Abstract
The detection of severe malaria in resource–constrained settings is often difficult because of requirements for laboratory infrastructure and/or clinical expertise. The aim of this study, therefore, was to explore the utility of a multiple antigen (HRP–2/pLDH) rapid diagnostic test (RDT) as a low–cost, surrogate marker of patients at high risk for complications of severe malaria. We reviewed programmatic data at a peripheral health center in Western Uganda. Available demographic and clinical data on all individuals presenting to the center who underwent an RDT for suspected malaria infection were reviewed. We fit logistic regression models to identify correlates of two outcomes of interest: 1) severe malaria–related anemia, defined here as hemoglobin ≤7g/dL and 2) receipt of parenteral quinine. 1509 patients underwent malaria testing with an SD FK60 RDT during the observation period. A total of 637 (42%) RDTs were positive for at least one species of malaria, of which 326 (51%) exhibited a single HRP–2 band and 307 (48%) exhibited both HRP–2 and pLDH bands, while 4 exhibited only a single pLDH band. There was a trend towards more severe anemia in patients with a HRP–2/pLDH positive RDT compared to a HRP–2 only RDT (β = –0.99 g/dl, 95% CI –1.99 to 0.02, P = 0.055). A HRP–2/pLDH positive RDT was associated with an increased risk of severe malaria–related anemia compared to a negative RDT (adjusted odds ratio (AOR) 18.8, 95% CI 4.32 to 82.0, P < 0.001) and to a HRP–2 only RDT (AOR 2.46, 95% CI 0.75 to 8.04, P = 0.14). There was no significant association between RDT result and the administration of parenteral quinine. These results offer preliminary evidence that specific patterns of antigen positivity on RDTs could be utilized to identify patients at an increased risk for complications of severe malaria.
Each year millions of African children are infected with P. falciparum malaria, and approximately 550 000 die from manifestations of severe disease [1]. In areas of high transmission intensity, severe anemia is the most common complication of severe malaria, which primarily affects infants and young children [2]. While delays in the diagnosis and treatment of malaria are associated with poor outcomes [3-5], effective management requires well–equipped hospitals with highly trained clinicians, which are lacking in many malaria endemic settings [6,7]. Mortality among children with severe malaria anemia who receive care at hospitals and referral centers has been reported as high as 10–20% [8-11], and it may be even higher among those presenting to peripheral health facilities, where human resources and acute care services, including access to mechanical ventilation, hemodialysis, and blood transfusion, are limited [12,13].
A major challenge to the identification of severe malaria stems from the rigorous laboratory–based diagnostic criteria [2,14,15]. Hemoglobin, glucose, creatinine, and lactate levels are rarely available at peripheral health facilities in resource–constrained settings [14,15]. Even light microscopy is often inaccessible because of the requisite infrastructure and technical expertise [7,13,16,17]. Without proper diagnostic tools, health care workers may misidentify and inappropriately triage cases of severe malaria [7]. An ideal diagnostic test would enable health care workers, especially nurses, midwives, and community health workers, to accurately and quickly identify patients either with or at risk for complications of severe malaria and initiate expedited management practices.
Rapid diagnostic tests (RDTs) are low–cost, simple tools for the diagnosis of malaria and are particularly valuable in settings where microscopy is not readily available [18-21]. Newer generation RDTs that incorporate multiple antigens on a single membrane have been developed to differentiate between various Plasmodium species infections. Additionally, these RDTs have shown some ability to provide a quantitative estimate of parasite density due to the differing sensitivities of the respective antigens [22-24]. Because parasite density can be a correlate of disease severity, the use of multiple antigens on a single membrane has the potential to aid in detection of severe malaria. No study, however, has examined the association of RDT band positivity with clinical parameters. Thus, we reviewed data from a peripheral health center in rural Uganda to assess for a correlation between band RDT positivity and markers of disease severity. Our overarching goal was to explore the potential use of a multiple–antigen RDT as a low–cost, scalable marker of patients at risk of severe malaria–related anemia.
METHODS
Study site
We evaluated programmatic data during the period January–March 2013, collected from the Bugoye Level III Health Center (BHC), in the Kasese District of Western Uganda (0° 18’ North, 30° 5′ East). The health center functions as a referral center for the Bugoye sub–county, and serves a rural population of approximately 50 000 residents. The center houses a small laboratory with staff trained to perform light microscopy and RDTs, as well other basic tests, such as hemoglobin estimation.
Study overview
The objective of this retrospective, observational pilot study was to assess the diagnostic utility of a three–band (HRP–2/pLDH) RDT at a resource–limited, rural health center in malaria–endemic Western Uganda.
Study procedures
We reviewed data on all individuals with fever who underwent an RDT for suspected malaria infection. We abstracted data on patient age, sex, village of residence, patient disposition, laboratory results, and medication prescriptions from clinic registers.
Laboratory procedures
Laboratory diagnosis of malaria was made using the Standard Diagnostics FK60 Malaria Ag P. falciparum/Pan RDT (Standard Diagnostics, Hagal–Dong, Korea). The RDT is a validated antigen detection test with three individual bands signifying the control, the HRP–2, and the pLDH antigens [23,25,26]. The presence of a control band in the absence of either a HRP–2 or pLDH result indicates a negative test. The presence of a single HRP–2 line denotes infection with P. falciparum, whereas a unique pLDH line indicates infection with one or more of the other Plasmodium species. The presence of a positive HRP–2 line together with a pLDH line indicates an infection with either P. falciparum or a mixed–species infection.
RDTs (Lot #090192) for the study were obtained from Kampala Pharmaceutical Industries (Kampala, Uganda), and stored in their original packaging at room temperature. RDTs were performed and interpreted by two members of the BHC laboratory staff in accordance with the manufacturer’s instructions. In cases in which the control line did not appear, the result was considered invalid and the test was repeated. Faint HRP–2 and pLDH test lines were considered positive.
When available, we reviewed matched thick and thin smears for specimens with three–band positive tests. Smears were prepared using a modified Field’s Stain and examined by light microscopy [27]. Asexual parasitemia of any level was reported as a positive smear. Blood smears were transported to the Epicentre Mbarara Research Center for confirmatory review, where two expert microscopists, who were blinded to the field results, independently read the slides. Hemoglobin (Hb) levels were estimated using Sahli’s method [28] as is the standard of care at BHC.
Statistical analysis
Data were entered into Microsoft Excel (Redmond, WA) and analyzed with Stata 12.1 (College Station, TX, USA). We summarized patient characteristics and compared those with two– and three–band positive RDTs using Pearson chi–squared testing. We fit univariable logistic regression models to identify correlates for two outcomes of interest: 1) severe anemia, defined as a hemoglobin <7 g/deciliter (g/dL) and 2) receipt of parenteral quinine. While the WHO traditionally define severe malaria-related anemia a normocytic anemia of Hb <5 g/dL in children and <7 g/dL in adults, we used hemoglobin values of <7 g/dL as the clinically relevant outcome as it represents the transfusion threshold for severe malaria in adults and approaches the threshold at which transfusion should be considered in children when present with other clinical features [2,29].
Our primary explanatory variable of interest was RDT test result, defined as HRP–2 vs HRP–2/pLDH positive. Secondary explanatory variables included sex, age, village distance from the health center, village elevation, and transmission season, defined as high (January) or low (February, March). We fit multivariable models including all variables that were significant in univariable models with a pre–specified P–value of <0.25 [30]. A resulting P–value of <0.05 was considered statistically significant in the final models.
Ethics statement
Ethical approval for study procedures and data collection was provided by the institutional review boards of Partners Healthcare and the Mbarara University of Science and Technology. Informed consent was not required by the ethical review committees due to the programmatic nature of the project.
RESULTS
During the observation period, 1509 patients underwent malaria testing with an RDT. A total of 637 RDTs (42%) were positive for malaria. Of the positive RDTs, 326 (51.2%) exhibited a single HRP–2 band, 307 RDTs (48.2%) exhibited both HRP–2 and pLDH bands, while only 4 RDTs (0.6%) exhibited a single pLDH band. Characteristics of the study sample, stratified by RDT result, are summarized in Table 1.
Table 1.
Univariable comparison of patients with positive RDT by antigen category
| Characteristic | HRP–2 positive | HRP–2/pLDH positive | P–value |
|---|---|---|---|
|
Number (n, %) |
326 (51.5) |
307 (48.5) |
– |
|
Sex (Male/Female) |
124/202 |
123/184 |
0.60 |
|
Age (median, interquartile range): |
31 (15, 58) |
25 (12, 58) |
0.22 |
| <5 years (n, %) |
87 (26.7) |
87 (28.3) |
0.64 |
| <15 years (n, %) |
192 (58.9) |
216 (70.4) |
0.003 |
|
Village (n, %): |
|||
| distant from Clinic |
22 (6.8) |
18 (5.9) |
0.69 |
| dow elevation |
152 (46.6) |
141 45.9) |
0.03 |
|
Transmission season: |
|||
| high (n, %) |
119 (36.5) |
164 (53.4) |
<0.001 |
| low (n, %) | 207 (63.5) | 143 (46.6) |
Notably, the prevalence rate of HRP–2/pLDH positive results was twice as high among patients <15 years compared to those ≥15 years. The absolute number and proportion of HRP–2/pLDH positive RDTs declined during the transition from high to low transmission seasons, while that of HRP–2 only positive RDTs remained relatively stable.
Of the 307 patients with HRP–2/pLDH positive RDTs, 90 (29.3%) had corresponding blood smears available for interpretation. The majority (83/90, 92.2%) of smears from patients with HRP–2/pLDH positive RDT results demonstrated P. falciparum mono–infections, although there were 4 mixed–species infections (2 Pf/Pv, 1 Pf/Po, 1 Pf/Pm). In addition, there were two non–P. falciparum mono–infections (1 Po, 1 Pm), which may have represented sub–patent P. falciparum co–infection not seen on microscopy. All four of the pLDH–only positive RDTs were identified as P. ovale mono–infections. Parasite density was reported for 89 of 90 of the three–band positive individuals. The mean density was 73 105 parasites/μL with a median density of 40 200 parasites/μL (IQR = 12 100–103 238). Parasite densities were only available for three patients with HRP–2 positive RDTs.
The sensitivity for P. falciparum infections (including mixed–species infections) was 99.1% (95% CI 94.5% to 99.9%). The specificity was 75% (95% CI 53.0% to 89.4%), although only a small number of smears were prepared in patients with paired HRP–2 positive (n = 19) and negative (n = 19) RDTs. If, however, we assume that the two RDTs that were three–band positive but negative on microscopy represented true P. falciparum infections, as seen when confirmed by PCR in other studies [31,32], the specificity improves to 81.8%.
During the observation period, a total of 85 paired hemoglobin (Hb) levels were assessed in patients also undergoing an SD FK60 RDT. Children <5 years of age were more likely to have a Hb performed and had a higher proportion of values <7 g/dL (56.4% vs 26.1%) compared to those individuals ≥5 years of age. The mean Hb was 7.3g/dL (95% CI 6.6 to 8.1) in patients with a HRP–2 only positive RDT, and 6.3g/dL (95% CI 5.6 to 7.1) in patients with a three–band positive RDT (β = –0.99 g/dL, 95% CI –1.99 to 0.02, P = 0.055, Figure 1).
Figure 1.
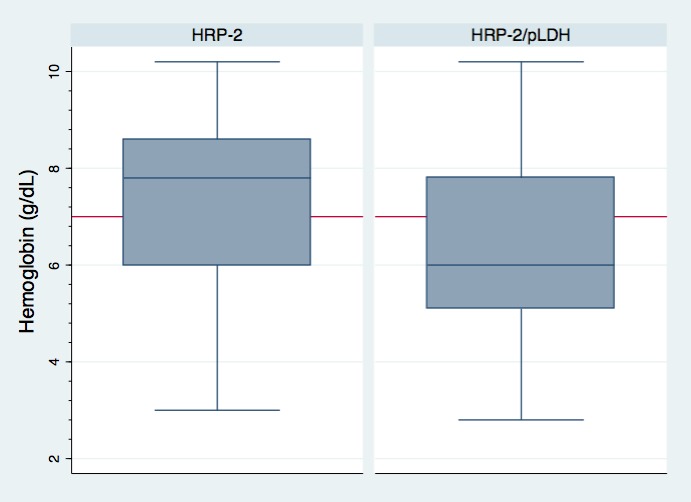
Box plot showing distribution of hemoglobin level in HRP–2 vs HRP–2/pLDH positive rapid diagnostic tests.
In multivariate logistic regression models, a HRP–2/pLDH positive RDT was the strongest predictor of severe anemia (Table 2). The odds of severe malaria anemia were more than double in those patients with a HRP–2/pLDH positive RDT compared to those with an HRP–2 only positive RDT (AOR 2.46, 95% CI 0.75 to 8.04, P = 0.14) with a trend toward statistical significance.
Table 2.
Logistic regression models for correlates of hemoglobin <7 mg/dL
| Univariable model | Multivariate model | |||||
|---|---|---|---|---|---|---|
|
Characteristic |
OR |
95% CI |
P–value |
AOR |
95% CI |
P–value |
|
Male sex |
1.51 |
0.62–3.72 |
0.37 |
– |
– |
– |
|
Age <15 years |
4.47 |
1.49–13.38 |
0.007 |
4.55 |
1.32–15.6 |
0.016 |
|
High season |
0.85 |
0.35–2.05 |
0.72 |
– |
– |
– |
|
RDT result: |
||||||
| negative |
Reference |
Reference |
||||
| HRP–2 only |
7.69 |
1.81–32.6 |
0.006 |
7.49 |
1.70–33.1 |
0.008 |
| HRP–2/pLDH | 18.0 | 4.37–74.2 | <0.001 | 18.8 | 4.32–82.0 | <0.001 |
OR – odds ratio, CI – confidence interval, AOR – adjusted odds ratio, RDT – rapid diagnostic test, HRP–2 – histidine rich protein–2, pLDH – pan–lactate dehydrogenase.
*Explanatory variables with a P–value of <0.25 in the univariable model were included in the multivariable model.
We identified 179 patients who were admitted to the inpatient ward with an admission diagnosis of malaria. RDT results were available for 116 (64.8%) of these patients. There were no differences in rates of inpatient admission between those with HRP–2 only and HRP–2/pLDH positive RDTs. Patients with HRP–2/pLDH positive results were more likely to receive treatment with parenteral quinine and anti–pyretics. In the multivariate analysis for correlates of receipt of parenteral quinine, the strongest associations were seen with three–band positive RDT and infection during high transmission season, although neither of these reached statistical significance (Table 3).
Table 3.
Logistic regression models for correlates of parenteral quinine
| Univariable model | Multivariate model | |||||
|---|---|---|---|---|---|---|
|
Characteristic |
OR |
95% CI |
P–value |
AOR |
95% CI |
P–value |
|
Male sex |
1.47 |
0.43–4.98 |
0.54 |
– |
– |
– |
|
Age <15 years |
0.89 |
0.25–3.19 |
0.85 |
– |
– |
– |
|
Comorbidity |
3.44 |
0.81–2.43 |
0.35 |
– |
– |
– |
|
Antibiotics |
0.63 |
0.18–2.26 |
0.48 |
– |
– |
– |
|
High season |
6.52 |
0.80–52.9 |
0.079 |
5.59 |
0.68–46.0 |
0.11 |
| 3–band RDT | 2.36 | 0.66–8.43 | 0.19 | 1.92 | 0.52–7.06 | 0.33 |
OR – odds ratio, CI – confidence interval, AOR – adjusted odds ratio, RDT – rapid diagnostic test, HRP–2 – histidine rich protein–2, pLDH – pan–lactate dehydrogenase.
*Explanatory variables with a P–value of <0.25 in the univariable model were included in the multivariable model.
DISCUSSION
Our results show a trend towards patients with HRP–2/pLDH positive RDTs having more severe malaria–related anemia that those patients with an HRP–2 only positive RDT. While previous work with the SD FK60 in a reference laboratory found a correlation between parasite density and the presence of the pLDH band [23], our findings linking three–band positivity to anemia are novel. Our findings provide preliminary evidence that multiple band RDTs, with antigens of differing sensitivity, may have the potential to serve as an adjunctive tool for the identification of patients at high risk of severe malaria–related anemia. Further studies are needed to determine if incorporating three–band positivity into malaria case management algorithms at peripheral health facilities might accurately identify patients either with or at risk for complications of severe malaria.
We postulate that the positive pLDH band, which is less sensitive than HRP–2, is a marker of higher parasite density [33-35]. Findings from our study that would support this association include: 1) nearly a quarter of three–band positive smears demonstrated parasite densities >100 000/μl; 2) a higher prevalence of three–band positive RDTs among individuals <15 years of age who have not yet acquired protective immunity [36-39]; 3) high relative rates of three–band positive disease during the traditional high transmission season; and 4) the higher degree of anemia among patients with a HRP–2/pLDH positive RDT. We note that previous studies have shown that the degree of anemia correlated with both parasitemia and schizontemia [40].
An alternate explanation for the relationship could be HRP–2 antibody persistence leading to false–positive HRP–2 positive RDTs. Prior studies in Uganda have reported HRP–2 band specificities as low as 62% [31]. These false–positive HRP–2 tests could result after recent treatment with antimalarials, making those patients with acute illness as indicated by HRP–2/pLDH band positivity appear relatively ill.
However, our clinical experience in Bugoye suggests that very few patients seek treatment for malaria outside of the government health center, given difficult terrain and high costs associated with private facilities. In settings where there is a low probability of prior treatment, such as our site, the specificity of the HRP–2 antigen is typically higher than that associated with studies conducted at urban referral centers [31,41,42]. As evidence of this, studies from more rural sites in Uganda that have utilized polymerase chain reaction (PCR) to investigate discordant RDT and microscopy results have found the PCR–corrected specificity to be significantly higher [32,43]. Additionally, validation studies with the SD FK 60 in field conditions from other regions have reported a specificity for P. falciparum malaria exceeding 95% [26].
Our study, which was hypothesis generating in nature and primarily involved a retrospective review of routinely collected data, has a number of limitations. Foremost among these is the lack of available paired slides for all specimens. The available slides and Hb results were prepared in a non–random manner at the direction of the supervising clinical officer. We did, however, review smears for nearly 30% of those patients with three–band positive RDTs and Hb values for nearly 15% of patients with positive RDTs. The second major limitation of our study was that the use of the Sahlis method for Hb estimation, which while simple and inexpensive, can be prone to dilution and reading errors. We believe there should be minimal bias stemming from this method because errors would be stochastic and a single staff member interpreted all results. Finally, we performed this study at a single site and with a relatively small sample size, which reinforces the need for corroboration of these findings in larger, and more diverse patient populations. A follow up, prospective study is now under way.
CONCLUSIONS
In summary, our findings suggest that patients with HRP–2/pLDH positive RDTs have more severe malaria–related anemia than patients with HRP–2 only positive RDTs. We postulate that the positive pLDH band, which is less sensitive than HRP–2, is a marker of higher parasite density and correspondingly, more severe anemia. These results offer preliminary evidence that multiple antigen RDTs could be utilized to identify patients at an increased risk for complications of severe malaria. If confirmed, multiple antigen RDTs may play a role in case management algorithms at peripheral health facilities. Further studies with more comprehensive data collection methods, larger sample sizes, and longer timeframes should be pursued before this approach is adopted.
Acknowledgements
We would like to thank the patients and clinical staff at Bugoye Health Centre III, especially Shem Bwambale, Biira Yolecy, and Joaqim Bathimwa, who were critical in identifying these trends. Additionally, we would like to recognize Gus Ruchman and Jen Zhu, who collected initial data for this report, along with Juliet Mwanga–Amumpaire and Dan Nyehangane from Epicentre Mbarara assisted with supplies and logistics.
Funding: None.
Authorship declarations: RB conceived the study, collected the data, participated in the design of the study, performed the statistical analysis, and drafted the manuscript. RR conceived the study with RB, participated in the design of the study and helped to draft the manuscript. MM, MN, and EM helped collect the data and draft the manuscript. YB helped draft the manuscript. MS participated in the design of the study, reviewed the statistical analysis, and helped draft the manuscript. All authors read and approved the final manuscript.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflict of interest.
REFERENCES
- 1.WHO. World malaria report. Geneva: World Health Organization, 2013. [Google Scholar]
- 2.Severe malaria. Trop Med Int Health. 2014;19(Suppl 1):7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 3.de Savigny D, Mayombana C, Mwageni E, Masanja H, Minhaj A, Mkilindi Y, et al. Care-seeking patterns for fatal malaria in Tanzania. Malar J. 2004;3:27. doi: 10.1186/1475-2875-3-27. Published online 07 30, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Checkley AM, Smith A, Smith V, Blaze M, Bradley D, Chiodini PL, et al. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ. 2012;344:e2116. doi: 10.1136/bmj.e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141:547–55. doi: 10.7326/0003-4819-141-7-200410050-00012. [DOI] [PubMed] [Google Scholar]
- 6.Day N. The management of patients with severe malaria. Am J Trop Med Hyg. 2007;77(Suppl):29–35. [PubMed] [Google Scholar]
- 7.Achan J, Tibenderana J, Kyabayinze D, Mawejje H, Mugizi R, Mpeka B, et al. Case management of severe malaria–a forgotten practice: experiences from health facilities in Uganda. PLoS ONE. 2011;6:e17053. doi: 10.1371/journal.pone.0017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg AE, Ntumbanzondo M, Ntula N, Mawa L, Howell J, Davachi F. Hospital-based surveillance of malaria-related paediatric morbidity and mortality in Kinshasa, Zaire. Bull World Health Organ. 1989;67:189–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–43. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- 10.Jaffar S, Van Hensbroek MB, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. Am J Trop Med Hyg. 1997;57:20–4. doi: 10.4269/ajtmh.1997.57.20. [DOI] [PubMed] [Google Scholar]
- 11.Biemba G, Thuma PE, Weiss G, Gordeuk VR. Severe anaemia in Zambian children with Plasmodium falciparum malaria. Trop Med Int Health. 2000;5:9–16. doi: 10.1046/j.1365-3156.2000.00506.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood B, Marsh K, Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991;7:277–81. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 13.Abreha T, Alemayehu B, Tadesse Y, Gebresillassie S, Tadesse A, Demeke L, et al. Malaria diagnostic capacity in health facilities in Ethiopia. Malar J. 2014;13:292. doi: 10.1186/1475-2875-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Severe falciparum malaria. Geneva: WHO, 2000. [Google Scholar]
- 15.Bejon P, Berkley JA, Mwangi T, Ogada E, Mwangi I, Maitland K, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moerman F, Lengeler C, Chimumbwa J, Talisuna A, Erhart A, Coosemans M, et al. The contribution of health-care services to a sound and sustainable malaria-control policy. Lancet Infect Dis. 2003;3:99–102. doi: 10.1016/S1473-3099(03)00518-8. [DOI] [PubMed] [Google Scholar]
- 17.Bloland PB, Kachur SP, Williams HA. Trends in antimalarial drug deployment in sub-Saharan Africa. J Exp Biol. 2003;206:3761–9. doi: 10.1242/jeb.00637. [DOI] [PubMed] [Google Scholar]
- 18.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011;7:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clin Microbiol Infect. 2013;19:399–407. 2. doi: 10.1111/1469-0691.12151. [DOI] [PubMed] [Google Scholar]
- 20.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505. doi: 10.1099/jmm.0.052506-0. [DOI] [PubMed] [Google Scholar]
- 22.Richter J, Muller-Stover I, Hoppenheit B, Haussinger D. Co-reactivity of plasmodial histidine-rich protein 2 and aldolase on a combined immuno-chromographic- malaria dipstick (ICT) as a potential semi-quantitative marker of high Plasmodium falciparum parasitaemia. Parasitol Res. 2004;94:384–5. doi: 10.1007/s00436-004-1213-6. [DOI] [PubMed] [Google Scholar]
- 23.Van der Palen M, Gillet P, Bottieau E, Cnops L, Van Esbroeck M, Jacobs J. Test characteristics of two rapid antigen detection tests (SD FK50 and SD FK60) for the diagnosis of malaria in returned travellers. Malar J. 2009;8:90. doi: 10.1186/1475-2875-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatton ML, Rees-Channer R, Glenn J, Barnwell J, Cheng Q, Chiodini P, et al. Pan-Plasmodium band sensitivity for Plasmodium falciparum detection in combination malaria rapid diagnostic tests and implications for clinical management. Malar J. 2015;14:115. doi: 10.1186/s12936-015-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Malaria Rapid Diagnostic Test Performance, Results of WHO product testing of malaria RDTs: Round 3 (2010–2011). Geneva: WHO, 2012. [Google Scholar]
- 26.Kosack CS, Naing WT, Piriou E, Shanks L. Routine parallel diagnosis of malaria using microscopy and the malaria rapid diagnostic test SD 05FK60: the experience of Medecins Sans Frontieres in Myanmar. Malar J. 2013;12:167. doi: 10.1186/1475-2875-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Malaria Working Party of The General Haematology Task Force of the British Committee for Standards in Haematology The laboratory diagnosis of malaria. Clin Lab Haematol. 1997;19:165–70. doi: 10.1111/j.1365-2257.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 28.van Lerberghe W, Keegels G, Cornelis G, Ancona C, Mangelschots E, van Balen H. Haemoglobin measurement: the reliability of some simple techniques for use in a primary health care setting. Bull World Health Organ. 1983;61:957–65. [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Management of severe malaria: a practical handbook. Geneva: WHO, 2012. [Google Scholar]
- 30.Hosmer D, Lemeshow S. Applied logistic regression. New York: Wiley; 2000. [Google Scholar]
- 31.Hawkes M, Conroy AL, Opoka RO, Namasopo S, Liles WC, John CC, et al. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar J. 2014;13:43. doi: 10.1186/1475-2875-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–8. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 33.Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacol Ther. 2003;99:221–60. doi: 10.1016/S0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 35.Gonçalves BP, Huang CY, Morrison R, Holte S, Kabyemela E, Prevots DR, et al. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med. 2014;370:1799–808. doi: 10.1056/NEJMoa1303944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aponte JJ, Menendez C, Schellenberg D, Kahigwa E, Mshinda H, Vountasou P, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–3. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 39.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 40.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 41.Amuasi JH, Diap G, Nguah SB, Karikari P, Boakye I, Jambai A, et al. Access to artemisinin-combination therapy (ACT) and other anti-malarials: national policy and markets in Sierra Leone. PLoS ONE. 2012;7:e47733. doi: 10.1371/journal.pone.0047733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tipke M, Louis VR, Ye M, De Allegri M, Beiersmann C, Sie A, et al. Access to malaria treatment in young children of rural Burkina Faso. Malar J. 2009;8:266. doi: 10.1186/1475-2875-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilian AH, Kabagambe G, Byamukama W, Langi P, Weis P, von Sonnenburg F. Application of the ParaSight-F dipstick test for malaria diagnosis in a district control program. Acta Trop. 1999;72:281–93. doi: 10.1016/S0001-706X(99)00003-0. [DOI] [PubMed] [Google Scholar]


