Highlights
-
•
We analyzed mental stress, fibrinogen, and CVD within an integrated framework.
-
•
Fibrinogen is not a positive mediator between mental stress and CVD.
-
•
The higher the fibrinogen response to mental stress is, the lower the risk of CVD, assessed by detectable HS-CTnT levels.
Keywords: Stress, Psychological, Fibrinogen, Troponin T, Atherosclerosis, Allostasis
Summary
Background
Plasma fibrinogen is considered as a positive mediator between mental stress and cardiovascular disease because it is an acute-phase protein released in response to mental stress and a coagulation factor. However those three factors have never been studied together within a single integrated framework, using cardiac troponin T as a marker of cardiovascular risk.
Methods
491 disease-free men and women aged 53–76 were tested for fibrinogen levels before, immediately after, and following recovery from standardized mental stress tasks. We measured plasma cardiac troponin T using a high-sensitivity assay (HS-CTnT) and coronary calcification using electron-beam dual-source computed tomography.
Results
The average fibrinogen concentration increased by 5.1% (s.d. = 7.3) in response to stress and then tended to return to baseline values. People with higher baseline fibrinogen values had smaller increases (blunted responses) following the stress task (P = 0.001), and people with higher stress responses showed better recovery (P < 0.001). In unadjusted analyses, higher baseline fibrinogen was associated with higher chances of having detectable HS-CTnT (P = 0.072) but, conversely, higher fibrinogen response was associated with lower chances of having detectable HS-CTnT (P = 0.007). The adjustment for clinical, inflammatory, and haemostatic factors, as well as for coronary calcification eliminated the effect of baseline fibrinogen, whereas the negative association between fibrinogen response and HS-CTnT remained robust: the odds of detectable HS-CTnT halved for each 10% increase in fibrinogen concentration due to stress (OR = 0.49, P = 0.007, 95% CI = 0.30–0.82).
Conclusions
Greater fibrinogen responses to mental stress are associated with lower likelihood of detectable high-sensitivity troponin T plasma concentration. A more dynamic fibrinogen response appears to be advantageous for cardiovascular health.
1. Introduction
Mental stress is a recognized risk factor for cardiovascular disease (CVD) in acute conditions, when sudden stressful events trigger acute CVD events, as well as in chronic conditions, when poor adaptation to recurrent daily stressors produces a pathophysiological substratum for CVD (Brotman et al., 2007; Steptoe and Kivimäki, 2012; Surtees et al., 2008). Although the mechanisms underlying these associations have not been completely clarified, proinflammatory factors, cortisol levels, heart rate variability, and haemostatic processes have been identified as key mediators (Dimsdale, 2008; Lazzarino et al., 2013; Steptoe and Vögele, 1991).
Fibrinogen is a plasma protein produced by the liver and is a major coagulation factor. It is a positive acute-phase reactant protein, i.e. its concentration increases with inflammation, and it is traditionally considered as a risk factor for CVD because it promotes formation of thrombus (Lowe et al., 2004; Stulnig, 2013).
A number of studies have found positive associations between mental stress and plasma fibrinogen concentration (Brunner et al., 1996; Kittel et al., 2002; Steptoe et al., 2003, 2004; Von Känel et al., 2001; Wirtz et al., 2008, 2009). These include large-scale population surveys linking single measurements of plasma fibrinogen with questionnaire-based self-reported levels of chronic stress, and more focused psychophysiological studies that have measured fibrinogen secretion in response to acute mental stress in laboratory-based experimental settings. Regardless of the study design and the reference population, higher stress was associated with higher fibrinogen plasma concentration (Brunner et al., 1996; Kittel et al., 2002; Steptoe et al., 2003, 2004; Von Känel et al., 2001; Wirtz et al., 2008, 2009).
The pathway linking mental stress, fibrinogen, and CVD might therefore appear to be defined: the greater the stress (or poor adaptation to stress), the higher the fibrinogen, and the greater the occurrence of CVD (Austin et al., 2013).
Nevertheless this causal sequence has not been completely disentangled because the three components have never been studied together in a single framework. Instead, the current literature is made of two separate groups of studies: one considering heightened fibrinogen as a function of mental stress, and another set considering it as a risk factor for CVD. This separation inevitably dictates a monophasic approach to a phenomenon which may rather be better described within an integrated multiphasic framework due to the complex dynamics of fibrinogen metabolism and of coagulation pathways in general.
The notion of allostasis has drawn attention to the complex system of neuroendocrine responses to environmental challenges that is characteristic of living organisms. It has been conceptualized as the process through which organisms actively adjust to both predictable and unpredictable external events through the anabolism and catabolism of mediators, i.e. the way in which they maintain stability through change (McEwen and Wingfield, 2003). From this perspective, serious pathophysiology may occur when chronic overload resulting from sustained stress stimulates prolonged allostatic actions that in the long term lose their effectiveness and ability to respond (McEwen and Wingfield, 2010). The allostatic model suggests that sustained load is characterized by changes in the morphology of responses that are manifest in chronically heightened basal levels, inadequate biological responses (blunted stress reactivity), and impaired post-stress recovery (McEwen, 1998).
We have recently used cardiac Troponin T (CTnT) as a marker of CVD risk in conjunction with mental stress (Lazzarino et al., 2013). CTnT is a plasma protein routinely tested for the diagnosis of acute myocardial infarction (AMI), since it is a marker of myocardial cell damage (Thygesen et al., 2007). In clinical settings, CTnT is measured using standard assays that have a lower detection limit of 10 ng/L (Wallace et al., 2006) and a diagnostic threshold of 35 ng/L (Thygesen et al., 2007; Wallace et al., 2006). However, high-sensitivity assays have recently been developed (HS-CTnT) with a lower detection limit of 3 ng/L (Collinson, 2011; Collinson et al., 2012; Giannitsis et al., 2010). In healthy people not fulfilling any diagnostic criterion for AMI, greater HS-CTnT is associated with greater incidence of AMI, other structural and functional heart diseases, cardiovascular mortality, and all-cause mortality, and can be therefore considered the most proximal sentinel marker of heart disease (deFilippi et al., 2010; De Lemos et al., 2010). We have shown that dysregulated response to mental stress – marked using salivary cortisol release in response to mental stress – was associated with detectable plasma levels of HS-CTnT. If HS-CTnT is a good marker of CVD risk linked to mental stress, it may therefore be a valid outcome in the study of fibrinogen release in response to mental stress (Lazzarino et al., 2013).
Fibrinogen can be considered as an important component of the network of mediators relevant to cardiovascular allostasis because of its association with both mental stress and CVD, and because of its role within the coagulation system and in inflammation. Therefore we aimed to study the associations between basal fibrinogen, fibrinogen secretion in response to stress tasks, fibrinogen recovery from stress, and high-sensitivity cardiac troponin T (as a marker of CVD risk) within a single integrated framework.
2. Methods
2.1. Study design
Our study involved participants drawn from the Whitehall II epidemiological cohort (Marmot et al., 1991) for psychophysiological testing between 2006 and 2008 (Hamer et al., 2010). The criteria for entry into the study included no history or objective signs of clinical or subclinical CVD, no previous diagnosis or treatment for hypertension, inflammatory diseases, allergies, or kidney disease. CVD was defined as prior myocardial infarction, stable or unstable angina, revascularization procedure, heart failure, transient ischaemic attack, stroke, or electrocardiographic abnormalities (resting 12-lead electrocardiograms were taken). This information was confirmed by a telephone interview and verified from clinical data collected from the previous seven phases of the Whitehall II study. Volunteers were of white European origin, aged 53–76 years, and 56.5% were in full-time employment. Selection was stratified by grade of employment (current or most recent) to include higher and lower socioeconomic status participants. From the initially invited participants (n = 1169), 27.6% were not eligible (mainly because of prescribed medications) and 25.9% declined to take part. Participants were prohibited from using any anti-histamine or anti-inflammatory medication 7 days before testing and were rescheduled if they reported colds or other infections on the day of testing. Participants gave full informed consent to participate in the study and ethical approval was obtained from the UCLH committee on the Ethics of Human Research.
2.2. Data collection
We carried out psychophysiological stress testing in either the morning or afternoon in a light temperature-controlled laboratory. This procedure was based on a protocol previously used in this laboratory (Steptoe et al., 2002). Participants were instructed to refrain from drinking caffeinated beverages or smoking for at least 2 h before the study and not to have performed vigorous physical activity or consumed alcohol the previous evening. Venipuncture was performed using a butterfly needle that was inserted before the beginning of the session and removed after the end of it. After a 30 min rest period from needle insertion, baseline blood pressure (using an automated UA-779 digital monitor) was taken as well as saliva and blood samples. Two behavioural tasks, designed to induce mental stress, were then administered in random order. The tasks were a computerized version of the Stroop task and mirror tracing, both of which have been used extensively in psychophysiological research (Steptoe et al., 2002). The tasks each lasted for 5 min. Saliva and blood samples were then collected immediately and at 45 min after the tasks. The saliva samples were collected using Salivettes (Sarsted, Leicester, UK), which were stored at −30 °C until analysis. Levels of cortisol were assessed using a time resolved immunoassay with fluorescence detection, at the University of Dresden. The intra- and inter-assay coefficients of variation were less than 8%. We assayed plasma IL-6 using a Quantikine® high sensitivity two-site enzyme-linked immunosorbent assay (ELISA) from R&D Systems (Oxford, UK). The sensitivity of the assay ranged from 0.016 to 0.110 pg/ml and the intra and inter assay coefficients of variation (CV) were 7.3% and 7.7% respectively. C-reactive protein (CRP) was measured using high-sensitivity ELISA (R&D Systems, Oxford, UK; CVs <8%). Monocyte chemotactic protein-1 (MCP-1) was assayed using the Magnetic Luminex Performance Assay Human CCL2/MCP-1 multiplexed IL-1RA kit (R&D Systems®) read on a Luminex 200 (Bio-Rad®) with a CV <8%. Von Willebrand factor (vWF) was determined using a double sandwiched antibody enzyme-linked immunoassay (DakoCytomation Ltd., Cambridge, UK; CVs <5%) and fibrinogen by an automated Clauss assay in a MDA-180 coagulometer (Organon Teknika, Cambridge, UK) using the manufacturer's reagents and the International fibrinogen standard (inter- and intra-individual CV is 8%) (Gaffney and Wong, 1992).
We measured cardiac troponin T plasma concentration at 75 min after the end of the mental stress task using a highly sensitive assay on an automated platform (Elecsys 2010 Troponin T hs STAT, Roche Diagnostics). The lower detection limit was 3 ng/L with a reported 99th percentile value in apparently healthy individuals of 13.5 ng/L, at which the inter- and intra-individual CV is 9%, confirmed by in-house studies (Collinson, 2011; Collinson et al., 2012; Giannitsis et al., 2010).
We assessed coronary artery calcification (CAC) in separate sessions using electron beam computed tomography (GE Imatron C-150, San Francisco, CA, USA) as previously described (Anand et al., 2007). In brief, 40 contiguous 3 mm slices were obtained during a single breath-hold starting at the carina and proceeding to the level of the diaphragm. Scan time was 100 ms/slice, synchronized to 40% of the R–R interval. Agatston and volumetric calcium scores were calculated to quantify the extent of CAC by a single experienced investigator blinded to the psychophysiological and clinical data on an Aquarius workstation (TeraRecon Inc., San Mateo, CA, USA). Since calcified volume was very highly correlated with Agatston score (Spearman's rho = 0.99), we present data for Agatston score only.
Participants reported current smoking levels, weekly alcohol intake (units per week), employment grade (as a marker of social position), and hours of moderate or vigorous physical activity per week. We measured height and weight in light clothing for the calculation of body mass index (BMI) as kg/m2. Fasting blood samples were taken during a separate clinical assessment. Total and high-density lipoprotein (HDL) cholesterol and triglycerides were measured within 72 h in serum stored at 4 °C using enzymatic colorimetric methods. Low-density lipoprotein (LDL) cholesterol was derived using the Friedewald equation (Warnick et al., 1990). Glucose homeostasis was assessed from glycated haemoglobin (HbA1C) concentration, assayed using boronate affinity chromatography, a combination of boronate affinity and liquid chromatography.
2.3. Data analysis
We checked the data for missing, inconsistent, and duplicate information, as well as normality and digit preference for continuous variables.
We quantified the fibrinogen response to stress as the proportional increase in fibrinogen plasma concentration after the stress task ([immediate-post-task minus pre-task]/pre-task). Similarly, we quantified the fibrinogen recovery from stress as the proportional decrease in fibrinogen after the stress task ([immediate-post-task minus 45 min-post-task]/immediate-post-task). Agatston CAC score had a right-skewed distribution and for some analyses was transformed into an ordered categorical variable with four categories (cut-offs at 0, 100, and 400).
We conducted bivariate analyses and calculated P values using the likelihood ratio test. We then used three separate sets of multiple logistic regression models to examine the association between detectable HS-CTnT (outcome) with either baseline fibrinogen or fibrinogen stress response or fibrinogen recovery (alternative exposure variables). Fibrinogen response or recovery may differ according to baseline levels, so this parameter was included as a covariate when they were the exposure variable. We also adjusted for demographics such as age, gender, and employment grade, and for health behaviours such as smoking and alcohol intake because they are related to CVD and may confound the association between fibrinogen and CVD. We also took into account clinical variables that are known to be linked with CVD such as BMI, blood pressure, glycated haemoglobin (HbA1c), triglycerides, LDL, and total cholesterol/HDL ratio. Moreover, we adjusted for baseline and response values of circulating markers of inflammation and endothelial dysfunction such as CRP, cortisol, IL-6, vWF, and MCP-1 to account for vascular inflammation and endothelial dysfunction. Finally, we adjusted for Agatston CAC score to examine whether the association between fibrinogen and HS-CTnT was independent of underlying coronary atherosclerosis. We evaluated the extent of multicollinearity among covariates by computing one-to-one correlation coefficients and by examining variance inflation factors (VIF) (“Regression with Stata Web Book: Chapter 2 – Regression Diagnostics,” n.d.).
We performed several sensitivity analyses: we considered stress response and recovery as absolute changes in fibrinogen concentration rather than relative (the Spearman's rho coefficient of correlation between the two approaches was 0.98 for stress response and 0.99 for stress recovery); we adjusted for CAC using a continuous natural logarithmic scale (before the log-transformation, zero values were recoded to values equal to the half of the smallest value in the dataset); we repeated the analyses of stress responses and recovery without adjusting for baseline values of fibrinogen; we restricted the analysis to participants with no CAC; after having fitted the final models we carried out a backward stepwise estimation to eliminate unnecessary covariates (P value for retention = 0.05 or less); given the elevated number of undetectable values for HS-CTnT, we have transformed that measure into an ordered categorical variable scoring zero for undetectable levels (<3 ng/L), one for detectable levels up to 5.63 ng/L (median value for detectable levels), and two for detectable levels above 5.63 ng/L, and used multinomial logistic regression and linear regression instead of logistic regression.
Additional analyses involved blood pressure response and heart rate response as possible determinants of HS-CTnT detection.
3. Results
A total of 543 people participated in the study, but 52 (9.6%) had missing information for HS-CTnT or fibrinogen due to insufficient blood samples and were therefore removed. The final analytic sample comprised 491 disease-free participants aged 62.9 years on average (standard deviation [s.d.] = 5.7) of whom 54.1% were men. The excluded participants did not differ significantly from the main sample in any covariates. The mean fibrinogen concentration at baseline was 314.1 mg/dL (s.d. = 61.6), and on average increased to the value of 330.1 mg/dL (s.d. = 65.9) in response to the stress task, and then decreased during the recovery period to the mean value of 321.9 mg/dL (s.d. = 65.0) measured at 45 min post task. There was considerable inter-individual variation in fibrinogen response and recovery: the average fibrinogen increase in response to the stress tasks was 5.1% (s.d. = 7.3) with values ranging from −30.5% to +57.6% (therefore for some individuals fibrinogen plasma concentration decreased in response to the stress tasks), and the average decrease in fibrinogen during the recovery period was 2.3% (s.d. = 7.8) with values ranging from −68.5% to +23.1% (therefore for some individuals fibrinogen plasma concentration increased during the resting period). The prevalence of detectable levels of HS-CTnT concentrations was 17.1% (95% confidence interval [CI] = 13.8%–20.5%).
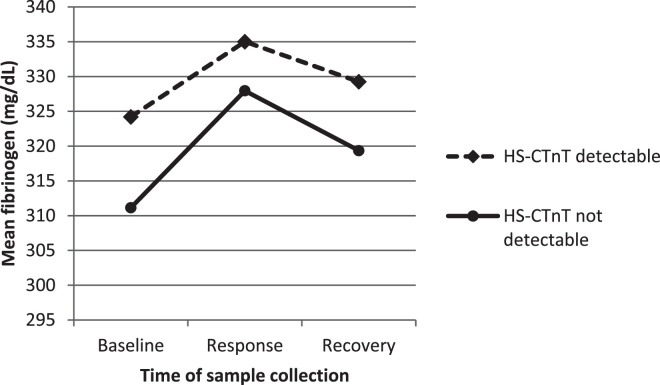
In unadjusted analysis, people with detectable levels of HS-CTnT appeared to have on average higher levels of baseline fibrinogen and flatter profile to the mental stress test, i.e. blunted response and slower recovery (Fig. 1).
Figure 1.
Mean concentration of fibrinogen in the peripheral blood plasma, measured at baseline (before the stress task), immediately after the stress task (response values), and following a 45 min rest period (recovery) in people with (n = 407) and without (n = 84) detectable levels of HS-CTnT peripheral blood plasma concentration, for 491 disease-free participants drawn from the Whitehall II epidemiological cohort between 2006 and 2008 in United Kingdom.
Table 1 describes the sample according to baseline fibrinogen. People with higher fibrinogen tended to be older, of lower socio-economic status as defined by grade of employment, were more likely to be female, smoker, and low alcohol consumers, and also tended to have higher levels of clinical and inflammatory factors such as BMI, blood pressure, glycated haemoglobin, LDL, total cholesterol, CRP, IL-6, and vWF. Higher levels of baseline fibrinogen were also strongly associated with smaller increases (blunted responses) in fibrinogen after the mental stress tasks (P = 0.001) and were marginally associated with positive HS-CTnT values (P = 0.072).
Table 1.
Characteristics of the study sample by tertiles of plasma fibrinogen concentration measured at baseline, for 491 disease-free participants drawn from the Whitehall II epidemiological cohort between 2006 and 2008 in United Kingdom.
| Factor and category | Tertiles of plasma fibrinogen at baseline |
P | |||||
|---|---|---|---|---|---|---|---|
| Lowest | Medium | Highest | |||||
| Age (mean years ± s.d.) | 62.4 | ±5.2 | 62.9 | ±5.6 | 63.6 | ±6.2 | 0.001 |
| Male (%) | 66.1 | 50.6 | 46.6 | <0.001 | |||
| Latest grade of employment (%) | |||||||
| Higher | 42.5 | 40.7 | 33.5 | <0.001 | |||
| Intermediate | 42.5 | 39.0 | 35.8 | ||||
| Lower | 14.9 | 20.3 | 30.7 | ||||
| Current smoker (%) | 3.4 | 4.1 | 9.1 | <0.001 | |||
| Alcohol consumption (%) | |||||||
| No alcohol | 9.8 | 15.7 | 21.6 | 0.005 | |||
| Below recommended levels | 74.7 | 73.3 | 65.3 | ||||
| Above recommended levels | 15.5 | 11.0 | 13.1 | ||||
| Hours of physical activity per week (%) | |||||||
| <1 h | 22.1 | 24.1 | 24.4 | 0.559 | |||
| 1–4 h | 29.7 | 36.7 | 30.2 | ||||
| 5–7 h | 23.8 | 21.7 | 22.7 | ||||
| >7 h | 24.4 | 17.5 | 22.7 | ||||
| Body mass index (mean kg/m2 ± s.d.) | 24.6 | ±3.2 | 25.6 | ±3.8 | 27.2 | ±4.3 | <0.001 |
| Systolic blood pressure (mean mmHg ± s.d.) | 127.0 | ±14.7 | 130.4 | ±16.4 | 129.6 | ±16.3 | 0.123 |
| Diastolic blood pressure (mean mmHg ± s.d.) | 68.2 | ±8.2 | 70.5 | ±8.7 | 70.5 | ±9.2 | 0.015 |
| Glycated haemoglobin (mean % ± s.d.) | 5.4 | ±0.6 | 5.5 | ±0.4 | 5.5 | ±0.4 | 0.001 |
| Triglycerides (median g/L ± IQR) | 1.2 | ±0.8 | 1.2 | ±1.0 | 1.2 | ±0.7 | 0.761 |
| HDL (mean mmol/L ± s.d.) | 1.7 | ±0.5 | 1.7 | ±0.5 | 1.7 | ±0.5 | 0.661 |
| LDL (mean mmol/L ± s.d.) | 2.9 | ±0.8 | 3.0 | ±0.8 | 3.1 | ±1.0 | 0.013 |
| Total cholesterol (mean mmol/L ± s.d.) | 5.2 | ±0.9 | 5.3 | ±0.9 | 5.4 | ±1.0 | 0.043 |
| Total cholesterol/HDL ratio (mean ± s.d) | 3.3 | ±1.0 | 3.4 | ±1.1 | 3.5 | ±1.2 | 0.074 |
| C-reactive protein (median mg/L ± IQR) | 0.6 | ±0.7 | 1.0 | ±1.1 | 1.7 | ±2.2 | <0.001 |
| Salivary cortisol (mean nmol/L ± s.d.) | 6.8 | ±5.1 | 6.4 | ±3.8 | 6.3 | ±4.0 | 0.566 |
| IL-6 (median pg/ml ± IQR) | 0.9 | ±0.5 | 1.1 | ±0.8 | 1.4 | ±1.1 | <0.001 |
| Von Willebrand factor (mean % ± s.d.) | 96.3 | ±35.3 | 102.9 | ±36.9 | 114.4 | ±44.1 | 0.001 |
| MCP-1 (median pg/mL ± IQR) | 134.8 | ±58.7 | 138.4 | ±54.5 | 141.8 | ±61.6 | 0.492 |
| Fibrinogen (mean mg/dL ± s.d.) | 251.8 | ±24.7 | 308.7 | ±13.2 | 380.9 | ±46.5 | |
| Salivary cortisol response (mean % increase ± s.d.) | 15.1 | ±65.4 | 16.7 | ±53.5 | 17.7 | ±60.2 | 0.520 |
| IL-6 response (mean % increase ± s.d.) | 46.2 | ±74.3 | 34.7 | ±44.9 | 35.5 | ±59.8 | 0.212 |
| Von Willebrand factor response (mean % increase ± s.d.) | 7.1 | ±22.6 | 4.3 | ±20.7 | 3.7 | ±18.5 | 0.554 |
| MCP-1 response (mean % increase ± s.d.) | 8.6 | ±24.5 | 9.2 | ±23.8 | 5.8 | ±20.6 | 0.325 |
| Fibrinogen response (mean % increase ± s.d.) | 6.6 | ±8.0 | 4.5 | ±7.2 | 4.3 | ±6.5 | 0.001 |
| Fibrinogen recovery (mean % decrease ± s.d.) | 2.6 | ±8.5 | 2.3 | ±7.6 | 2.0 | ±7.5 | 0.363 |
| Agatston coronary calcium score (%) | |||||||
| None | 40.8 | 44.8 | 44.3 | 0.513 | |||
| <100 | 35.6 | 32.6 | 29.5 | ||||
| <400 | 15.5 | 15.7 | 12.5 | ||||
| 400+ | 8.0 | 7.0 | 13.6 | ||||
| HS-CTnT detectable (>3 ng/L) (%) | 13.9 | 17.4 | 19.6 | 0.072 | |||
| HS-CTnT concentration if detectable (geom. mean ng/L ± s.d.) | 6.7 | ±1.8 | 6.0 | ±1.5 | 6.7 | ±2.0 | 0.578 |
P values were computed using the likelihood ratio test. All variables were measured at baseline with the exception of response variables (calculated as the proportional increase from baseline to immediately post task) and fibrinogen recovery (calculated as the proportional decrease from immediate post task to 45 min post task). HS-CTnT was measured at 75 min post task. Tertiles of plasma fibrinogen at baseline (mg/dL): lowest = from 166 to 283; medium = from 284 to 330; highest = from 331 to 573. IQR = interquartile range.
Table 2 shows the correlates of fibrinogen responses to mental stress tasks. In contrast to baseline, fibrinogen responses seemed to be independent from age, gender, and most of the other clinical and inflammatory covariates. Higher responses to stress were strongly associated with better recovery (P = 0.001) and with lower chances of testing positive for HS-CTnT (P = 0.007).
Table 2.
Characteristics of the study sample by tertiles of plasma fibrinogen response to mental stress task (proportional increase from immediate pre task to immediate post task), for 491 disease-free participants drawn from the Whitehall II epidemiological cohort between 2006 and 2008 in United Kingdom.
| Factor and category | Tertiles of plasma fibrinogen response (proportional increase) |
P value | |||||
|---|---|---|---|---|---|---|---|
| Lowest | Medium | Highest | |||||
| Age (mean years ± s.d.) | 63.2 | ±6.0 | 62.8 | ±5.5 | 62.9 | ±5.5 | 0.279 |
| Male (%) | 55.3 | 53.2 | 55.0 | 0.813 | |||
| Latest grade of employment (%) | |||||||
| Higher | 38.8 | 39.2 | 38.0 | 0.383 | |||
| Intermediate | 39.4 | 36.3 | 40.9 | ||||
| Lower | 21.8 | 24.6 | 21.1 | ||||
| Current smoker (%) | 5.9 | 4.7 | 6.4 | 0.470 | |||
| Alcohol consumption (%) | |||||||
| No alcohol | 17.6 | 14.6 | 15.2 | 0.832 | |||
| Below recommended levels | 65.9 | 74.3 | 73.1 | ||||
| Above recommended levels | 16.5 | 11.1 | 11.7 | ||||
| Hours of physical activity per week (%) | |||||||
| <1 h | 24.6 | 20.4 | 25.9 | 0.793 | |||
| 1–4 h | 38.3 | 29.3 | 28.3 | ||||
| 5–7 h | 19.8 | 25.1 | 23.5 | ||||
| >7 h | 17.4 | 25.1 | 22.3 | ||||
| Body mass index (mean kg/m2 ± s.d.) | 26.3 | ±3.7 | 25.7 | ±4.3 | 25.5 | ±3.7 | 0.294 |
| Systolic blood pressure (mean mmHg ± s.d.) | 129.7 | ±15.4 | 128.8 | ±17.6 | 128.8 | ±14.8 | 0.565 |
| Diastolic blood pressure (mean mmHg ± s.d.) | 70.7 | ±8.6 | 69.3 | ±8.3 | 69.3 | ±9.2 | 0.157 |
| Glycated haemoglobin (mean % ± s.d.) | 5.5 | ±0.7 | 5.5 | ±0.3 | 5.4 | ±0.4 | 0.072 |
| Triglycerides (median g/L ± IQR) | 1.3 | ±0.9 | 1.1 | ±0.8 | 1.2 | ±0.8 | 0.480 |
| HDL (mean mmol/L ± s.d.) | 1.6 | ±0.5 | 1.7 | ±0.4 | 1.7 | ±0.5 | 0.947 |
| LDL (mean mmol/L ± s.d.) | 3.1 | ±0.9 | 2.9 | ±0.8 | 3.0 | ±0.8 | 0.253 |
| Total cholesterol (mean mmol/L ± s.d.) | 5.4 | ±1.0 | 5.2 | ±0.8 | 5.3 | ±0.9 | 0.130 |
| Total cholesterol/HDL ratio (mean ± s.d) | 3.6 | ±1.2 | 3.2 | ±0.9 | 3.4 | ±1.1 | 0.221 |
| C-reactive protein (median mg/L ± IQR) | 1.0 | ±1.3 | 1.1 | ±1.5 | 0.9 | ±1.2 | 0.683 |
| Salivary cortisol (mean nmol/L ± s.d.) | 6.2 | ±3.7 | 6.8 | ±4.8 | 6.3 | ±3.7 | 0.935 |
| IL-6 (median pg/ml ± IQR) | 1.2 | ±0.7 | 1.1 | ±0.9 | 1.1 | ±0.7 | 0.095 |
| Von Willebrand factor (mean % ± s.d.) | 105.6 | ±39.9 | 107.4 | ±40.0 | 100.1 | ±38.2 | 0.264 |
| MCP-1 (median pg/mL ± IQR) | 143.5 | ±59.3 | 138.5 | ±57.4 | 128.7 | ±59.4 | 0.040 |
| Fibrinogen (mean mg/dL ± s.d.) | 321.6 | ±57.6 | 320.8 | ±59.8 | 302.0 | ±65.8 | 0.001 |
| Salivary cortisol response (mean % increase ± s.d.) | 16.1 | ±54.2 | 17.7 | ±60.3 | 14.9 | ±65.3 | 0.711 |
| IL-6 response (mean % increase ± s.d.) | 40.1 | ±60.7 | 35.1 | ±53.5 | 41.3 | ±68.8 | 0.329 |
| Von Willebrand factor response (mean % increase ± s.d.) | 2.8 | ±22.2 | 3.9 | ±19.4 | 8.4 | ±19.8 | <0.001 |
| MCP-1 response (mean % increase ± s.d.) | 9.4 | ±24.3 | 5.9 | ±19.8 | 7.7 | ±24.0 | 0.014 |
| Fibrinogen response (mean % increase ± s.d.) | −1.9 | ±5.1 | 5.0 | ±1.4 | 12.2 | ±5.8 | |
| Fibrinogen recovery (mean % decrease ± s.d.) | −1.7 | ±10.5 | 3.2 | ±4.6 | 5.2 | ±5.4 | <0.001 |
| Agatston coronary calcium score (%) | |||||||
| None | 41.2 | 46.2 | 43.9 | 0.340 | |||
| <100 | 32.4 | 31.6 | 32.7 | ||||
| <400 | 15.3 | 12.9 | 15.8 | ||||
| 400+ | 11.2 | 9.4 | 7.6 | ||||
| HS-CTnT detectable (>3 ng/L) (%) | 21.6 | 19.2 | 10.5 | 0.007 | |||
| HS-CTnT concentration if detectable (geom. mean ng/L ± s.d.) | 6.7 | ±1.6 | 6.7 | ±2.0 | 5.5 | ±1.5 | 0.393 |
P values were computed using the likelihood ratio test. All variables were measured at baseline with the exception of response variables (calculated as the proportional increase from baseline to immediately post task) and fibrinogen recovery (calculated as the proportional decrease from immediate post task to 45 min post task). HS-CTnT was measured at 75 min post task. Tertiles of plasma fibrinogen response (%): lowest = from −30.5 to 2.6; medium = from 2.7 to 7.4; highest = from 7.4 to 57.6.
The correlates of individual differences in post-stress fibrinogen recovery are shown in Table 3. Fibrinogen recovery rates appeared to be independent of most covariates, with the exceptions of MCP-1, IL-6, and vWf, and to be weakly associated with HS-CTnT (P = 0.365).
Table 3.
Characteristic of the study sample by tertiles of plasma fibrinogen recovery to mental stress task (proportional decrease from immediate post task to 45 min post task), for 491 disease-free participants drawn from the Whitehall II epidemiological cohort between 2006 and 2008 in United Kingdom.
| Factor and category | Tertiles of plasma fibrinogen recovery (proportional decrease) |
P value | |||||
|---|---|---|---|---|---|---|---|
| Lowest | Medium | Highest | |||||
| Age (mean years ± s.d.) | 63.9 | ±6.1 | 62.8 | ±5.7 | 62.3 | ±5.1 | 0.115 |
| Male (%) | 51.5 | 55.6 | 56.4 | 0.881 | |||
| Latest grade of employment (%) | |||||||
| Higher | 41.5 | 39.2 | 35.5 | 0.428 | |||
| Intermediate | 39.2 | 33.9 | 43.0 | ||||
| Lower | 19.3 | 26.9 | 21.5 | ||||
| Current smoker (%) | 6.4 | 7.0 | 3.5 | 0.085 | |||
| Alcohol consumption (%) | |||||||
| No alcohol | 15.2 | 17.0 | 15.7 | 0.875 | |||
| Below recommended levels | 70.2 | 72.5 | 70.3 | ||||
| Above recommended levels | 14.6 | 10.5 | 14.0 | ||||
| Hours of physical activity per week (%) | |||||||
| <1 h | 25.4 | 23.8 | 21.2 | 0.423 | |||
| 1–4 h | 32.5 | 33.9 | 29.1 | ||||
| 5–7 h | 20.1 | 22.0 | 26.7 | ||||
| >7 h | 21.9 | 20.2 | 23.0 | ||||
| Body mass index (mean kg/m2 ± s.d.) | 26.0 | ±4.1 | 25.9 | ±3.9 | 25.5 | ±3.8 | 0.357 |
| Systolic blood pressure (mean mmHg ± s.d.) | 128.6 | ±15.2 | 128.8 | ±16.2 | 129.9 | ±16.5 | 0.934 |
| Diastolic blood pressure (mean mmHg ± s.d.) | 69.4 | ±7.9 | 69.5 | ±8.2 | 70.3 | ±9.9 | 0.917 |
| Glycated haemoglobin (mean % ± s.d.) | 5.5 | ±0.6 | 5.5 | ±0.4 | 5.4 | ±0.4 | 0.895 |
| Triglycerides (median g/L ± IQR) | 1.3 | ±0.7 | 1.2 | ±0.8 | 1.0 | ±0.9 | 0.100 |
| HDL (mean mmol/L ± s.d.) | 1.7 | ±0.5 | 1.7 | ±0.5 | 1.6 | ±0.4 | 0.325 |
| LDL (mean mmol/L ± s.d.) | 3.1 | ±1.0 | 2.9 | ±0.7 | 3.0 | ±0.8 | 0.705 |
| Total cholesterol (mean mmol/L ± s.d.) | 5.4 | ±1.0 | 5.2 | ±0.8 | 5.3 | ±0.9 | 0.142 |
| Total cholesterol/HDL ratio (mean ± s.d) | 3.5 | ±1.2 | 3.4 | ±1.1 | 3.4 | ±1.0 | 0.825 |
| C-reactive protein (median mg/L ± IQR) | 1.1 | ±1.3 | 1.1 | ±1.6 | 0.9 | ±1.1 | 0.178 |
| Salivary cortisol (mean nmol/L ± s.d.) | 6.7 | ±4.4 | 6.5 | ±4.4 | 6.1 | ±3.5 | 0.586 |
| IL-6 (median pg/ml ± IQR) | 1.2 | ±0.8 | 1.2 | ±0.9 | 1.0 | ±0.7 | 0.206 |
| Von Willebrand factor (mean % ± s.d.) | 105.8 | ±38.0 | 105.9 | ±38.1 | 101.0 | ±42.3 | 0.230 |
| MCP-1 (median pg/mL ± IQR) | 145.3 | ±55.2 | 133.5 | ±51.8 | 135.9 | ±63.2 | 0.015 |
| Fibrinogen (mean mg/dL ± s.d.) | 316.5 | ±60.7 | 323.3 | ±63.7 | 304.6 | ±59.6 | 0.363 |
| Salivary cortisol response (mean % increase ± s.d.) | 8.5 | ±41.7 | 17.3 | ±66.8 | 22.9 | ±67.3 | 0.135 |
| IL-6 response (mean % increase ± s.d.) | 3.2 | ±16.1 | 2.4 | ±14.1 | 0.9 | ±12.4 | 0.011 |
| Von Willebrand factor response (mean % increase ± s.d.) | 3.3 | ±21.7 | 5.7 | ±24.6 | 7.1 | ±19.8 | 0.005 |
| MCP-1 response (mean % increase ± s.d.) | 12.6 | ±29.4 | 6.0 | ±18.3 | 4.3 | ±17.8 | <0.001 |
| Fibrinogen response (mean % increase ± s.d.) | 1.6 | ±7.5 | 5.5 | ±5.4 | 8.4 | ±7.2 | <0.001 |
| Fibrinogen recovery (mean % decrease ± s.d.) | −5.0 | ±8.7 | 2.9 | ±1.4 | 8.8 | ±3.3 | |
| Agatston coronary calcium score (%) | |||||||
| None | 43.9 | 45.0 | 42.4 | 0.999 | |||
| <100 | 31.0 | 33.9 | 31.4 | ||||
| <400 | 16.4 | 11.1 | 16.9 | ||||
| 400+ | 8.8 | 9.9 | 9.3 | ||||
| HS-CTnT detectable (>3 ng/L) (%) | 20.6 | 17.6 | 12.9 | 0.365 | |||
| HS-CTnT concentration if detectable (geom. mean ng/L ± s.d.) | 5.5 | ±1.6 | 6.7 | ±1.8 | 7.4 | ±2.0 | 0.105 |
P values were computed using the likelihood ratio test. All variables were measured at baseline with the exception of response variables (calculated as the proportional increase from baseline to immediately post task) and fibrinogen recovery (calculated as the proportional decrease from immediate post task to 45 min post task). HS-CTnT was measured at 75 min post task. Tertiles of plasma fibrinogen recovery (%): lowest = from −68.5 to 0.3; medium = from 0.4 to 5.2; highest = from 5.2 to 23.1.
Table 4 summarizes the results from the logistic regressions on HS-CTnT. The marginally significant positive association between baseline fibrinogen concentration and HS-CTnT was eliminated after the adjustment for covariates. In contrast, the negative association between stress response and detectable HS-CTnT remained very similar to the crude association in terms of both effect size and statistical significance after adjustment for demographic factors, cardiovascular risk factors, neuroendocrine and inflammatory variables, and extent of coronary calcification: the odds of detectable HS-CTnT halved for each 10% increase in fibrinogen response to stress task (odds ratio [OR] = 0.49; P = 0.007; 95% CI = 0.30–0.82).
Table 4.
Multiple logistic regression models for the association between baseline fibrinogen, fibrinogen response to mental stress tasks, or fibrinogen recovery and plasma detectable HS-CTnT, for 491 disease-free participants drawn from the Whitehall II epidemiological cohort between 2006 and 2008 in England.
| Model for detectable HS-cTnT (outcome variable) | Exposure variable |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline fibrinogen (OR for 1 g/L) |
Fibrinogen response (OR for 10% increase) |
Fibrinogen recovery (OR for 10% decrease) |
|||||||
| OR | (95% CI) | P | OR | (95% CI) | P | OR | (95% CI) | P | |
| 1. Crude association | 1.41 | (0.97–2.04) | 0.072 | 0.63 | (0.45–0.88) | 0.007 | 0.99 | (0.96–1.01) | 0.365 |
| 2. Adjusted for age, gender, and baseline fibrinogen when exposure is fibrinogen response or recovery | 1.35 | (0.89–2.05) | 0.154 | 0.61 | (0.41–0.90) | 0.014 | 0.99 | (0.96–1.03) | 0.601 |
| 3. With further adjustment for latest grade of employment, smoking, alcohol consumption, and physical activity | 1.27 | (0.83–1.95) | 0.265 | 0.61 | (0.41–0.91) | 0.015 | 0.99 | (0.96–1.03) | 0.683 |
| 4. With further adjustment for systolic and diastolic blood pressure, LDL, total cholesterol/HDL ratio, triglycerides, glycated haemoglobin, and BMI | 1.13 | (0.71–1.79) | 0.607 | 0.57 | (0.37–0.88) | 0.011 | 0.99 | (0.95–1.02) | 0.480 |
| 5. With further adjustment for baseline values of CRP, salivary cortisol, IL6, vWF, and MCP-1 | 1.05 | (0.58–1.91) | 0.878 | 0.53 | (0.34–0.83) | 0.006 | 0.98 | (0.94–1.02) | 0.250 |
| 6. With further adjustment for response values of salivary cortisol, IL6, vWF, and MCP-1 | 0.97 | (0.50–1.85) | 0.919 | 0.49 | (0.30–0.81) | 0.006 | 0.98 | (0.94–1.02) | 0.284 |
| 7. With further adjustment for coronary calcification (Agatston score in 4 categories) | 0.92 | (0.48–1.76) | 0.798 | 0.49 | (0.30–0.82) | 0.007 | 0.98 | (0.94–1.02) | 0.246 |
All variables were measured at baseline with the exception of response variables (calculated as the proportional increase from baseline to immediately post task) and fibrinogen recovery (calculated as the proportional decrease from immediate post task to 45 min post task). HS-CTnT was measured at 75 min post task.
No pair of covariates had a correlation coefficient >0.30, with the following exceptions: systolic and diastolic blood pressure (0.70); LDL and total cholesterol/HDL ratio (0.62); triglycerides and total cholesterol/HDL ratio (0.62); CRP and BMI (0.53); CRP and baseline IL-6 (0.45). All covariates had VIFs <3. The exclusion of one or both components of the mentioned pairs of covariates did not change the results. All sensitivity analyses gave similar results to the main analyses.
The additional analyses involving blood pressure response and heart rate response as possible determinants of HS-CTnT detection, gave null findings: although systolic blood pressure increased on average by 35.7 mmHg (s.d. = 16.0) due to the stress tests, diastolic blood pressure by 16.8 mmHg (s.d. = 7.5), and heart rate by 10.5 (s.d. = 9.2) beats per minute, the increase in those parameters was not associated with positive values of HS-CTnT.
4. Discussion
This study confirms prior evidence showing an increase in plasma fibrinogen in response to mental stress. However, in contrast with the notion that larger fibrinogen stress responses may be pathogenic, our results suggest that greater increases have a physiological protective role for cardiovascular health such that people with flat morphology of reaction to mental stress seem to be less protected. Among the three phases, i.e. basal fibrinogen levels, stress reactivity, and post-stress recovery, the reactivity seems to be the more robust marker of allostatic adaptation, showing very large effect size, statistical significance, and independence from covariates.
We have also shown that a sequence of two brief moderately challenging mental stress tasks, which are specifically designed to mimic mild every-day-life stressors, can influence fibrinogen release into the blood stream very efficiently for some individuals and less efficiently for others, even if they are drawn from a well-defined, low-risk, disease-free population. It follows that if one-off measurements of plasma fibrinogen are sampled at unspecified time points without taking environmental factors into account and, moreover, in individuals from heterogeneous populations, they would show large intra-individual variation and, as a consequence, their use as markers of risk may be limited. Our results therefore explain why some other authors have not found robust associations between single measurements of fibrinogen and CVD. For example, Kaptoge et al. found that fibrinogen was associated with CVD but was marginally important in predictive models for CVD after the inclusion of the standard risk factors for CVD prediction (Emerging Risk Factors Collaboration et al., 2012; Stulnig, 2013). Similarly, Mendelian randomization studies that were focused on the genetic determinants of fibrinogen plasma concentration, and not on the reactivity, found no evidence for a causal relationship between fibrinogen levels and cardiac disease (Keavney et al., 2006; Ken-Dror et al., 2012).
Thus the novelty of our analysis lies particularly in the fact that we have linked fibrinogen response to laboratory-induced acute mental stress with a robust CVD outcome such as HS-CTnT. One previous study adopted a very similar approach but identified hypertension as the main clinical outcome and found a positive association between fibrinogen response and hypertension (Brydon and Steptoe, 2005). We think that our new finding is not in contradiction with this previous study because hypertension is closely associated with mental stress (Armario et al., 2003) and is a more distal risk factor for CVD compared to HS-CTnT, which is a robust proximal marker of CVD.
Allostasis is a convenient framework within which to examine the association between mental stress and cardiovascular health. Unfortunately, in this study we could not explore what the actual causes of altered allostatic profile are. Stress reactivity seemed to be independent from age, gender, and other clinical and non-clinical factors related to CVD and to health in general. Also atherosclerosis seemed not to interfere with allostatic regulation, although it was found that tissue factor (extrinsic coagulation pathway) is present in the matrix of the necrotic core of the atherosclerotic plaque (Wilcox et al., 1989).
The prevalence of detectable HS-CTnT in our British sample was 17.1%, which is similar to levels reported (15.7%) in a nationally representative CVD-free population sample in USA (De Lemos et al., 2010).
Detectable HS-CTnT is associated with noncardiac conditions such as severe renal disease (Irfan et al., 2012; Sharma et al., 2006) and, theoretically, our results could be due to confounding if patients with renal disease are more likely to test positive at mental stress tests. However, it is unlikely that this mechanism underlies our results since the study participants were free from any chronic conditions at the time of testing, as explained in Section 2.
Non-calcified coronary plaques are less detectable using cardiac computerized tomography and that may partly explain why CAC did not attenuate the association between fibrinogen and HS-CTnT. However, there is a direct relationship between the number of calcified plaques present and total plaque burden, and CAC correlates highly with the severity of coronary artery disease, so the absence of calcification implies that there is probably little significant coronary artery disease (Arad et al., 2000). On the other hand, it has been argued that raised troponin T may be due to occult or undetected plaque rupture (Korosoglou et al., 2011) and it is known that plaque rupture is a relatively common event that is usually not followed by an acute cardiac event (Arbab-Zadeh et al., 2012). This process may have operated in our patients with minimal CAC score levels and detectable HS-CTnT. However this is unlikely to invalidate our results because atherosclerosis may lie on the causal pathway between fibrinogen and HS-CTnT and also it cannot give an alternative explanation to the dynamics of association that we found.
Fibrinogen was not associated with CAC in our sample. This may be due to the fact that fibrinogen does not directly interfere with the phenomenon of plaque calcification and/or that fibrinogen and CAC can also operate on parallel pathways leading to troponin T release.
A single measure of plasma HS-CTnT concentration cannot be regarded as a robust test if it is not stable over time, i.e. if it shows high intra-individual short-term variation. However, the results from the ARIC study showed that HS-CTnT intra-individual variability over 6 weeks is almost null, with a correlation coefficient of 0.94 (Agarwal et al., 2011). Thus, although our study collected HS-CTnT after a brief moderately stressful behavioural challenge, it is improbable that troponin T was released in response to this task. To the contrary, we hypothesized that higher baseline fibrinogen is indicative of chronic allostatic load that might lead to blunted reactivity and impaired recovery, and this process is likely to be relevant to the aetiology of heart disease, which can be detected by chronic elevation in HS-CTnT concentration.
Our study involved participants free of CVD because we are interested in the primary prevention of heart disease. This approach has the disadvantage of generating results that are not necessarily applicable to the general population. Our results could be due to selection bias if people at the initial stages of the disease or people who already had CVD episodes have different patterns of associations compared with healthy individuals.
The assay that we have used to measure the concentration of cardiac troponin T in the peripheral blood plasma had high sensitivity but we cannot be sure that the participants who scored zero really had no troponin T in their plasma. Although the lower detection limit of the assay was very low (3 ng/L), some participants may have had lower non-zero but undetectable concentrations. These measurement issues may have distorted our results if such participants tended to have higher fibrinogen reaction to the mental stress tests.
This is a cross-sectional study and therefore we cannot determine the causal sequence. Blunted fibrinogen stress responsivity may contribute to early signs of CVD, or people at an early stage of cardiac disease may be more prone to disturbed stress responses. In fact, cardiac troponins are the most sensitive and specific biochemical markers of myocardial damage (Jaffe et al., 2000), but their elevation can be due to a variety of reasons such as pericarditis, myocarditis, and pulmonary embolism (Roongsritong et al., 2004). However, it is unlikely that the undetected presence of those conditions can explain our findings since no participants reported any symptoms or signs of cardiac or kidney disease, had any previous diagnosis or treatment for hypertension, inflammatory disease or allergies, and did not show any electrocardiographic indications of cardiac disease on tests carried out over more than 20 years in the Whitehall II study.
The role of fibrinogen in the coagulation cascade is complex because fibrinogen is not only the product but also the origin of several reactions within a system of mutually reinforcing and mutually rebalancing agents. For example, the breakdown of fibrinogen has a pro-haemostatic function on the one hand, given that this phenomenon is the only route for the formation of fibrin, and has an anti-haemostatic function on the other hand, since unbroken fibrinogen induces platelet adhesion and activation (Chiumiento et al., 2007; Kamath and Lip, 2003). In our study, for some people fibrinogen levels increased by more than 10% after a brief stress test and it is plausible that the released fibrinogen came from activated platelets rather than from liver production, which can take several hours to be completed. In fact, platelets exhibit both adrenergic and dopaminergic receptors that are influenced by different catecholamines and it has been shown that physiological and pathological conditions causing sympathoadrenal activation in vivo, e.g. mental stress, modify the circulating platelet populations and modulate platelet reactivity through an increase in circulating catecholamines (Anfossi and Trovati, 1996).
The fact that heart rate response and blood pressure response were not associated with detectable plasma levels of HS-CTnT suggests that the stress response dynamics leading to heart disease may be peculiar for some CVD risk factors only.
In conclusion, greater fibrinogen responses to mental stress are associated with lower likelihood of detectable high-sensitivity troponin T plasma concentration, independently of basal fibrinogen. Thus, a more dynamic fibrinogen response appears to be advantageous for cardiovascular health. Further research is needed to ascertain the role of fibrinogen in the pathophysiology of cardiovascular disease and the causes of impaired allostatic adaptation to mental stress.
Contributors
All authors have made substantial contribution to the conception and design of the study, the acquisition of data, the interpretation of the results, and the critical review of the article. AL drafted the article and carried out the data analysis and takes responsibility for the accuracy of the analysis. All authors have approved the final article.
Role of the funding source
This research was supported by the British Heart Foundation, United Kingdom. The funders played no role in any phase of the study.
Conflict of interest
All authors declare no conflict of interest of any kind.
Acknowledgement
None.
References
- Agarwal S.K., Avery C.L., Ballantyne C.M., Catellier D., Nambi V., Saunders J., Sharrett A.R., Coresh J., Heiss G., Hoogeveen R.C. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin. Chem. 2011;57:891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand D.V., Lim E., Darko D., Bassett P., Hopkins D., Lipkin D., Corder R., Lahiri A. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J. Am. Coll. Cardiol. 2007;50:2218–2225. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Anfossi G., Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur. J. Clin. Invest. 1996;26:353–370. doi: 10.1046/j.1365-2362.1996.150293.x. [DOI] [PubMed] [Google Scholar]
- Arad Y., Spadaro L.A., Goodman K., Newstein D., Guerci A.D. Prediction of coronary events with electron beam computed tomography. J. Am. Coll. Cardiol. 2000;36:1253–1260. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A., Nakano M., Virmani R., Fuster V. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario P., del Rey R.H., Martin-Baranera M., Almendros M.C., Ceresuela L.M., Pardell H. Blood pressure reactivity to mental stress task as a determinant of sustained hypertension after 5 years of follow-up. J. Hum. Hypertens. 2003;17:181–186. doi: 10.1038/sj.jhh.1001530. [DOI] [PubMed] [Google Scholar]
- Austin A.W., Wissmann T., von Kanel R. Stress and hemostasis: an update. Semin. Thromb. Hemost. 2013;39:902–912. doi: 10.1055/s-0033-1357487. [DOI] [PubMed] [Google Scholar]
- Brotman D.J., Golden S.H., Wittstein I.S. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- Brunner E., Davey Smith G., Marmot M., Canner R., Beksinska M., O’Brien J. Childhood social circumstances and psychosocial and behavioural factors as determinants of plasma fibrinogen. Lancet. 1996;347:1008–1013. doi: 10.1016/s0140-6736(96)90147-6. [DOI] [PubMed] [Google Scholar]
- Brydon L., Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J. Hypertens. 2005;23:1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- Chiumiento A., Lamponi S., Barbucci R. Role of fibrinogen conformation in platelet activation. Biomacromolecules. 2007;8:523–531. doi: 10.1021/bm060664m. [DOI] [PubMed] [Google Scholar]
- Collinson P.O. Sensitive troponin assays. J. Clin. Pathol. 2011;64:845–849. doi: 10.1136/jclinpath-2011-200164. [DOI] [PubMed] [Google Scholar]
- Collinson P.O., Heung Y.M., Gaze D., Boa F., Senior R., Christenson R., Apple F.S. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin. Chem. 2012;58:219–225. doi: 10.1373/clinchem.2011.171082. [DOI] [PubMed] [Google Scholar]
- deFilippi C.R., de Lemos J.A., Christenson R.H., Gottdiener J.S., Kop W.J., Zhan M., Seliger S.L. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. J. Am. Med. Assoc. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lemos J.A., Drazner M.H., Omland T., Ayers C.R., Khera A., Rohatgi A., Hashim I., Berry J.D., Das S.R., Morrow D.A., McGuire D.K. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. J. Am. Med. Assoc. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration, Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P., Walker M., Thompson A., Sarwar N., Caslake M., Butterworth A.S., Amouyel P., Assmann G., Bakker S.J.L., Barr E.L.M., Barrett-Connor E., Benjamin E.J., Björkelund C., Brenner H., Brunner E., Clarke R., Cooper J.A., Cremer P., Cushman M., Dagenais G.R., D’Agostino R.B., Sr., Dankner R., Davey-Smith G., Deeg D., Dekker J.M., Engström G., Folsom A.R., Fowkes F.G.R., Gallacher J., Gaziano J.M., Giampaoli S., Gillum R.F., Hofman A., Howard B.V., Ingelsson E., Iso H., Jørgensen T., Kiechl S., Kitamura A., Kiyohara Y., Koenig W., Kromhout D., Kuller L.H., Lawlor D.A., Meade T.W., Nissinen A., Nordestgaard B.G., Onat A., Panagiotakos D.B., Psaty B.M., Rodriguez B., Rosengren A., Salomaa V., Kauhanen J., Salonen J.T., Shaffer J.A., Shea S., Ford I., Stehouwer C.D.A., Strandberg T.E., Tipping R.W., Tosetto A., Wassertheil-Smoller S., Wennberg P., Westendorp R.G., Whincup P.H., Wilhelmsen L., Woodward M., Lowe G.D.O., Wareham N.J., Khaw K.-T., Sattar N., Packard C.J., Gudnason V., Ridker P.M., Pepys M.B., Thompson S.G., Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney P.J., Wong M.Y. Collaborative study of a proposed international standard for plasma fibrinogen measurement. Thromb. Haemost. 1992;68:428–432. [PubMed] [Google Scholar]
- Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A.S., Katus H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- Hamer M., O’Donnell K., Lahiri A., Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur. Heart J. 2010;31:424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Irfan A., Twerenbold R., Reiter M., Reichlin T., Stelzig C., Freese M., Haaf P., Hochholzer W., Steuer S., Bassetti S., Zellweger C., Freidank H., Peter F., Campodarve I., Meune C., Mueller C. Determinants of high-sensitivity troponin T among patients with a noncardiac cause of chest pain. Am. J. Med. 2012;125:491–498.e1. doi: 10.1016/j.amjmed.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Jaffe A.S., Ravkilde J., Roberts R., Naslund U., Apple F.S., Galvani M., Katus H. It's time for a change to a troponin standard. Circulation. 2000;102:1216–1220. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- Kamath S., Lip G.Y.H. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96:711–729. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
- Keavney B., Danesh J., Parish S., Palmer A., Clark S., Youngman L., Delépine M., Lathrop M., Peto R., Collins R. Fibrinogen and coronary heart disease: test of causality by “Mendelian randomization”. Int. J. Epidemiol. 2006;35:935–943. doi: 10.1093/ije/dyl114. [DOI] [PubMed] [Google Scholar]
- Ken-Dror G., Humphries S.E., Kumari M., Kivimaki M., Drenos F. A genetic instrument for Mendelian randomization of fibrinogen. Eur. J. Epidemiol. 2012;27:267–279. doi: 10.1007/s10654-012-9666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel F., Leynen F., Stam M., Dramaix M., de Smet P., Mak R., De Backer G., Kornitzer M. Job conditions and fibrinogen in 14226 Belgian workers: the Belstress study. Eur. Heart J. 2002;23:1841–1848. doi: 10.1053/euhj.2002.3258. [DOI] [PubMed] [Google Scholar]
- Korosoglou G., Lehrke S., Mueller D., Hosch W., Kauczor H.-U., Humpert P.M., Giannitsis E., Katus H.A. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart Br. Card. Soc. 2011;97:823–831. doi: 10.1136/hrt.2010.193201. [DOI] [PubMed] [Google Scholar]
- Lazzarino A.I., Hamer M., Gaze D., Collinson P., Steptoe A. The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. J. Am. Coll. Cardiol. 2013;62:1694–1701. doi: 10.1016/j.jacc.2013.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G.D.O., Rumley A., Mackie I.J. Plasma fibrinogen. Ann. Clin. Biochem. 2004;41:430–440. doi: 10.1258/0004563042466884. [DOI] [PubMed] [Google Scholar]
- Marmot M.G., Smith G.D., Stansfeld S., Patel C., North F., Head J., White I., Brunner E., Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Regression with Stata Web Book: Chapter 2 – Regression Diagnostics [WWW Document], n.d. URL http://www.ats.ucla.edu/stat/stata/webbooks/reg/chapter2/statareg2.htm (accessed 30.04.14).
- Roongsritong C., Warraich I., Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. 2004;125:1877–1884. doi: 10.1378/chest.125.5.1877. [DOI] [PubMed] [Google Scholar]
- Sharma R., Gaze D.C., Pellerin D., Mehta R.L., Gregson H., Streather C.P., Collinson P.O., Brecker S.J.D. Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T. Heart Br. Card. Soc. 2006;92:804–809. doi: 10.1136/hrt.2005.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Kivimäki M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Kunz-Ebrecht S., Owen N., Feldman P.J., Rumley A., Lowe G.D.O., Marmot M. Influence of socioeconomic status and job control on plasma fibrinogen responses to acute mental stress. Psychosom. Med. 2003;65:137–144. doi: 10.1097/01.psy.0000039755.23250.a7. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Owen N., Kunz-Ebrecht S., Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain. Behav. Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Owen N., Kunz-Ebrecht S.R., Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Vögele C. Methodology of mental stress testing in cardiovascular research. Circulation. 1991;83:II14–II24. [PubMed] [Google Scholar]
- Stulnig T.M. C-reactive protein, fibrinogen, and cardiovascular risk. N. Engl. J. Med. 2013;368:84–85. doi: 10.1056/NEJMc1213688. [DOI] [PubMed] [Google Scholar]
- Surtees P.G., Wainwright N.W.J., Luben R.N., Wareham N.J., Bingham S.A., Khaw K.-T. Psychological distress, major depressive disorder, and risk of stroke. Neurology. 2008;70:788–794. doi: 10.1212/01.wnl.0000304109.18563.81. [DOI] [PubMed] [Google Scholar]
- Thygesen K., Alpert J.S., White H.D., Jaffe A.S., Apple F.S., Galvani M., Katus H.A., Newby L.K., Ravkilde J., Chaitman B., Clemmensen P.M., Dellborg M., Hod H., Porela P., Underwood R., Bax J.J., Beller G.A., Bonow R., Van der Wall E.E., Bassand J.-P., Wijns W., Ferguson T.B., Steg P.G., Uretsky B.F., Williams D.O., Armstrong P.W., Antman E.M., Fox K.A., Hamm C.W., Ohman E.M., Simoons M.L., Poole-Wilson P.A., Gurfinkel E.P., Lopez-Sendon J.-L., Pais P., Mendis S., Zhu J.-R., Wallentin L.C., Fernández-Avilés F., Fox K.M., Parkhomenko A.N., Priori S.G., Tendera M., Voipio-Pulkki L.-M., Vahanian A., Camm A.J., De Caterina R., Dean V., Dickstein K., Filippatos G., Funck-Brentano C., Hellemans I., Kristensen S.D., McGregor K., Sechtem U., Silber S., Tendera M., Widimsky P., Zamorano J.L., Morais J., Brener S., Harrington R., Morrow D., Lim M., Martinez-Rios M.A., Steinhubl S., Levine G.N., Gibler W.B., Goff D., Tubaro M., Dudek D., Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- Von Känel R., Mills P.J., Fainman C., Dimsdale J.E. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom. Med. 2001;63:531–544. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Wallace T.W., Abdullah S.M., Drazner M.H., Das S.R., Khera A., McGuire D.K., Wians F., Sabatine M.S., Morrow D.A., de Lemos J.A. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- Warnick G.R., Knopp R.H., Fitzpatrick V., Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- Wilcox J.N., Smith K.M., Schwartz S.M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz P.H., Redwine L.S., Baertschi C., Spillmann M., Ehlert U., von Känel R. Coagulation activity before and after acute psychosocial stress increases with age. Psychosom. Med. 2008;70:476–481. doi: 10.1097/PSY.0b013e31816e03a5. [DOI] [PubMed] [Google Scholar]
- Wirtz P.H., Redwine L.S., Ehlert U., von Känel R. Independent association between lower level of social support and higher coagulation activity before and after acute psychosocial stress. Psychosom. Med. 2009;71:30–37. doi: 10.1097/PSY.0b013e31818f6868. [DOI] [PubMed] [Google Scholar]