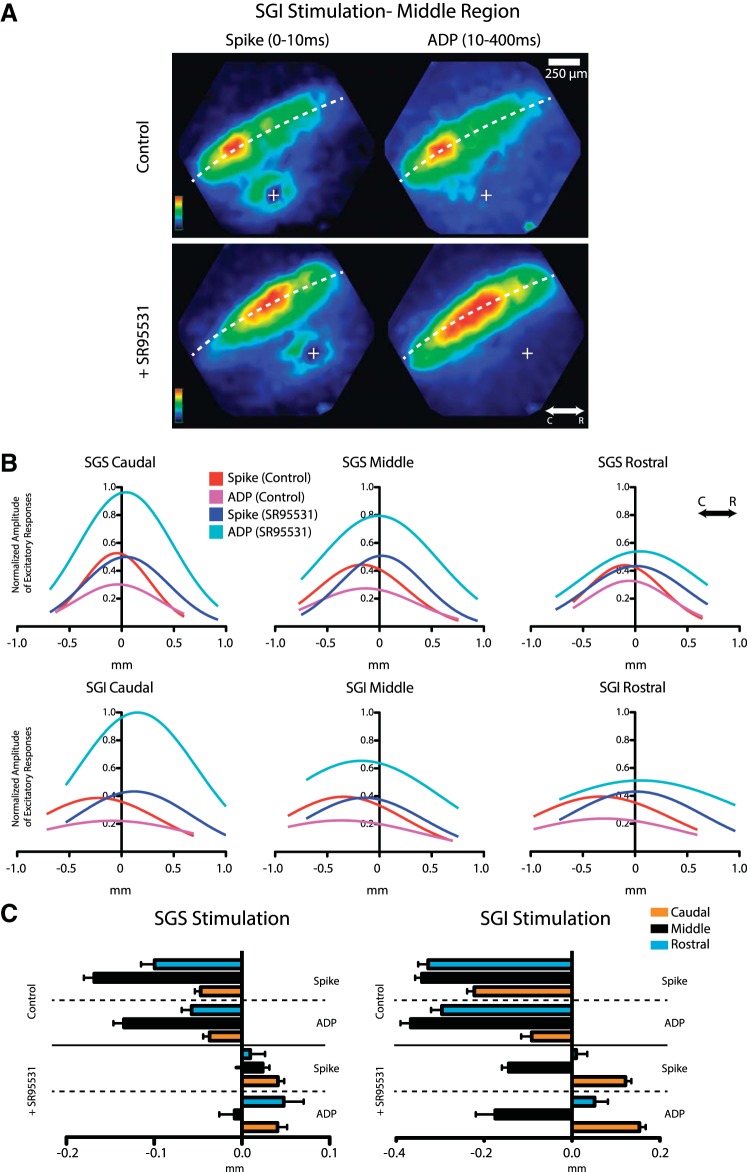
The visuosensory layers of the SC have a high density of GABAA and GABAB receptors (Bowery et al. 1987; Mize 1992), and GABAergic inhibition has been shown to play distinct roles in SC behaviors (Binns and Salt 1997). We therefore tested the role of GABA-mediated inhibition in shaping the distinctive spatial patterns of spread seen in these parasagittal SC slices. We found that blocking GABAA receptors with the antagonist SR-95531 (5 μM) reduced and even nearly eliminated the asymmetry of response maps, resulting in approximately equal spread in the rostral and caudal directions (Fig. 3A). However, as noted for Fig. 1 above, the control map in Fig. 3A reflects the initial spike, and we wanted to assess the role of GABAA receptors in the asymmetry of both response components. GABAA receptor blockade increases responses to electrical stimulation in coronal SC slices, but the increase in the amplitude of the ADP was much larger than the increase in the amplitude of the initial spike (Vokoun et al. 2010). In parasagittal slices, we obtained a similar result; SR-95531 had a greater effect on the ADP and increased its amplitude above that of the initial spike. However, these effects varied with location (Fig. 3B). The increases in the ADP were in general larger than the increases in the initial spike, and the increases in the responses rostral to the site of stimulation were larger than in responses caudal to the site of stimulation. To visualize the spatial variations in the effect of SR-95531 on both components, spatiotemporal maps were constructed in which the amplitude within a band of interest was averaged along a roughly horizontal trajectory through the visuosensory layer (x-axis) and plotted vs. time (y-axis; Fig. 3C). The ADP is barely visible in the control spatiotemporal plot because of its small amplitude compared with the initial spike, but following SR-95531 addition both components become clear and revealed nearly symmetric distributions. GABAA receptor blockade enhanced the spread of the initial spike and ADP preferentially in the rostral direction and reduced the asymmetry. This loss of asymmetry with SR-95531 application was also illustrated by the position of the maximal response within the visuosensory layers, which was much closer to the site of stimulation in SR-95531 than in control experiments for both the initial spike and ADP (Fig. 3, A and C).
Fig. 3.
Role of GABAA receptors in directing signal spread. A, left: charge-coupled device (CCD) image of an SC slice with a stimulating electrode in the middle region of the SGI. Right: color maps of maximal response (above) and 50% thresholded image (below) before (Control) and after SR-95531 application. Control responses show a greater caudally directed spread, and this asymmetry was reduced by the drug. The approximately horizontal white (above) and red (below) dashed curves represent the dorsal surface of the slice. The approximately vertical dashed line represents the perpendicular from the slice surface through the stimulus site. B: optical traces from locations rostral and caudal to the site of stimulation show responses to electrical stimulation before (black) and after application of the GABAA receptor antagonist SR-95531 (5 μM). Locations 1 and 2 are near the site of stimulation (approximately 150–200 μm), and locations 3 and 4 are more distant (∼500 μm). Note the large increase in ADP amplitude following drug application. C: spatiotemporal maps with average amplitude within the region of interest (dashed rectangle in the CCD image in A) encoded as color and plotted against distance from the site of stimulation (vertical dashed white line) as the x-axis and time as the y-axis. These maps show the shift in response profile from a peak caudal to the site of stimulation to a peak more central with an increase in rostrally directed spread. The map also shows the spatial register between the initial spike and ADP as well as the substantial increase in the ADP following SR-95531 application.
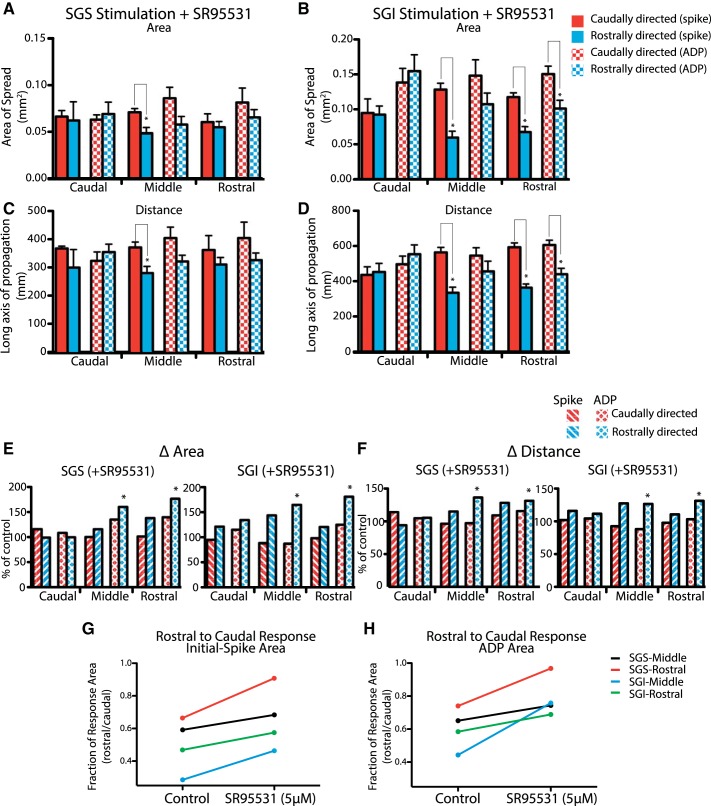
To quantify the effect of GABAA receptor-mediated inhibition on response spread and asymmetry, we evaluated the area and distance of spread in the caudal, middle, and rostral thirds of the SC (as defined in Fig. 1B) of both the initial spike and the ADP. In the caudal region, control responses were already symmetric for both response components (Figs. 1 and 2), and SR-95531 left both areas and distances of spread in the two directions statistically indistinguishable for both visuosensory layer stimulation (Fig. 4, A and C; P values for all between 0.35 and 0.85; N = 4) and motor layer stimulation (Fig. 4, B and D; P values 0.2–0.82; N = 4). Visuosensory layer responses to stimulation of either the visuosensory or motor layers in the caudal region of the SC showed little change from controls (Fig. 4, E and F). GABAA receptor blockade, however, had much greater effects on the spatial dynamics of the ADP in the middle and rostral thirds of the SC. The reduction in skew of the ADP compared with control reflected statistically significant increases in the area (Fig. 4E; middle region: P = 0.017, N = 7; rostral region: P = 0.007, N = 8) and distance of propagation in the rostral direction (Fig. 4F; middle region: P = 0.012, N = 14; rostral region: P = 0.013, N = 8). This produced responses that spread symmetrically along the rostrocaudal axis in terms of area and long axis of propagation (P values 0.07–0.38; N = 8). GABAA receptor blockade produced no significant changes in spread in the caudal direction throughout the SC in terms of area or distance. Similar results were seen with motor layer stimulation where in the rostral third of the SC a small but statistically significant asymmetry remained in the presence of SR-95531 (Fig. 4, B and D). However, responses in areas rostral to the stimulus were significantly larger than controls (Fig. 4, E and F). Thus GABAergic inhibition biased the spread of responses, and GABAA receptor blockade eliminated this bias in the slow component of the responses.
Fig. 4.
GABAA receptors and asymmetry. Spread in the rostral and caudal directions of the initial spike and ADP in the presence of SR-95531. Asymmetry is represented by the relative areas within the 50% response contour falling to the caudal or rostral side of the perpendicular line. Red, caudal spread (spike); blue, rostral spread (spike); red checkers, caudal spread (ADP); blue checkers, rostral spread (ADP). A and B: areas of responses to SGS stimulation (A) and SGI stimulation (B). C and D: distance of spread in response to SGS stimulation (C) and SGI stimulation (D). E: relative increase in the area of responses following SR-95531 application in the caudal spike (red-striped), rostral spike (blue-striped), caudal ADP (red diamond), and rostral ADP (blue diamond) with stimulation in the SGS (left) or SGI (right). F: same analysis as in E, for the changes in distance of spread. G: ratio of rostral to caudal responses of the initial spike area before and after SR-95531 application. H: ratio of rostral to caudal responses of the ADP area before and after SR-95531 application. *P < 0.05.
Application of SR-95531 had a less pronounced effect on the spatial distribution of the initial spike. The observed increase in area (Fig. 4E) and distance (Fig. 4F) in rostrally directed spread in the middle and rostral regions with both SGS and SGI stimulation were not significantly larger than control. In the presence of SR-95531, spike distributions with motor layer stimulation maintained a significant caudal bias in both area (Fig. 4B; P = 0.001, middle region; P = 0.0005, rostral region) and distance (Fig. 4D; P = 0.0001, middle region; P = 0.001, rostral region) in the middle and rostral regions. Superficial layer stimulation revealed a similar asymmetry in initial spike area (Fig. 4A; P = 0.008) and distance (Fig. 4C; P = 0.01; N = 8) in the middle region, but in the rostral region initial spikes spread symmetrically. Application of SR-95531 thus had no statistically significant effect on the spatial distribution of the initial spike, although the effective asymmetry was reduced due to the increase in the area and distance of initial spike spread rostral to the site of stimulation.
This trend toward symmetry can be seen when the rostral-to-caudal ratio is evaluated before and after SR-95531 for the middle and rostral regions. Both the initial spike (Fig. 4G) and the ADP (Fig. 4H) ratios trended toward symmetry, indicating an increase in rostrally directed excitation. This analysis further supports the hypothesis that rostrally directed GABAergic inhibition underlies the asymmetric spread of activity that is seen in control conditions.
As a final comparison of the initial spike and the ADP in their asymmetry and sensitivity to GABAA receptor blockade, we focused on different time windows for the initial spike (<10 ms) and the ADP (10–200 ms). We evaluated the response amplitudes along a path parallel to the dorsal surface of a slice (Fig. 5A) and fitted a Gaussian to the response amplitude vs. position data (Fig. 5B). These fits revealed that SR-95531 produced shifts of variable magnitude in the rostral (positive) direction for both the initial spike and ADP with both SGI and SGS stimulation. The distances from the response peak to the site of stimulation provided a measure of asymmetry (Fig. 5C). Under control conditions, we observed caudal displacements of the peak response with either visuosensory or motor layer stimulation with displacements of similar magnitude in both the initial spike and ADP. In all cases, SR-95531 shifted these displacements in the rostral (positive) direction; in the caudal region, SR-95531 reversed the asymmetry and shifted the displacements to positive values. This comparison of the two components indicated that the rostrocaudal asymmetry of responses in the sensory layers was manifest similarly in both the initial spike and ADP. This result suggests a tight coupling between these two temporal phases of the response to electrical stimulation.
Fig. 5.
Spread of initial spike and ADP. A: representative response amplitude maps of the initial spike (left column) and the ADP (right column) before (top) and after (bottom) application of SR-95531 following SGI stimulation in the middle region. The dashed white line traces the middle of the SGS, which was used to track the distance of spread plotted in B. B: distributions of initial spike and ADP amplitude represented by Gaussian fits in response to SGS and SGI stimulation in the caudal, middle, and rostral regions. X-axis origin represents the line perpendicular to the dorsal surface through the site of stimulation with negative x-values representing caudal and positive representing rostral. C: mean values of the position of the maximal initial spike and ADP from the Gaussian fits in B. N = 9.
We found no evidence of a role for GABAB receptors. The GABAB receptor antagonist CGP 52432 (3 μM) produced no changes in the pattern of activation (N = 3), although it did produce a small (∼5%) uniform increase in the amplitude of both the initial spike and ADP (data not shown). Thus the inhibitory circuitry responsible for the asymmetry in response spread employs GABAA receptors rather than GABAB receptors. The SC contains all three major classes of GABA receptors, ionotropic GABAA and GABAC receptors and metabotropic GABAB receptors (Bowery et al. 1987; Chebib and Johnston 1999; Mize 1992; Nicoll et al. 1990). Recent evidence indicates that GABAC receptors are exclusively on GABAergic interneurons, which disinhibit visuosensory layer neurons projecting to thalamic nuclei (Schmidt et al. 2001). Therefore, GABAC receptors are unlikely to be involved in the circuit reported here. The highly selective GABAA receptor antagonist SR-95531 induced profound increases in response amplitude and spread in the rostral direction within SC, whereas GABAB receptor blockade had little effect. Thus the rostrally directed inhibition revealed here depends on GABAA receptor-dependent inhibition.
Orthodromic excitatory and inhibitory responses.
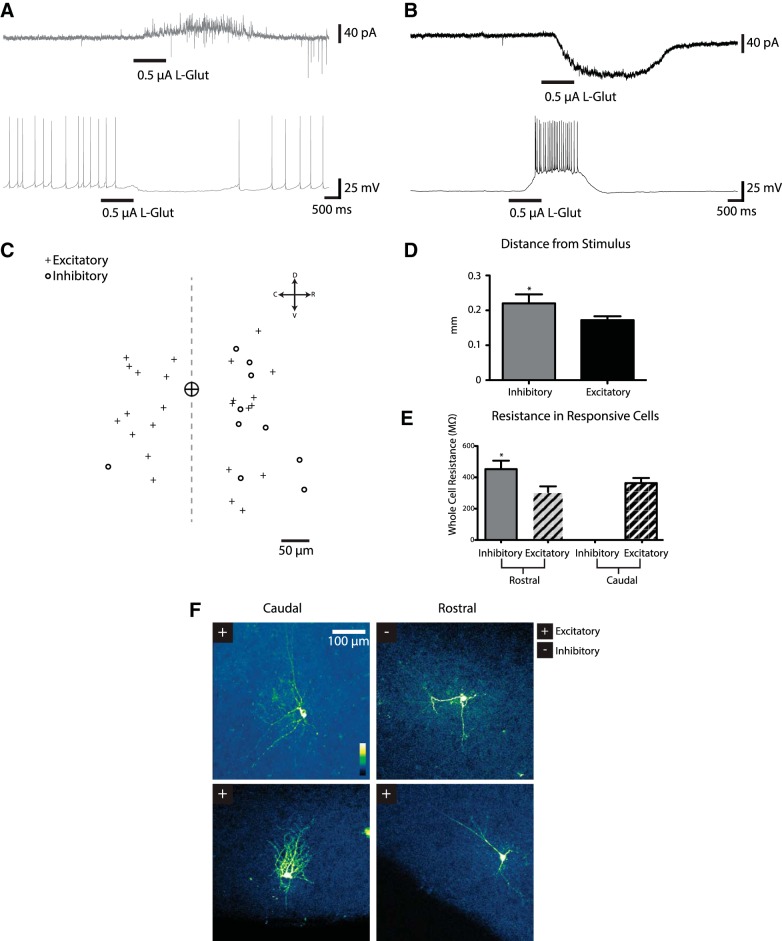
Voltage imaging monitors population responses summated from various sources. A depolarization at any given site can reflect orthodromic responses to synaptic inputs from neurons for which cell bodies and dendrites were activated by electrical stimulation, synaptic responses to axons of passage, or antidromic action potentials in axons activated by the stimulus. To distinguish between these possibilities, we used glutamate iontophoresis combined with patch-clamp recording. We used this approach previously to reveal a novel ascending excitatory pathway from the motor layers to the visuosensory layers (Ghitani et al. 2014). Glutamate iontophoresis directly depolarizes neurons close to the site of glutamate application and elicits action potentials but only elicits synaptic responses from neurons at greater distances from the site of application. We patch-clamped neurons in the visuosensory layers of the SC and applied glutamate iontophoretically in the visuosensory layers at locations either rostral or caudal to the neuron from which we recorded. Because iontophoresis uses electrical current to eject glutamate ions from the pipette, controls with reverse polarity ruled out the possibility that iontophoresis activated axons of passage. Furthermore, patch-clamp recordings readily distinguish between orthodromic synaptic inputs and antidromic action potentials.
Glutamate iontophoresis (0.5–1 μA, 1-s duration) applied in the visuosensory layers sufficiently far (mean distance 186.3 ± 11 μm) from the recorded neuron to avoid direct activation (Ghitani et al. 2014) elicited two distinct responses in visuosensory layer neurons. One population (12 of 78 neurons) produced small outward currents when held at −70 or −60 mV. Holding these neurons at higher potentials (−50 mV), well above the reversal potential of Cl− (ECl = −76 mV), revealed robust outward synaptic currents (Fig. 6A, top trace). Under current-clamp, these neurons often spiked spontaneously when held at approximately −45 mV, and glutamate iontophoresis reversibly abolished this spiking (Fig. 6A, bottom trace). Glutamate iontophoresis moved the voltage in the negative direction when a holding current held the membrane above −45 mV. Hyperpolarizing a cell eliminated the synaptic responses elicited by glutamate (data not shown). These results indicated that glutamate iontophoresis can activate subsets of neurons that make inhibitory synaptic connections with other visuosensory layer neurons in an intralaminar inhibitory circuit.
Fig. 6.
Single-neuron responses to glutamate. Patch-clamp recordings from neurons show responses to l-glutamate (1 M) iontophoresis at sites rostral or caudal to the neuron under recording. A: a neuron receiving inhibitory inputs from a stimulation site caudal to the cell under recording. Top trace shows current in a cell voltage-clamped at −50 mV. Glutamate (L-Glut) iontophoresis (0.5 μA, 1 s) elicited an outward current. Bottom trace shows the same cell under current-clamp (holding current adjusted to bring the neuron to −45 mV), displaying spontaneous spikes that were abolished by glutamate. B: a neuron displaying excitatory synaptic responses when glutamate was applied to a rostral location. Top trace: current from a cell voltage-clamped at −70 mV. Glutamate elicited inward synaptic currents. Bottom trace: voltage under current-clamp (adjusted to −55 mV). Glutamate elicited a depolarization. C: spatial distribution of responsive cells with respect to the site of glutamate application. Distances for cells receiving excitatory (pluses) and inhibitory (open circles) synaptic inputs were plotted. Sites left of the origin represent glutamate application to a rostral location, whereas sites right of the origin represent glutamate application to a caudal location. All neurons recorded from were in the SGS. D: distance from the stimulus site to neurons displaying inhibitory or excitatory responses. Analysis includes neurons from both the rostral and the caudal side of a stimulus. E: mean whole cell resistances of neurons displaying excitatory and inhibitory responses. Neurons with excitatory responses from caudal and rostral sites had the same resistance. Neurons receiving inhibitory inputs from a rostral site had a higher resistance than neurons receiving excitatory inputs from a rostral site. F: morphology of neurons receiving excitatory (+) and inhibitory (−) responses on each side of the stimulus revealed with Lucifer yellow in a 2-photon microscope. *P < 0.05.
Another population responded to glutamate with robust inward currents when voltage-clamped at −70, −60, and −50 mV (24 of 78 neurons). When current-clamped, these neurons responded with a slow depolarization, which at times drove the neuron above threshold and elicited a burst of action potentials (Fig. 6B). These neurons were classified as receiving excitatory inputs from neurons located near the site of glutamate application. Slightly more than half of the neurons from which we recorded failed to respond to glutamate iontophoresis (54%, 42 of 78 neurons). Since we could not ascertain whether inputs to these neurons had been severed during slice preparation or were not contacted by neurons at the site of glutamate application, we omitted them from our analyses.
Of 48 visuosensory layer neurons rostral to the site of glutamate application, 11 displayed inhibitory responses, 12 displayed excitatory responses, and the rest were unresponsive. In contrast, of 30 visuosensory layer neurons caudal to the site of glutamate application, 14 had excitatory responses, and only 1 had an inhibitory response; the rest were unresponsive (Fig. 6C). For 4 neurons, we had no measurement of the distances from the stimulus, and these cells were omitted from this plot. These results thus support our voltage imaging results and indicate that inhibitory visuosensory layer neurons preferentially innervate neurons in the rostral direction.
To compare the neurons receiving inhibitory and excitatory innervation, we investigated their electrophysiological and morphological properties. The mean distance from the site of glutamate application was similar for neurons receiving excitatory vs. inhibitory inputs (P = 0.41). Similarly, neurons received excitatory inputs from caudal and rostral sites in roughly equal numbers (P = 0.12). However, a comparison of the distances from all responsive neurons suggested that neurons receiving excitatory inputs may be slightly closer to the site of glutamate application compared with neurons receiving inhibitory inputs (0.172 ± 0.011 vs. 0.220 ± 0.026 mm, respectively; P = 0.049; Fig. 6D). Additionally, neurons receiving inhibitory inputs had significantly higher resistances than neurons receiving excitatory inputs (470 ± 51.3 vs. 335 ± 24.7 MΩ; P = 0.012; Fig. 6E), suggesting that inhibition preferentially targets smaller neurons.
In neurons filled with Lucifer yellow and viewed with 2-photon microscopy we observed a variety of morphologically distinct cell types, but these cell types could not be distinguished on the basis of their responses to glutamate iontophoresis. Figure 6F, left column (Caudal), shows neurons with different morphologies receiving excitatory inputs. Morphologically similar neurons had divergent responses. Figure 6F, right column (Rostral), shows two morphologically similar neurons, one receiving excitatory inputs and the other receiving inhibitory inputs. Because of this variation, we were unable to assign either inhibitory or excitatory synaptic responses to distinct morphological classes of neurons. The higher resistance (Fig. 6F) may indicate that neurons receiving intralaminar inhibition also receive excitatory inputs from the motor layers (Ghitani et al. 2014), but more experiments will be needed to clarify the specificity of this circuitry.
Caudal over rostral priority.
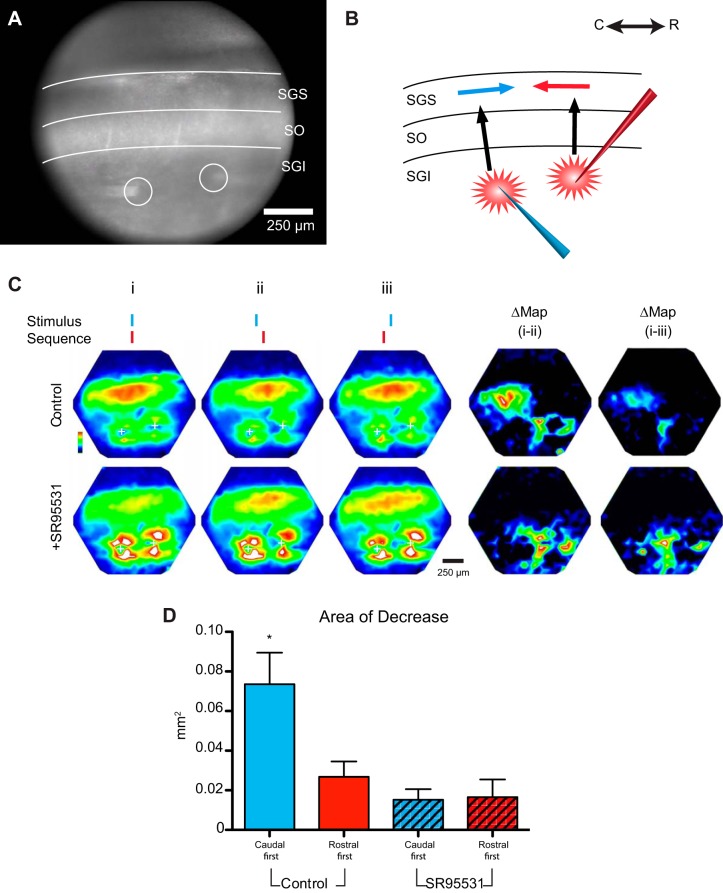
The sensory layers perform the first step of integrating visual inputs for the guidance of attention and gaze shifts. The inhibitory circuit described here could contribute to this integration to favor inputs representing more peripheral locations. This would suppress visual inputs arising from the nasal/central visual field in preparation for orienting movements to the periphery. We tested this hypothesis with dual-site experiments in which we stimulated two sites along the rostrocaudal axis, one associated with peripheral visual field locations and one associated with central visual field locations. We varied the timing of these two inputs to compare the influence of activity corresponding to a peripheral site occurring before, during, or after activity corresponding to a central site. We predicted that only when the activation of the peripheral site occurred before activation of the central site would we see inhibition of responses at rostral sites.
With two sites in the motor layer in the middle region of an SC slice, we stimulated with temporal offsets of 5–10 ms (Fig. 7A). We hypothesized that initial stimulation at a caudal site (Fig. 7B, blue electrode) would inhibit rostral sites (Fig. 7B, blue arrow) to reduce the depolarization (Fig. 7B, red arrow) evoked by subsequent stimulation of a rostral site (Fig. 7B, red electrode). By contrast, we expected that simultaneous stimulation or stimulation of the rostral site before caudal site would not.
Fig. 7.
Rostrally directed inhibition impedes spread of responses to delayed stimulation at a 2nd site. CCD image (A) and schematic (B) of the dual-site stimulation experiments are shown. Electrical stimulation was applied either to both sites in the SGI simultaneously or with 5- to 10-ms offsets. C: simultaneous stimulation (i) in the SGI produced a caudally propagating response from the rostral (right) stimulation site. ii: Stimulating the caudal site 5 ms before the rostral site impeded the caudal propagation and reduced responses to the 2nd stimulus. iii: Applying the caudal stimulus 5 ms after the rostral stimulus did not diminish responses. The difference map (Δ) between simultaneous and caudal-first stimulation shows a zone of inhibition near the rostral site, whereas the Δ map on the right shows no effect of caudal-first stimulation. Application of SR-95531 (5 μM) abolished this inhibition, and temporal offsets resulted in no discernible changes in the amplitude and extent of caudally directed propagation. D: area of Δ map (within 50% threshold contour) in control (blue) following the application of SR-95531 (blue striped bar; N = 7; P = 0.0028) in caudal-first experiments. Areas of Δ maps of rostral-first experiments are shown for control (red) and following SR-95531 application (red stripped bar; N = 5; P = 0.41). *P < 0.05.
Imaging responses with this dual-site stimulation protocol indicated that rostrally directed inhibition dominated the circuit when the two stimuli were offset by 5 ms. (The domination was much less evident with a 10-ms offset, data not shown.) Simultaneous stimulation of the two sites led to spread in the caudal direction (Fig. 7Ci) with spatiotemporal properties and response amplitudes similar to those evoked by stimulation at the rostral site alone. When the caudal stimulus preceded the rostral stimulus by 5 ms (Fig. 7Cii), we observed a decreased caudal spread of responses to stimulation of the rostral site. Mapping the difference between maximal response amplitudes to simultaneous or offset stimuli revealed an area of decreased responses (Fig. 7C, left Δ Map) and demonstrated that inhibition is concentrated in a region rostral to the initial stimulus. Reversing the order (i.e., stimulating the rostral site 1st) produced minimal inhibition (Fig. 7C, right Δ Map).
Blockade of GABAA receptors abolished the caudal-over-rostral priority (Fig. 7C, bottom row). We calculated the area of the region inhibited by thresholding the difference maps of responses from simultaneous vs. caudal-first stimulation. SR-95531 (5 μM) reduced the area of rostrally directed inhibition from 0.073 ± 0.0160 mm2 in control experiments to 0.015 ± 0.0054 mm2 (P = 0.0028; n = 7; Fig. 7D) when the caudal stimulus preceded the rostral stimulus by 5 ms. Reversing the order of stimulation to rostral-first significantly reduced the area of inhibition compared with caudal-first stimulation (0.026 ± 0.0078 mm2; P = 0.042), and this area was indistinguishable from that following SR-95531 addition (0.016 ± 0.0089 mm2; P = 0.41). Thus activation of a site in the SC associated with peripheral locations suppressed activity in regions of the SC associated with more central/nasal location in the visual field.
DISCUSSION
The SC plays an integral role in establishing priority maps that guide shifts in spatial attention and orienting. The visual layers of the SC are tightly interconnected to higher visual processing centers, including the primary visual cortex (Fries 1984), the frontal eye fields (Leichnetz et al. 1981), and the lateral intraparietal area (Lynch et al. 1985), which are involved in goal-directed, top-down attention and eye movement control (Fecteau and Munoz 2006; Itti and Koch 2001; Krauzlis et al. 2013). The SC, along with the pulvinar, are the primary centers of stimulus-driven, bottom-up spatial attention in visual processing (Baluch and Itti 2011; Guillery 1995; Harting et al. 1980; Shipp 2004) with both structures forming maps in response to salient visual stimuli. The contributions of intrinsic circuitry to the resultant saliency maps have thus far not been explored. Our data, however, suggest that in the SC, circuits encoding the amplitude of the stimulus are primed to skew saliency maps toward more peripheral targets. The intrinsic circuitry within the SC presents a hard-wired bias in shifts of spatial attention.
These results add a significant new element to our understanding of the circuitry of the SC. Excitatory responses in the rostrocaudal axis of the visual layers in the SC are shaped by an intralaminar GABAergic circuit, which impedes excitatory spread in the rostral direction. Physiologically, such inhibition would give rise to sensory responses that preferentially activate visual layer areas encoding more peripheral locations while suppressing more central cues. Asymmetric spread was seen either in response to direct stimulation of the sensory layers or, indirectly, in response to stimulation of the deeper, motor layers through an ascending excitatory input (Ghitani et al. 2014). This indicates that the circuit could be activated by visual cues directly or through feedback from the deeper layers, which may indirectly involve inputs from top-down attention centers (Krauzlis et al. 2013; May 2006). Furthermore, dual-site stimulation experiments demonstrated the ability of this circuit to impede subsequent excitatory responses and indicated that the rostrally directed inhibitory circuit enables activity at a caudal site to dominate the population responses within the visual layers.
Inhibitory SC circuitry.
There are two other well-established inhibitory circuits in the SC, anatomically and functionally distinct from the intralaminar circuit described here. An interlaminar inhibitory feedback circuit from motor to visuosensory layers has previously been reported (Lee et al. 2007; Phongphanphanee et al. 2011; Richmond et al. 1983), and it is accepted that this circuit functions in saccadic suppression, whereby motor layer activation inhibits subsequent sensory inputs in the direction of the selected saccade by inhibiting visuosensory layer activity. Previous studies in rodent SC slices also demonstrated widespread intralaminar inhibition but without the asymmetry we observed (Lee and Hall 2006; Phongphanphanee et al. 2014; Sooksawate et al. 2011). These studies focused on comparing the lateral extents of excitation and inhibition to test the role of inhibition in the winner-takes-all model of saccade choice. Lee and Hall (2006) used glutamate uncaging in both coronal and parasagittal slices and observed symmetric inhibition. Phongphanphanee et al. (2014) studied horizontal slices and saw asymmetry in some select cases but did not report the spatial distribution of isolated excitation and inhibition along the rostrocaudal axis. However, slices of visuosensory layers prepared by cutting in the horizontal plane will lose their motor inputs, and in our hands motor layer stimulation provided the most striking demonstration of asymmetry. Age could also be a factor. The age of the animals used in these prior studies (as young as 13 and 16 days) extends to stages of mouse and rat development where visual systems have not reached maturity.
Roles for asymmetric inhibition in the SC.
In all vertebrate species studies so far, the superficial, visuosensory layers perform the first step of integrating visual information for the guidance of attention and orienting movements (Kardamakis et al. 2015; Vanegas 1984). Indeed, recent work in the rat is consistent with a role for the SC in attention (Clements et al. 2014; Mitchinson and Prescott 2013). If this asymmetric inhibition described here in the rat also exists in the monkey SC, it may explain express saccade phenomena. For example, activation of a more caudal site in the SC would inhibit the rostral SC, allowing a more peripheral location in the visual field to take priority over a more central location. In monkeys, a delay between the offset of a fixation stimulus and the onset of a peripheral target usually reduces the latency of saccades to the peripheral target in what is referred to as the gap effect, and this delay often leads to ultrashort latency express saccades (Boch 1986; Fischer and Boch 1983; Fischer et al. 1984; Paré and Munoz 1996; Schiller et al. 1987; Sommer 1994). Physiologically, this would present as decreased excitability of neurons encoding more central locations and increased excitability of neurons encoding more peripheral locations. The asymmetric inhibition we observed is tailored to precisely this kind of distribution. Interestingly, neurons in the rostral SC encoding target location close to the fovea showed concomitant reductions in activity during the gap period (Dorris et al. 1997).
We found that activation of a caudal site in the sensory layers of the SC inhibited visuosensory layer activity rostral to the site of activation. This GABAA receptor-mediated suppression develops rapidly, in a few milliseconds. If this hard-wired circuit is also present in monkeys, activity in the sensory layers will travel to the motor layers via the descending pathway (Behan and Appell 1992; Lee and Hall 1995; Mooney et al. 1988; Moschovakis et al. 1988) to activate neurons encoding eye movements to peripheral locations, whereas the inhibitory circuit described here will suppress the sensory activation of neurons representing central locations. Indeed, this inhibitory circuit could contribute to the disengagement of attention at an initial location (usually more central/nasal) that precedes a shift of attention to a peripheral field (Posner 1980). In this case, the changes in excitability in the visuosensory layers would be transmitted to attentional circuits in the cortex via the ascending collicular projections through the thalamus (Chomsung et al. 2008; Karten et al. 1997) to mediate rapid shifts of attention to a new location and disengagement of attention from the current location. Consistent with these ideas, increased excitability of visuosensory neurons in the SC was recently reported in a rat model of attention deficit disorder (Clements et al. 2014).
Conclusion.
Saliency maps are generally viewed as the result of integration of stimulus-driven responses from various visual processing areas. How intrinsic circuitry contributes to this processing remains poorly understood. Our data indicate that a GABAergic intralaminar pathway residing within the SC visuosensory layers assigns value based on location. The novel organization of this inhibitory circuit is unlikely to serve in one of the more established sensory roles of inhibition such as response tuning/sharpening, gain control/normalization, or edge/feature detection (Isaacson and Scanziani 2011). Instead, this biased inhibitory circuit gives the SC a built-in system for favoring peripheral locations of the visual field over central locations and conferring priority in attention and orienting movements.
GRANTS
This work was supported by National Institutes of Health Grants EY-019963, EY-024153, NS-072905.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.O.B., N.G., M.B.J., and M.A.B. conception and design of research; P.O.B. performed experiments; P.O.B. analyzed data; P.O.B., N.G., M.B.J., and M.A.B. interpreted results of experiments; P.O.B. and M.B.J. prepared figures; P.O.B., M.B.J., and M.A.B. drafted manuscript; P.O.B., N.G., M.B.J., and M.A.B. edited and revised manuscript; P.O.B., N.G., M.B.J., and M.A.B. approved final version of manuscript.
REFERENCES
- Baluch F, Itti L. Mechanisms of top-down attention. Trends Neurosci 34: 210–224, 2011. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Appell PP. Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol 315: 230–243, 1992. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol 504: 629–639, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW. The neural basis of visual attention. J Physiol 589: 49–57, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch R. Behavioral modulation of neuronal activity in monkey striate cortex: excitation in the absence of active central fixation. Exp Brain Res 64: 610–614, 1986. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20: 365–383, 1987. [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron 43: 575–583, 2004. [DOI] [PubMed] [Google Scholar]
- Chang PY. Hetergeneous Spatial Patterns of Long-Term Potentiation in Hippocampal Slices (PhD thesis) Madison, WI: Univ. of Wisconsin, Madison, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PY, Jackson MB. Heterogeneous spatial patterns of long-term potentiation in rat hippocampal slices. J Physiol 576: 427–443, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol 26: 937–940, 1999. [DOI] [PubMed] [Google Scholar]
- Chomsung RD, Petry HM, Bickford ME. Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. J Comp Neurol 510: 24–46, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements KM, Devonshire IM, Reynolds JN, Overton PG. Enhanced visual responses in the superior colliculus in an animal model of attention-deficit hyperactivity disorder and their suppression by d-amphetamine. Neuroscience 274: 289–298, 2014. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Salzberg BM. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol 83: 35–88, 1978. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol 38: 690–713, 1975. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Goldberg ME, Krishna BS. Spatial representation and cognitive modulation of response variability in the lateral intraparietal area priority map. J Neurosci 33: 16117–16130, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10: 382–390, 2006. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26, 1983. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R, Ramsperger E. Express-saccades of the monkey: effect of daily training on probability of occurrence and reaction time. Exp Brain Res 55: 232–242, 1984. [DOI] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230: 55–76, 1984. [DOI] [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, Basso MA. Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J Neurosci 34: 6822–6833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron 54: 677–696, 2007. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat 187: 583–592, 1995. [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Frankfurter AJ, Strominger NL, Royce GJ. Ascending pathways from the monkey superior colliculus: an autoradiographic analysis. J Comp Neurol 192: 853–882, 1980. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci 3: 284–291, 2000. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. J Neurophysiol 86: 2527–2542, 2001. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci 2: 194–203, 2001. [DOI] [PubMed] [Google Scholar]
- Kardamakis AA, Saitoh K, Grillner S. Tectal microcircuit generating visual selection commands on gaze-controlling neurons. Proc Natl Acad Sci USA 112: E1956–E1965, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Cox K, Mpodozis J. Two distinct populations of tectal neurons have unique connections within the retinotectorotundal pathway of the pigeon (Columba livia). J Comp Neurol 387: 449–465, 1997. [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol 4: 219–227, 1985. [PubMed] [Google Scholar]
- Kojima S, Nakamura T, Nidaira T, Nakamura K, Ooashi N, Ito E, Watase K, Tanaka K, Wada K, Kudo Y, Miyakawa H. Optical detection of synaptically induced glutamate transport in hippocampal slices. J Neurosci 19: 2580–2588, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Obaid AL, Salzberg BM. Optical recording of electrical activity from parallel fibres and other cell types in skate cerebellar slices in vitro. J Physiol 393: 681–702, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci 26: 4763–4768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Hall WC. Interlaminar connections of the superior colliculus in the tree shrew. II: Projections from the superficial gray to the optic layer. Vis Neurosci 12: 573–588, 1995. [DOI] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. Proc Natl Acad Sci USA 104: 6824–6827, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Hardy SG, Astruc J. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience 6: 1023–1041, 1981. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol 235: 241–254, 1985. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151: 321–378, 2006. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res 247: 243–253, 1982. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. J Neurophysiol 79: 1597–1602, 1998. [DOI] [PubMed] [Google Scholar]
- Mitchinson B, Prescott TJ. Whisker movements reveal spatial attention: a unified computational model of active sensing control in the rat. PLoS Comput Biol 9: e1003236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res 90: 219–248, 1992. [DOI] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, Hess PR, Allen Z, Lewin AC, Rhoades RW. The projection from the superficial to the deep layers of the superior colliculus: an intracellular horseradish peroxidase injection study in the hamster. J Neurosci 8: 1384–1399, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol 60: 263–302, 1988. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev 70: 513–565, 1990. [DOI] [PubMed] [Google Scholar]
- Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76: 3666–3681, 1996. [DOI] [PubMed] [Google Scholar]
- Phongphanphanee P, Marino RA, Kaneda K, Yanagawa Y, Munoz DP, Isa T. Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. Eur J Neurosci 40: 2329–2343, 2014. [DOI] [PubMed] [Google Scholar]
- Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, Hall WC. A circuit model for saccadic suppression in the superior colliculus. J Neurosci 31: 1949–1954, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol 32: 3–25, 1980. [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Wurtz RH, Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. J Neurophysiol 50: 1415–1432, 1983. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808, 1972. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J Neurosci 23: 5854–5864, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol 34: 920–936, 1971. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049, 1987. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Boller M, Ozen G, Hall WC. Disinhibition in rat superior colliculus mediated by GABAc receptors. J Neurosci 21: 691–699, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci 10: 38–45, 2006. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci 8: 223–230, 2004. [DOI] [PubMed] [Google Scholar]
- Sommer MA. Express saccades elicited during visual scan in the monkey. Vision Res 34: 2023–2038, 1994. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, Behan M, Yanagawa Y, Isa T. Organization of GABAergic inhibition in the motor output layer of the superior colliculus. Eur J Neurosci 33: 421–432, 2011. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev 66: 118–171, 1986. [DOI] [PubMed] [Google Scholar]
- Tiao YC, Blakemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol 168: 483–503, 1976. [DOI] [PubMed] [Google Scholar]
- Vanegas H. (editor). Comparative Neurology of the Optic Tectum. New York: Plenum, 1984. [Google Scholar]
- Vokoun CR, Jackson MB, Basso MA. Intralaminar and interlaminar activity within the rodent superior colliculus visualized with voltage imaging. J Neurosci 30: 10667–10682, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Cohen LB. Fast multisite optical measurement of membrane potential. In: Fluorescent and Luminescent Probes of Biological Activity, edited by Mason WT. San Diego, CA: Academic Press, 1993. [Google Scholar]
- Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science 171: 82–84, 1971. [DOI] [PubMed] [Google Scholar]