Abstract
GLP-1R agonists are novel new drugs for patients with type 2 diabetes mellitus (T2DM). An intriguing connection between these agents such as liraglutide and native GLP-1 from the gut and the release of the blood pressure lowering cardiac hormone atrial natriuretic peptide (ANP) establishes a new gut – heart connection not only for glycemic control in diabetes, but in overall cardiovascular homeostasis.
The heart plays a fundamental part in blood pressure regulation through the production and release of the peptide hormone atrial natriuretic peptide (ANP) (1). As originally described, and as we know today, ANP is synthesized in secretory granules of cardiomyocytes located in the atria of the heart as a prohormone, which is processed into the mature biologically active ANP, which functions via guanylyl cyclase A (GC-A) that then produces the second messenger 3’,5, cyclic guanosine monophosphate (cGMP) (2). Released in response to an increase in dietary sodium and/or an increase in intravascular volume with atrial stretch, ANP activates GC-A in endothelial cells, vascular smooth muscle and collecting duct cells to increase blood vessel permeability, induce vasorelaxation and inhibit sodium ion reabsorption that results in natriuresis, respectively. In addition, GC-A is highly expressed in zona glomerulosa cells of the adrenal glands where ANP inhibits the production and release of aldosterone that contributes to blood pressure reduction. All these actions come together to establish ANP as a fundamental physiologic regulator of blood pressure homeostasis through these pleotropic actions. Most recently, genetic variants of the ANP gene (NPPA) that result in an increase in circulating levels of ANP are associated with lower blood pressure, decreased risk of hypertension and protection from the metabolic syndrome in healthy individuals (3,4).
In this current issue of Nature Medicine, Kim et al provides an entirely new mechanism in the control of ANP release from the heart by a gut-released hormone, thuse revealing for the first time a new gut–heart connection in the control of blood pressure homeostasis (5). Previously, diabetic patients receiving long acting glucagon like peptide 1 (GLP-1) analogues, including liraglutide and exenatide, showed a reduction in blood pressure (6). In the study by Kim et al the authors demonstrate that the effect of liraglutide may be linked to ANP and GLP-1 receptors (GLP1R) in the atria. This study changes our view of the physiology of ANP and the molecular mechanisms mediating its release from the atrial cardiomyocyte. Clinically, the findings further our understanding of how long acting GLP-1 agonists, widely used in T2DM, may exert a beneficial effect in this disease syndrome that is associated with high cardiovascular risk and that occurs often in the setting of hypertension.
Liraglutide is an innovative long acting GLP-1 agonist that belongs to the new therapeutic group of mimetics of incretins, gut-released hormones that include GLP-1, that are currently used in T2DM given their potential cardioprotective and blood pressure lowering effect (6). The authors demonstrated in normotensive and hypertensive mice a specific cardiac localization of the GLP-1R, limited to atria (GLP-1R was previously shown in the human heart without chamber-specific localization). Importantly, after food intake, liraglutide and GLP-1 stimulated the secretion of ANP from atrial cardiomyocytes and this was dependent upon the GLP-1R. After infusion of angiotensin II to induce hypertension, liraglutide treatment did not lower the blood pressure in mice lacking GLP-1R, whereas in wild type mice it was substantially reduced. The vasodilatory effects of liraglutide on blood vessels did not seem to be direct and were not mediated by the vasodilator acetylcholine, which functions through nitric oxide signaling in the endothelium; instead, a vasodilatory response was observed on the inner surface of vessels indicating a vascular smooth muscle relaxation after GLP-1R activation, also independent of nitric oxide (5).
Kim et al (5) also found that in normotensive and hypertensive NPPA knockout mice liraglutide failed to reduce the blood pressure and did not increase urine sodium excretion, indicating an indirect ANP-dependent effect. The ANP secretion from atrial cardiomyocytes induced by liraglutide activation of GLP-1R was mediated by activation and translocation of the downstream effector Epac2 in both normotensive and hypertensive mice. Germline deletion of the Epac2 gene (Rapgef4) in mice did not increase ANP levels after GLP-1R agonist infusion. Thus, liraglutide is an ANP secretagogue functioning to release the blood pressure regulator ANP via a GLP-1R/Epac2 mechanism resulting in biological actions such as vasodilation and natriuresis. In contrast, liraglutide does not seem to exert changes in plasma BNP levels in either saline- or Angiotensin II-infusion WT or GLP-1R knockout mice.
The study by Kim et al (5) raises some intriguing questions. The study showed the hypotensive and natriuretic effects of liraglutide in normotensive mice and in two types of hypertensive mouse models (hypertension induced by angiotensin II infusion and hypertension secondary to overload due to transthoracic constriction). In vivo, native GLP-1 seemed to be rapidly degraded and inactivated as there were only modest increases in ANP levels after injection (5). However, after a meal ANP levels increased in wild type mice after 10 min, in contrast to GLP-1R-deficient mice where food ingestion had no effect on plasma ANP level. One wonders about the exact molecular form of native GLP-1 that is released after a meal. Could it be anextended molecular form that is more resistant to enzymatic degradation? The acute administration of liraglutide in normotensive and hypertensive mice increased ANP levels and lowered blood pressure. Indeed, a prolonged and sustained administration of liraglutide twice per day for 3 weeks in Angiotensin II-infused mice continued to increase ANP levels and decrease blood pressure, mostly during the evening lights off period. This observation is of clinical significance as it indicates that the blood pressure-lowering action of liraglutide may be long lasting. As hypertension is frequently present in diabetic patients, this property of long acting GLP-1 agonist would also benefit such patients. In addition, this reduction in blood pressure, especially during nighttime, might be due to a physiological response which is observed in humans in which blood pressure reductions are greater during the night.
It would be very interesting to investigate whether other GLP-1R agonists, such as exenatide, might also lead to the activation of the same pathway with the same effectiveness. Further, it will be important to determine whether the liraglutide actions continue beyond the period reported in the current study.
As one searches for the physiological meaning of this gut-heart axis, it could be speculated that the vasodilatory response after GLP-1R activation, secondary to ANP release, promotes an increased blood supply to target organs including the gut, pancreas, kidney and adipose tissue. This vascular response could result in better absorption and distribution of nutrients, optimized renal handling of sodium so as to regulate intravascular volume, and the effective regulation of insulin and glucagon release (Fig. 1).
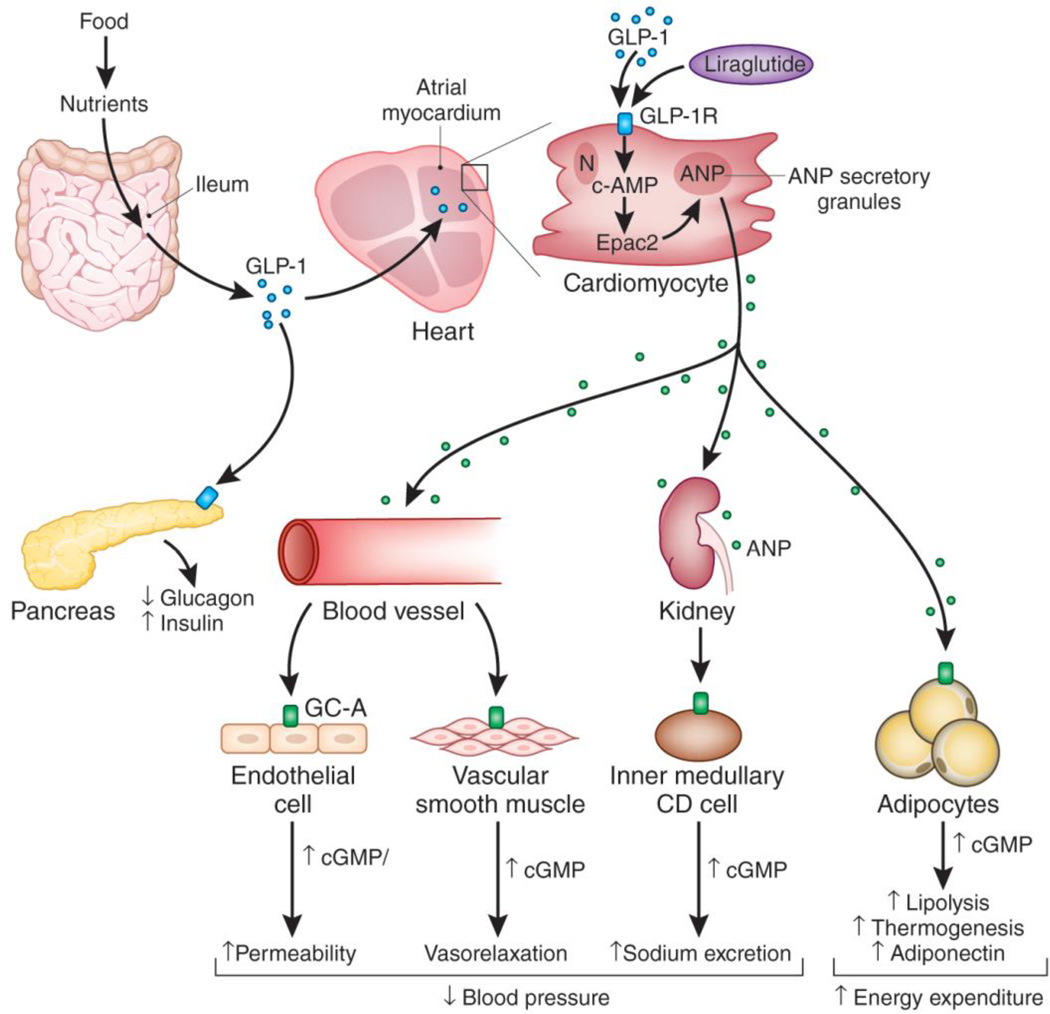
Fig. 1. Cardiometabolic actions of GLP-1 and GLP-1 agonists through activation of atrial ANP secretion.
Nutrients from meals are absorbed by the ileum followed by release of GLP-1 from secretory vesicles in ileum epithelial cells. In the pancreas, GLP-1 reduces glucagon secretion and increases insulin release via GLP-1R to regulate optimal glucose homeostasis. GLP-1 activates GLP-1Rs in the atrial myocardium, which induces ANP secretion from atrial secretory granules; this action is mimicked by the long acting GLP-1 agonist liraglutide via activation of Epac2, which stimulates secretion of ANP. ANP mediates pleotropic actions via GC-A/cGMP signaling to reduce blood pressure by augmenting endothelial permeability and inducing vascular smooth muscle relaxation in the vessel wall, and enhancing sodium excretion and, inhibiting sodium ion reabsorption in the kidney. ANP may also improve metabolic homeostasis through activating lipolysis and energy utilization in adipocytes, resulting in increased energy expenditure.
Nonetheless, the effect of liraglutide with regard to this gut-heart-GLP-1/ANP axis now needs further investigations in humans. It should be noted that subjects with T2DM in the majority of cases are overweight or obese, hypertensive, dyslipidemic and also display metabolic syndrome features. These conditions are characterized by lower natriuretic peptide levels and over-activation of the renin-angiotensin-aldosterone system which may contribute to hypertension (7, 8). Kim et al (5) also studied mice with hypertension secondary to subcutaneous infusion of Angiotensin II as well as in a model of hypertension produced by aortic constriction. The importance of establishing the action on ANP release by liraglutide in two models of hypertension may suggest that the probability of translating these findings to human hypertension is high. Thus, it will be important to analyze the liraglutide effect in humans in the context of TWDM together with these comorbidities to verify whether these actions persist.
The authors have clearly advanced our understanding of therapeutics related to both diabetes and hypertension, as well as providing us new insights into a new endocrine axis between the gut and the heart, which then links to the to the kidney. Here ANP binds to natriuretic peptide-A receptor (NPR-A) that, via 3',5'-cyclic guanosine monophosphate (cGMP), mediates natriuresis at the level of the inner medullary collecting duct in which GC-A is highly expressed (9); additional mechanisms of ANP mediated natriuresis include increases in glomerular filtration rate, direct inhibition of renin, and suppression of aldosterone secretion from the adrenal zona glomerulosa cells. With the explosion in diabetes worldwide and the availability of a long-acting GLP-1 receptor agonist, such as liraglutide, to treat affected individuals, it is reassuring to know that this innovative diabetic drug has pleiotrophic actions—beyond the regulation of insulin and glucagon—that now include a link to the heart to release the blood-pressure lowering and natriuretic peptide ANP.
The findings of the study by Kim et al (5) hint at the ‘wisdom of the body’ so well articulated decades ago by Walter Cannon in his studies of the physiologic role of the gut in overall homeostasis wherein an increase in nutrients to the gut after a meal translates into the release of a gut hormone, GLP-1 (10). Now the link from the gut to the pancreas can include a gut-heart connection. But, as the authors suggest in their study, the wisdom of the body and the use of GLP-1-like agonists go beyond merely blood pressure regulation, as ANP also regulates important metabolic actions such as lipolysis, lipid oxidation, enhanced thermogenesis, and modulation of insulin release (11,12) (Fig. 1).
The paper by Kim et al (5) has important clinical implications. It is not uncommon that T2DM develops in the setting of hypertension, obesity and/or metabolic syndrome. Liraglutide acts via GLP-1R/Epac2-effector to induce ANP secretion. ANP then exerts a vasodilator effect and reduces intravascular volume, thereby reducing arterial hypertension by these cardiorenal actions. Furthermore, ANP antagonizes the renin-angiotensin-aldosterone system that could result in a reduction of the mineralocorticoid hormone actions, recognizing that aldosterone has been shown to be inappropriately high in cardio-metabolic disorders. One could predict as well possible beneficial effects in the improvement in insulin sensitivity (in addition to the direct insulin secretory effects of liraglutide), reduction in salt sensitivity, reduction in cardiac and renal injury and fibrosis, increase in lipolysis, thermogenesis and energy expenditure with secondary weight loss, would also be expected. Indeed, the physician caring for the diabetic patient particularily with hypertension and/or metabolic syndrome may begin to even personalize therapy with long acting GLP-R agonists to gain the benefit of this link to the heart and ANP should future clinical studies support the observations in mice. Clearly, we are entering an exciting period in which new therapeutics with widespread pleiotrophic properties modulating a number of endocrine systems will be the focus of continuing basic clinical research.
Acknowledgments
Financial disclosure: No financial disclosures.
REFRENCES
- 1.Cataliotti A, Costello-Boerrigter LC, Chen HH, Textor SC, Burnett JC., Jr Sustained blood pressure–lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin Proc. 2012;87:412–415. doi: 10.1016/j.mayocp.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Nat. Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC., Jr J. Am. Coll. Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M, Platt M, Shibasaki T, Quaggin S, Backx PH, Seino S, Simpson J, Drucker DJ. Nat Med. 2013 doi: 10.1038/nm.3128. current issue. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Diabetes, Obesity and Metabolism. 2013;22 doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip E, Wilson P, Vasan RS. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 8.Ingelsson E, Pencina M, Tofler H, Benjamin E, Lanier K, Jacques P, Fox C, Meigs J, Levy D, Larson MG, Selhub J, D'Agostino RB, Wang TJ, Vasan RS. Circulation. 2007;28(9):984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. 116. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Nonoguchi H, Tomit K. Physiological effects of vasopressin and atrial natriuretic peptide in the collecting duct. Cardiovasc Res. 2001;51:470–480. doi: 10.1016/s0008-6363(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 10.Walter B. Cannon, The Wisdom of the Body. W.W. Norton & Company; 1932. [Google Scholar]
- 11.Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 12.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]