Abstract
The present investigation tested the role of ATP-activated P2X7 receptors (P2X7Rs) in alcohol-induced brain damage using a model that combines intragastric (iG) ethanol feeding and high fat diet in C57BL/6J mice (Hybrid). The Hybrid paradigm caused increased levels of pro-inflammatory markers, changes in microglia and astrocytes, reduced levels of neuronal marker NeuN and increased P2X7R expression in ethanol-sensitive brain regions. Observed changes in P2X7R and NeuN expression were more pronounced in Hybrid paradigm with inclusion of additional weekly binges. In addition, high fat diet during Hybrid exposure aggravated the increase in P2X7R expression and activation of glial cells.
Keywords: Purinergic P2X7 receptors, Pro-inflammatory cytokines, Microglia, Astrocytes, Neuroinflammation and neuronal loss, Intragastric ethanol feeding, High fat diet
1. INTRODUCTION
Neurological and cognitive impairments leading to permanent brain damage are major consequences of chronic alcohol abuse (Vetreno et al., 2011). Studies indicate that 50% of detoxified alcoholics have measurable cognitive impairments and over 75% of chronic alcoholics have significant brain damage at autopsy (Vetreno et al., 2011). At the neuroanatomical level, adverse effects of chronic alcohol intake include reduction of brain volume due to white matter loss, thinning of the corpus callosum and atrophy of the mammilary bodies. Regional volume differences are, in part, due to neuronal loss and gliosis in the thalamus, forebrain, hippocampus and cerebellum (Obernier et al., 2002; Zahr et al., 2011).
Nutritional deficiencies are associated with severe conditions of alcohol related brain damage such as Wernicke encephalopathy-Korsakoff syndrome (Nardone et al., 2013). In contrast, increased caloric intake and obesity in the Western societies often accompany alcohol consumption and abuse. There is evidence that together, alcohol abuse and obesity, may lead to higher rates of liver damage compared to the effects of either factor alone (Deng et al., 2005; Xu et al., 2011). Moreover, alcohol abuse and obesity separately are among etiologies of neurocognitive deficiencies. However, little is known if there is a synergy between ethanol and obesity in causing brain damage.
Recent evidence regarding the molecular mechanisms of alcohol (ethanol)-induced brain damage suggests the involvement of ethanol-induced neuroinflammation (Crews and Nixon, 2009; Crews and Vetreno, 2014; He and Crews, 2008; Kelley and Dantzer, 2011). These studies demonstrated increases in the number of pro-inflammatory cytokines as well as oxidative stress markers in both animal models of chronic ethanol exposure as well as in postmortem alcoholic human brain (Crews et al., 2006; Crews and Nixon, 2009; He and Crews, 2008; Qin et al., 2008). The important role of toll-like receptors (TLR4, TLR2) in ethanol-induced neuroinflammatory signaling cascades in glial cells and ethanol-related brain damage has been demonstrated (Alfonso-Loeches et al., 2010; Fernandez-Lizarbe et al., 2009). Moreover, the releases of pro- as well as anti-inflammatory cytokines were linked to long-term changes in ethanol-induced behaviors and neurodegeneration (Blednov et al., 2012; Crews et al., 2006; Crews and Nixon, 2009; Qin et al., 2008).
Neuroinflammation and related cognitive decline are also currently recognized as negative consequences of obesity (reviewed in (Miller and Spencer, 2014). Elevated expression of pro-inflammatory cytokines and transcription factor NFkB, activation and infiltration of microglia have been found in hypothalamus of rats fed high fat diet (De Souza et al., 2005), in hippocampus of mice on 60% high fat diet for 20 weeks (Jeon et al., 2012) and of db/db mouse model of metabolic syndrome (Dinel et al., 2011). Neuroinflammation caused by high fat diet may, in part, be related to activation of central processes. As such, a role of central TLR4-signalling in pro-inflammatory cascades has been suggested (Milanski et al., 2009). Despite this knowledge, there remains a paucity of information regarding other receptor systems that are important for the development of ethanol- as well as obesity-induced neuroinflammation.
Purinergic P2X7 receptors (P2X7Rs) have recently become a focus of investigation in the areas of chronic inflammation, neurodegeneration, neuropsychiatric disorders (e.g. depression) and pain (reviewed in (Skaper et al., 2010)). P2X7Rs belong to the ATP-gated P2XR superfamily of ligand gated ion channels (P2X1-P2X7) (Khakh et al., 2001; North, 2002). In contrast to the other P2X subtypes, P2X7R subtype is unique in that it: 1) is activated at high, millimolar ATP concentrations such as released during a CNS insult; 2) is able to form a pore that allows passage of molecules of up to 900 Da (North, 2002) and 3) interacts with many intracellular adaptor and signaling proteins due to their unusual long C-terminus (Kim et al., 2001). Notably, P2X7Rs are widely expressed in neurons, astrocytes, microglia, oligodendrocytes and Schwann cells (Skaper et al., 2010; Takenouchi et al., 2010).
Signaling through P2X7Rs plays a vital role in the activation of neuroimmune cells (Monif et al., 2010). Most importantly, P2X7Rs were shown to modulate the release of IL-1β, a recognized mediator of neurodegeneration (Bernardino et al., 2008; Clark et al., 2010; Honore et al., 2009; Takenouchi et al., 2008). P2X7Rs are also mediators of TNFα and CC-chemokine ligand 3 secretion (Kataoka et al., 2009; Suzuki et al., 2004), production of superoxide (Parvathenani et al., 2003) and nitric oxide (NO) (Gendron et al., 2003) and matrix metalloproteinase 9 (Choi et al., 2010; Shin et al., 2010). The presence of P2X7Rs has been demonstrated within the β-amyloid plaques suggesting a role in the processes of neurodegeneration in Alzheimer’s Disease (McLarnon et al., 2006; Parvathenani et al., 2003). P2X7Rs has been further implicated in neurodegenerative disorders through receptor up-regulation which is evident in epileptic brain, cerebral ischemia, amyotrophic lateral sclerosis, multiple sclerosis and chronic neuropathic pain (reviewed in (Volonte et al., 2011)). Involvement of P2X7Rs in obesity-induced renal inflammation and beta cell dysfunction has also been demonstrated (Glas et al., 2009; Solini et al., 2013). To date no information is available on the role of P2X7Rs in ethanol- and/or obesity-related brain damage.
To this end, the present study investigated the effects of concurrent administration of ethanol and high fat on the development of neuroinflammation and parallel changes in P2X7R expression. This was accomplished using a mouse model that combines an intragastric (iG) chronic ethanol exposure and high fat diet (Hybrid) in C57BL/6J mice. This model produces clinically relevant liver pathology of chronic alcoholic steatohepatitis with macrophage inflammation and features of alcoholic hepatitis (Lazaro et al., 2015).
2. MATERIALS AND METHODS
2.1. Materials
All reagents were of research grade unless otherwise indicated. Ethanol at 200 proof (Rossville Gold Shield) was used for intragastric feeding of mice.
2.2. Methods
2.2.1. Animals, Exposure to Ethanol and Diet
The experiments were performed on age matched, male C57BL/6J mice obtained from Jackson laboratories (Bar Harbor, ME). All animals were treated in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
For the majority of the studies, we used a model that combines exposure to ethanol (EtOH) and Western type high fat diet termed as Hybrid throughout the manuscript. This model was developed at the Southern California Center for Alcoholic Liver and Pancreatic Disease and Cirrhosis (Director Dr. Tsukamoto). Briefly, 8 weeks old C57BL/6J mice were started on ad libitum high fat solid diet designated as HCFD (HCFD pellets composed of 1% w/w cholesterol, 20%Cal lard, 17% corn oil:HCFD, Dyets Inc #180724) for 2 weeks followed by a surgery for placement of iG catheters. After recovery from the surgery (~1 week), mice were divided into 3 groups. The first group denoted as HCFD-EtOH received continuous infusion of ethanol (~27 g/kg/day, 35%Cal) plus liquid high fat diet (corn oil, 25%Cal) via iG catheters at 60% of total required calories for the duration of 6 weeks. Second group, denoted as HCFD-EtOH+Binge, was similar to the first with additional exposure to a binge bolus dose (3.5–5 g/kg) which was administered once a week. This bolus dose was injected through the iG catheters during the dark cycle after the ethanol infusion was withdrawn for 5–6 hours. The third control group denoted as HCFD-Glucose was infused with dextrose to account for the 35% of calories from EtOH. Mice in all 3 groups continued to consume solid HCFD for the remaining 40% calories throughout the duration of the experiment. In addition, for some experiments, a subset of EtOH+Binge mice were also fed ad libitum regular Chow at 40% of total calories (denoted respectively Chow-EtOH+Binge). The corresponding glucose controls were fed ad libitum Chow and received dextrose (denoted as Chow-Glucose). Shortly after the exposures, the iG tubes were removed, mice anesthetized using xylene and ketamine, decapitated and brain tissues removed for further processing.
Blood ethanol concentrations (BECs) measured using ANALOX GM7 Analyzer (Analox Instruments USA, MA) achieved in both Hybrid models (HCFD-EtOH and HCFD-EtOH+Binge) were ~200–400 mg% or 50–100 mM. This approach normally causes significant liver fibrosis with neutrophil infiltration which is characteristic of alcoholic hepatitis (Deng et al., 2005; Xu et al., 2011).
2.2.2. RT-PCR
Right brain hemispheres were snap frozen and kept in −80°C till RNA isolation. RNA was isolated using RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA) after tissue homogenization with microbeads in the TissueLyser (Qiagen, Valencia, CA). cDNA was then synthesized from 1 μg RNA using High Capacity RNA-to-cDNA Kit (Life Technologies, Grand Island, NY). The real-time PCR was performed using ABI 7900 fast real-time system (Life Technologies). GAPDH was used as normalization control. The following sets of primers were used: IL-1β forward – 5′-TCGCTCAGGGTCACAAGAAA-3′, IL-1β reverse – 5′-CATCAGAGGCAAGGAGGAAAAC-3′; IL-6 forward – 5′-TCGGAGGCTTAATTACACATGTTC-3′, IL-6 reverse – 5′-CAAGTGCATCATCGTTGTTCATAC-3′; TNFα forward - 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, TNFα reverse– 5′-TGGGAGTAGACAAGGTACAACCC-3′; MCP-1 forward – 5′-CCACTCACCTGCTGCTACTCAT-3′, MCP-1 reverse – 5′-TGGTGATCCTCTTGTAGCTCTCC-3′, iNOS forward -5′-CCTGGTACGGGCATTGCT-3′, iNOS reverse – 5′-GCTCATGCGGCCTCCTT-3′.
2.2.3. Processing and Immunofluorescence of Brain Tissues
Whole brains or brain hemispheres were cut into 4 coronal segments of 0.8–1.0 cm thickness each, which were then fixed by incubation in 4% paraformaldehyde for 48 hours. Tissues were then sucrose cryopreserved and kept in −80°C or paraffin-embedded. Sectioning was performed using a MicroM HM525 cryostat for frozen tissues (at 12 μm thickness) or MicroM HM310 rotary microtome for paraffin-embedded tissues (at 5 μm thickness). All procedures were carried out at the Histology Core Lab of the USC School of Pharmacy.
For immunofluorescence staining performed on sucrose cryopreserved tissues, sections were acetone fixed, blocked with 1% BSA. Paraffin-embedded tissue sections were de-paraffinized and hydrated, antigen retrieved by heating in citrate buffer (10mM sodium citrate, 0.05% Tween 20, pH 6.0) using a common pressure cooker and blocked in 5 % goat serum and 1 % BSA. Sections were then permeabilized using 0.25% Triton X100 and incubated with primary antibodies as follows: polyclonal anti-Iba1, 1:250, 24–48 hours at 4°C (Wako Chemicals, Richmond, VA); polyclonal anti-GFAP, 1:2000, 2 hours at room temperature (Millipore,Temecula, CA). Secondary anti-rabbit antibodies conjugated with DyLight550 were used and fluorescence visualized using a fluorescence microscope (Zeiss, Germany).
2.2.4. Western Blotting
Brains were dissected into different regions, fast frozen on dry ice and kept in −80°C until processed. Protein was obtained by brief sonication in RIPA buffer (10 mM Tris, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1 % sodium deoxycholate, pH 8.0). Protein separation of the homogenates was performed on 10% SDS-PAGE followed by Western blotting using antibodies to P2X7 (rabbit anti-P2X7, 1:1000, overnight, Alomone Labs, Israel) and NeuN (mouse anti-NeuN, 1:250, overnight, Millipore). Protein bands were visualized by incubation with secondary anti-rabbit or anti-mouse HRP conjugated antibodies and subsequent treatment using enhanced chemiluminescence (Clarity Western ECL substrate, Bio-Rad, Hercules, CA).
2.2.5. Data Analysis
Densitometry data for immunofluorescence and Western blots were analyzed using Image J software (NIH) and data presented as mean ± SEM using GraphPAD Prism (San Diego, CA). 2(−Delta Ct) method was used for the analyses of the RT-PCR data (Schmittgen and Livak, 2008). Significant differences between mouse groups were determined using Student’s t-test and significance set at P < 0.05.
3. RESULTS
3.1. Exposure to combined iG ethanol and Western diet (Hybrid) increased neuroinflammatory response in mouse brain
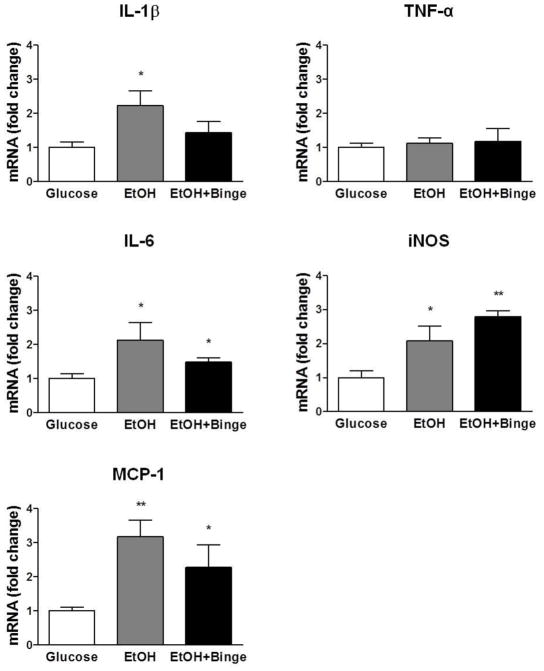
Using a Hybrid exposure paradigm, we found a significant neuroinflammatory response in C57BL/6J male mice that were treated using the HCFD-EtOH exposure model (Fig. 1). This included a significant, 2-fold increase in total brain mRNAs for pro-inflammatory cytokines IL-1β, IL-6, and a 3-fold increase in MCP-1. HCFD-EtOH+Binge exposure paradigm resulted in smaller compared to HCFD-EtOH exposure but significant increases in total brain mRNAs for pro-inflammatory cytokines IL-6 and MCP-1 (Fig. 1). mRNA for IL-1β was also increased in HCFD-EtOH+Binge paradigm, however this increase was not significant. TNFα production was not changed during both Hybrid exposures. Finally, we found a significant increase in iNOS mRNA levels in both exposure paradigms (Fig. 1).
Fig. 1. Combination of iG ethanol exposure and high fat diet (Hybrid) increased levels of inflammatory markers.
Results of RT-PCR, fold-changes compared to Glucose levels presented as mean ± SEM, n=3–4. The extent of the changes is higher in IL-1β, IL-6 and MCP-1 for HCFD-EtOH vs HCFD-EtOH+Binge. ** P < 0.01, *** P < 0.001 compared to Glucose.
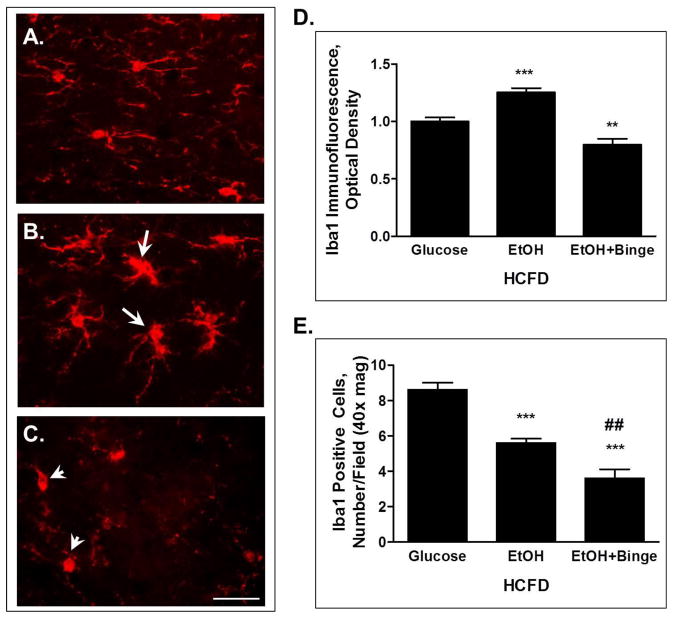
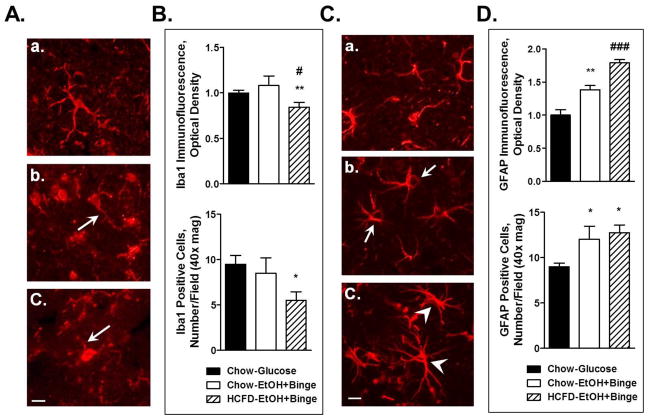
The neuroinflammatory response was also observed at the cellular level. Immunofluorescence staining of the microglial marker Iba1 on the hippocampal slices demonstrated a change in the morphology of these cells. Microglia in the HCFD-EtOH exposure group demonstrated more round morphology with enlarged cell bodies and less processes compared to the ramified resting microglia with more and thinner extensions in the HCFD-Glucose controls (Fig. 2B vs 2A). Moreover, some of the microglia appeared to be connected or fused (Fig. 2B, arrows). In contrast, exposure to the HCFD- EtOH+Binge paradigm resulted in a visible loss of morphological integrity of microglia (Fig. 2C, arrowheads). Changes in the Iba1 staining intensity paralleled morphological appearance. Thus, Iba1 immunofluorescence intensity obtained from cell bodies of a similar number of cells from each experimental condition revealed a significant increase during HCFD-EtOH compared to the HCFD-Glucose (Fig. 2D). In contrast, there was a small but significant decrease in Iba1 fluorescence for HCFD-EtOH+Binge (Fig. 2D). Interestingly, the numbers of Iba1 positive cells were significantly reduced during both EtOH exposures (Fig. 2E). In addition, HCFD-EtOH+Binge exposure resulted in a significantly fewer number of microglia compared to that of HCFD-EtOH (Fig. 2E).
Fig. 2. iG ethanol paradigms combined with high fat diet (Hybrid) caused changes in microglia in hippocampal slices visualized by Iba1 immunofluorescence.
A – C – microglia phenotypes; A– HCFD-Glucose, mostly resting microglia; B - HCFD-EtOH, the presence of activated and phagocytic microglia (arrows), C – HCFD-EtOH+Binge, loss of morphological integrity (arrowheads). Bar, 10 μm. D – Analyses of Iba1 immunofluorescence intensity from cellular bodies presented as mean ± SEM, n=14–15 cells/condition. E - Iba1-positive cells presented as number/ field, 40x magnification; n=43, 28 and 18 respectively for HCFD-Glucose, HCFD-EtOH and HCFD-EtOH+Binge. * P < 0.05, *** P < 0.001 compared to Glucose; ## P < 0.01 compared to HCFD-EtOH.
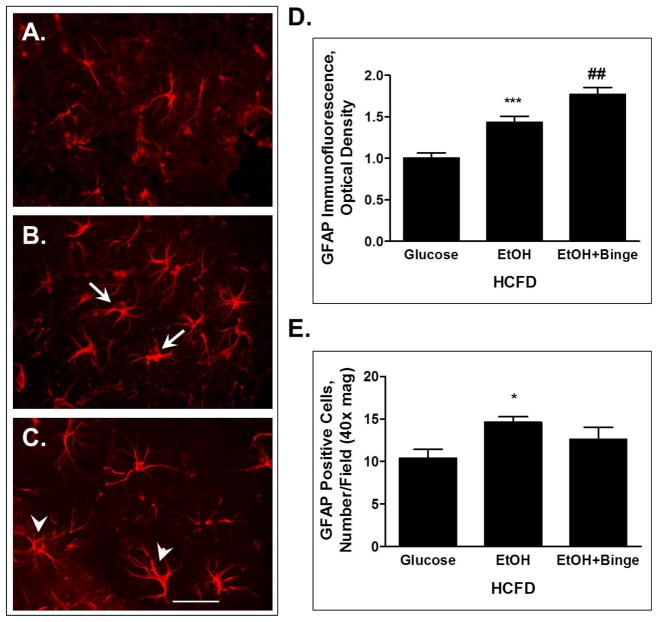
Hybrid exposures also caused changes in astrocyte activation state as illustrated by staining of hippocampal slices for astrocytic marker GFAP (Fig. 3). Morphologically, the astrocytes appeared to have enlarged cellular bodies during HCFD-EtOH compared to the HCFD-Glucose (Fig. 3B vs 3A, arrows). In addition, HCFD-EtOH+Binge resulted in longer and more branched cellular extensions, typical of reactive astrocytes (Fig. 3C, arrowheads). These phenotypic changes paralleled changes in the GFAP immunofluorescence intensity analyzed from cellular bodies of similar number of cells per each treatment group. GFAP immunofluorescence intensity was significantly increased in both EtOH groups with stronger effect in HCFD-EtOH+Binge (Fig. 3D). There was a small increase in the number of astrocytes during both EtOH exposures reaching significance for HCFD-EtOH (Fig. 3E).
Fig. 3. Exposure to combined iG ethanol paradigms and high fat diet (Hybrid) resulted in changes in hippocampal astrocytes visualized by GFAP immunofluorescence.
A – C – astrocyte phenotypes; A– HCFD-Glucose, astrocytes at rest; B– HCFD-EtOH, activated astrocytes (arrows); C– HCFD-EtOH+Binge, reactive astrocytes (arrowheads). Bar, 10 μm. D - Analyses of GFAP immunofluorescence intensity from cellular bodies presented as mean ± SEM, n=20 cells/condition. E – GFAP-positive cells presented as number/ field, 40x magnification; n=52, 73 and 63 respectively for HCFD-Glucose, HCFD-EtOH and HCFD-EtOH+Binge. ** P < 0.01, *** P < 0.001 compared to Glucose; ## P < 0.01 compared to HCFD-EtOH.
Taken together, the results showing a significant increase in the mRNA levels of pro-inflammatory mediators and changes in the number and morphology as well as the intensity of immunofluorescence staining of specific cellular markers of glial cells suggest that the Hybrid iG ethanol exposures induce neuroimmune responses in mice.
3.2. Exposure to Hybrid paradigms produced neuronal loss in mouse ethanol-sensitive brain regions
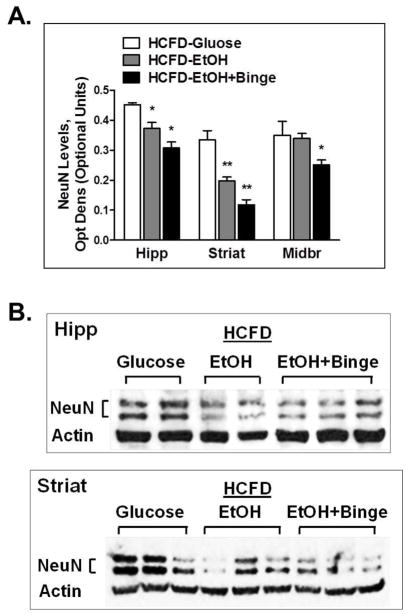
To evaluate the effects of Hybrid exposures on neurons, we isolated brain regions and tested for the expression of neuronal marker NeuN using Western immunoblotting. There was a significant decrease in NeuN expression in hippocampus, striatum and midbrain regions in both HCFD-EtOH and HCFD-EtOH+Binge groups (Fig. 4A). The magnitude of the effect was greater in HCFD-EtOH+Binge group for all 3 brain regions. In addition, marked changes were seen in the striatum region (Fig. 4A,B). These findings suggested that Hybrid exposures may result in neuronal loss in ethanol-sensitive brain regions.
Fig. 4. Exposure to combined iG ethanol paradigms and high fat diet (Hybrid) caused decreased NeuN levels in hippocampus.
A,B – Neuronal marker NeuN expression levels in ethanol-sensitive mouse brain regions. A - Data represent densitometry analysis of Western blots, mean ± SEM, n=4–6. * P < 0.05, ** P < 0.01 compared to HCFD-Glucose. B – Representative blots showing protein bands for NeuN and β-Actin in mouse hippocampus and striatum. Hipp – hippocampus, Striat – striatum, MidBr - midbrain.
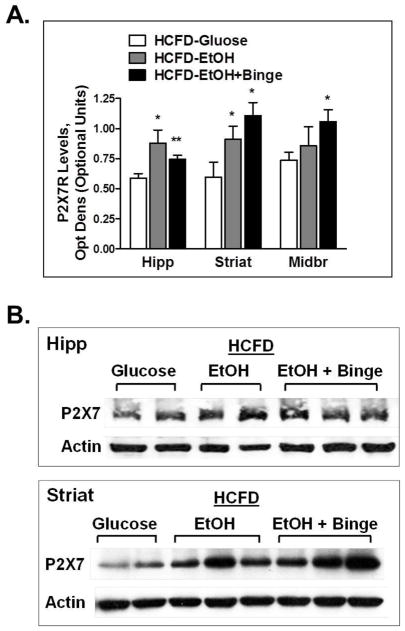
3.3. Hybrid paradigms resulted in an increase in P2X7R expression in mouse ethanol-sensitive brain regions
Testing for changes in P2X7R expression in the same brain regions where changes in NeuN levels were observed, we found that the use of the Hybrid paradigms induced significant increases in P2X7R levels in hippocampus, striatum and midbrain (Fig. 5A). This effect was more pronounced during exposure to HCFD-EtOH+Binge for striatum and midbrain, however it was lower compared to that during HCFD-EtOH in hippocampus (Fig. 5A). In contrast, in striatum and midbrain greater extent of increase in P2X7R expression was found during HCFD-EtOH+Binge compared to HCFD-EtOH (Fig. 5A,B). Changes found in P2X7R expression paralleled those of neuroinflammatory markers and NeuN expression.
Fig. 5. Exposure to combination of iG ethanol and high fat diet (Hybrid) caused increased levels of P2X7R in ethanol-sensitive mouse brain regions.
Changes were more pronounced in striatum. A - Data represent densitometry analysis of Western blots, mean ± SEM, n=4–6. * P < 0.05, ** P < 0.01 compared to Glucose. B – Representative blots showing protein bands for P2X7 and β-Actin in mouse hippocampus and striatum. Hipp – hippocampus, Striat – striatum.
3.4. High fat diet contributed to neuroinflammation and P2X7R expression during Hybrid exposure
We also tested whether high fat diet (HCFD) used in the Hybrid iG ethanol exposure paradigm was a contributing factor for the observed effects on glial cell activation and P2X7R expression. There were no apparent differences of glial cells between the control groups, i.e. Chow-Glucose and HCFD-Glucose (Fig. 6A,C vs Fg. 2A and Fig. 3A). We then compared immunofluorescence staining with microglial marker Iba1 of hippocampal slices from EtOH+Binge exposed mice on regular Chow and HCFD. The staining demonstrated the presence of more round cells with enlarged cellular bodies and less processes after the exposure to Chow-EtOH+Binge compared to Chow-Glucose (Fig. 6Ab vs Aa, arrow). HCFD condition had stronger effect on microglia: cellular extensions were visibly shorter and less abundant during HCFD-EtOH+Binge compared to those of Chow-EtOH+Binge (Fig. 6Ac vs Ab, arrow). Iba1 immunofluorescence intensity analyzed from the cell bodies did not show a significant change for EtOH+Binge in the Chow group, whereas it was significantly decreased in the HCFD group as compared to Chow-Glucose (Fig. 6B, upper panel). Similarly, EtOH+Binge with Chow did not significantly alter the number of Iba1 positive cells, whereas in combination with HCFD there was a significant reduction in the number of Iba1 positive cells (Fig. 6B, lower panel).
Fig. 6. High fat diet (HCFD) aggravated ethanol-induced effects on microglia (A, B) and astrocytes (C,D).
A, B – Iba1 immunofluorescence; A - microglia phenotypes; Aa – Chow+Glucose, Ab - Chow-EtOH+Binge, Ac – HCFD-EtOH+Binge; bar, 5 μm. The arrows show microglial processes. B, upper panel – Analyses of Iba1 immunofluorescence intensity from cellular bodies presented as mean ± SEM, n=15–17 cells/condition. B, lower panel – Iba1-positive cells presented as number/ field, 40x magnification; n=19, 17 and 11 respectively for HCFD-Glucose, HCFD-EtOH and HCFD-EtOH+Binge. C, D - GFAP immunofluorescence; C – phenotypes of astrocytes; Ca - Chow+Glucose, Cb - Chow-EtOH+Binge, Cc - HCFD-EtOH+Binge; bar, 5 μm. Arrows point to the enlarged cell bodies, arrowheads indicate reactive astrocytes. D, upper panel – Analyses of GFAP immunofluorescence intensity from cellular bodies presented as mean ± SEM, n=14–18 cells/condition. D, lower panel – GFAP-positive cells presented as number/field, 40x magnification; n=36, 48 and 51 respectively for HCFD-Glucose, HCFD-EtOH and HCFD-EtOH+Binge. * P < 0.05, ** P < 0.01 compared to Glucose; # P < 0.05, ### P < 0.001 compared to Chow-EtOH+Binge.
We next compared the effects on astrocytes. Immunofluorescence staining with astrocytic marker GFAP demonstrated that Chow-EtOH+Binge exposure resulted in enlarged cell bodies (Fig. 6Cb, arrows) compared to the corresponding controls, Chow-Glucose (Fig. 6Ca). HCFD-EtOH+Binge exposure appeared to have an overall stronger effect on the morphology of the astrocytes resulting in longer and more prominent extensions (Fig. 6Cc, as well as Fig. 3C). In agreement, the GFAP immunofluorescence intensity analyzed from cellular bodies was significantly increased in EtOH+Binge exposures, with significantly greater effect in the HCFD group (Fig. 6D, upper panel). Finally, the number of GFAP positive cells was increased for EtOH+Binge, regardless of the diet (Fig. 6D, lower panel).
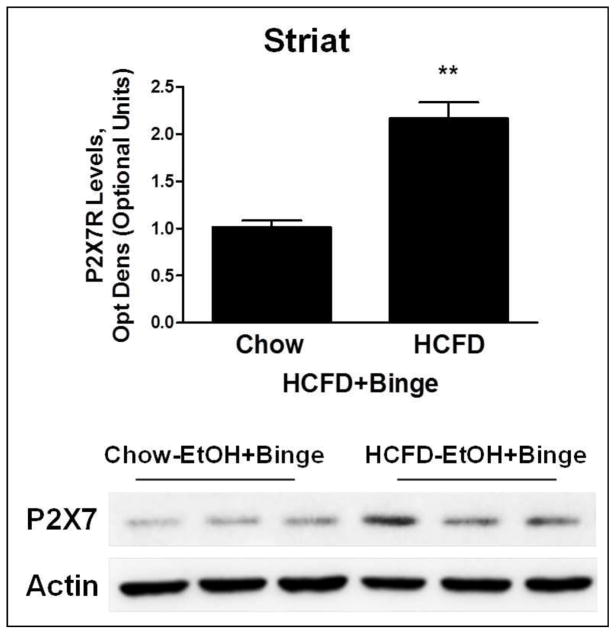
We also tested whether the high fat diet in the Hybrid exposure paradigms contributed to the changes in P2X7R expression. We compared P2X7R expression in striatum regions in two EtOH+Binge exposure groups, one on regular Chow (Chow-EtOH+Binge) and the other on HCFD (HCFD-EtOH+Binge). P2X7R expression was significantly higher (~2-fold) in the HCFD group compared to the Chow group (Fig. 7).
Fig. 7. High fat diet (HCFD) aggravated ethanol-induced P2X7R expression levels in striatum.
Densitometry analysis of Western blots for P2X7Rs. Data represent densitometry analysis of Western blots, mean ± SEM, n=3–4. * P < 0.05 compared to Chow. Lower panel shows protein bands for P2X7 and β-Actin in striatum (Striat).
4. DISCUSSION
The present investigation tested the combined effects of ethanol and high fat on the neuroimmune responses and P2X7R expression in brain a mouse model that combines iG ethanol exposure and HCFD (Hybrid). The results indicate that Hybrid exposure caused: 1) increase in pro-inflammatory markers and changes in microglia and astrocytes; 2) decrease in neuronal marker NeuN expression; 3) increased P2X7R expression in ethanol-sensitive brain regions of C57BL/6J mice. The observed changes in P2X7R and NeuN expression were more pronounced in the HCFD-EtOH+Binge model as compared to the HCFD-EtOH. Lastly, we found that high fat diet (HCFD) present during Hybrid EtOH exposure aggravated the increase in P2X7R expression and activation status of astroglia.
The iG ethanol exposure model in rodents was recently developed to aid in the investigation of the ethanol-induced liver damage (Tsukamoto et al., 2008; Ueno et al., 2012). Additional studies have reported a synergistic relationship between ethanol exposure and moderate adiposity such as found in the Hybrid paradigm in regards to causing liver damage (Deng et al., 2005; Xu et al., 2011). The addition of binge exposures in these models further shifted the chronic alcoholic steatohepatitis with macrophage inflammation, fibrosis, polymorphonuclear leukocyte infiltration, induction of chemokines and progenitor genes towards more clinically relevant features of alcoholic hepatitis such as hypoalbuminemia, bilirubinemia, and splenomegaly (Lazaro et al., 2015). The present study, for the first time, investigated the effects of the Hybrid paradigm on the brain.
We found that Hybrid exposures in mice, including HCFD-EtOH and HCFD-EtOH+Binge, caused significant neuroimmune response as evidenced by the increase in neuroinflammatory markers such as IL-1β, IL-6, MCP-1, iNOS. These findings are in good agreement with the existing knowledge that chronic ethanol exposure activates neuroimmune signaling leading to an increase in the inflammatory milieu in the brain. In this regard, pro-inflammatory markers such as monocyte chemoattractant protein 1 (MCP-1 or CCL-2) have been detected in postmortem human alcoholic brains (He and Crews, 2008). The release of pro- as well as anti-inflammatory cytokines such as IL-1β, IL-6, IL-10, TGFβ have been reported in rodent brains or brain slice cultures exposed to ethanol (Crews et al., 2006; Crews and Nixon, 2009; Qin et al., 2008). Moreover, the presence of reactive oxygen species, decreased glutathione levels and increased iNOS expression was detected in brain tissues after chronic ethanol administration (Collins and Neafsey, 2012a; Matsumoto and Matsumoto, 2008). Despite the previous reports on generation of TNFα in the models of chronic ethanol exposure (Crews et al., 2006; Qin et al., 2008), we did not find any changes in this pro-inflammatory mediator during the Hybrid exposure.
Microglia, resident immune cells of the CNS, and astrocytes, a subtype of glial cells, are mostly responsible for the neuroimmune responses in the brain and the secretion of various pro- and anti-inflammatory mediators (Gonzalez et al., 2014). In agreement, our study found that Hybrid paradigms caused significant changes in microglia and astrocytes. For example, the HCFD-EtOH paradigm was found to cause activation of microglia which was evident from the change in the phenotypic appearance with round cell bodies and shorter and less abundant extensions, as well as from the increase in the Iba1 immunofluorescence intensity. Despite these changes, there was a small decrease in the number of Iba1 positive cells with HCFD-EtOH. As identified on the Iba1 immunofluorescence images, some of the microglia appeared to be connected or fused, thus resulting in a lower total number of cells. Notably, inclusion of binges in HCFD-EtOH+Binge model further caused damaging effects to microglia. This was evident from the visible loss of morphological integrity of the cells, as well as lower Iba1 immunofluorescence intensity and substantially decreased numbers of microglia during HCFD-EtOH+Binge. Interestingly, microglia damage observed with the HCFD-EtOH+Binge may explain the lower degree of increases for IL-1β, IL-6 and MCP-1 in this paradigm suggesting that microglia are the primary source of these pro-inflammatory mediators. Findings of our study agree well with the previous findings that suggest partial or full activation of microglia in rodent ethanol exposure models or in post-mortem alcoholic human brain tissues (He and Crews, 2008; Marshall et al., 2013; McClain et al., 2011).
The Hybrid paradigms also caused activation of astrocytes illustrated by the apparent change in the cellular phenotypes including enlarged cellular bodies, increased GFAP immunofluorescence intensity and the number of GFAP-positive cells during HCFD-EtOH. Further, HCFD-EtOH+Binge exposure caused significantly stronger effects on astrocytes compared to those of HCFD-EtOH. The presence of bushier cellular extensions with stronger intensity for GFAP immunostaining suggested the presence of reactive astrocytes during HCFD-EtOH+Binge. In addition, substantially higher number of GFAP-positive cells suggested the activation of astrocytes. These findings are in good agreement with a recent report that demonstrated activation of astrocytes in mice exposed to ethanol via gavage (Kane et al., 2013). Taken together, the findings on the changes in pro-inflammatory mediators, in the number and morphology as well as expression of specific markers of glial cells, suggests that Hybrid exposures can lead to neuroimmune responses with increased neuroinflammation during HCFD-EtOH paradigm and a state with more cellular damage during HCFD-EtOH+Binge paradigm.
Results from recent investigations have identified functional alterations in hippocampal synaptic plasticity in adult and developing rodent models of ethanol exposure as a result of ethanol-induced neurotoxicity (reviewed in Collins and Neafsey, 2012a; Zorumski et al., 2014). In agreement, our current investigation found that exposure to the Hybrid exposure resulted in neuronal loss. This was evidenced by the decreased levels of neuronal marker NeuN expression in ethanol-sensitive brain regions including the hippocampus, striatum and midbrain. Notably, these brain regions have been linked to ethanol-induced alterations in cognition and memory (hippocampus) as well as behaviors associated with the reward pathway (striatum and midbrain). Importantly, we found that binge exposures caused significantly greater effect on NeuN levels in all brain regions. Taken together, our findings suggest that in addition to increased neuroinflammatory response, the Hybrid exposure caused significant neuronal damage in C57BL/6J mice. Moreover, the ethanol exposure paradigm with binge (that resembles binge drinking behavior in humans) caused more profound effects compared to the non-binge paradigm. Further work with the use of specific markers is needed to delineate neuronal apoptosis or necrosis during Hybrid paradigms.
Work performed over the last decade has started to shed light on the receptor systems that serve as danger-associated molecular patterns (DAMPs) and downstream signaling pathways that play an important role in the neuroimmune responses during chronic ethanol exposure. Recent studies with the use of silencing or knockout approaches implicate an important role for TLR4 in the innate immune responses that lead to ethanol related behavioral deficits and brain damage (reviewed in Crews and Vetreno, 2014;Szabo and Lippai, 2014). Suppression of TLR4 function or TLR4 deficiency have also led to reduction or abolishment of ethanol-induced activation of inflammasome complex components (Alfonso-Loeches et al., 2014; Lippai et al., 2013) and the resultant secretion of IL1-β and IL-18 (Alfonso-Loeches et al., 2014;Alfonso-Loeches et al., 2010; Kelley and Dantzer, 2011). P2X7Rs are in the list of DAMPs as they sense very high concentrations of ATP (≥ 1 mM) that are released as a result of an insult to the CNS. In our study both Hybrid exposures (HCFD-EtOH and HCFD-EtOH+Binge) caused a significant increase in P2X7R expression in ethanol-sensitive brain regions. The increases in P2X7Rs paralleled changes in the NeuN levels in the studied brain regions. Furthermore, the changes in P2X7R expression were more pronounced with the HCFD-EtOH+Binge model, especially for striatum and midbrain regions.
There is a broad distribution of P2X7Rs in the cells of the CNS which include glia as well as neurons (Skaper et al., 2010; Takenouchi et al., 2010;Gonzalez et al., 2014). Taken in the context of our findings on activation of microglia and astrocytes induced by Hybrid models (Fig. 4), we sought to identify the cell types that contribute to P2X7R changes. However, there are limitations to obtaining good quality immunostaining of brain tissues with P2X7R antibodies. Immunofluorescence staining demonstrated the presence of P2X7R in the DG hilus as well as CA3 region of hippocampus suggesting a neuronal presence of these receptors (data not shown). On the other hand, our initial findings in murine BV2 microglial cell line where ethanol induced a concentration-dependent increase in P2X7R protein expression (data not shown) suggested that microglia are important players in ethanol-induced P2X7R-signaling. Additional studies are needed to characterize/identify the CNS cell type(s) that contribute to ethanol-induced changes in P2X7R expression.
Lastly, our study begins to shed light on the contribution of the high fat diet component of the Hybrid exposure paradigms used in this study. Our findings suggested that HCFD paradigm aggravated the effects of Chow-EtOH+Binge on glial cells. EtOH+Binge exposure with Chow caused changes in microglia morphology consistent with activation, despite significant differences in terms of number of Iba1 positive microglia and Iba1 immunofluorescence. Chow-EtOH+Binge exposure also induced robust activation of astrocytes as seen with changes in morphology as well as significant increases in GFAP positive cells and GFAP immunofluorescence. Compared to the Chow, HCFD had significantly greater effects on both type of glial cells based on changes in morphology as well as changes in the immunofluorescence of the glial markers.
The presence of HCFD also resulted in a greater extent (~2-fold) of the induction of P2X7R levels caused by iG ethanol. These findings are in good agreement with the effects of the high fat diet on liver in the same iG mouse model as it causes significantly more steatosis as well as fibrosis (Deng et al., 2005; Xu et al., 2011). Growing evidence suggests that high fat diet can induce inflammation via NLRP3 inflammasome formation (Vandanmagsar et al., 2011). Given the known role of P2X7R in this pathway (Dubyak, 2012), our findings suggest that the combined effects of ethanol and high fat may emerge at P2X7Rs and its downstream signaling cascades.
5. CONCLUSION
In conclusion, we found increased neuroinflammation and neuronal loss in the mouse models of iG ethanol exposure combined with high fat diet, an established model used for studies of alcoholic liver damage. These findings add to the existing knowledge and suggest that the use of iG ethanol paradigms can represent novel suitable models for studies of ethanol-induced brain damage. Importantly, findings with P2X7Rs suggest that these receptors are involved in the pathology of ethanol-induced neuroinflammation and brain damage. Further approaches that include the use of pharmacological inhibitors or gene knockdown studies need to address the exact mechanism(s) underlying contribution of P2X7Rs in neuroinflammation and neurotoxicity caused by chronic ethanol exposure. Despite the lack of the pharmacological tools targeting specific P2XR subtypes, there are a few specific antagonists developed for P2X7Rs, albeit with very little information on their pre-clinical use. Our ongoing studies with the use of a P2X7 antagonist A804598 will begin to shed light on the role of this receptor in the pathology associated with chronic alcohol use. If successful, these studies will identify P2X7R as a novel target for treating or preventing ethanol-induced brain damage.
Highlights.
Hybrid model combining intragastric ethanol feeding and high fat diet in mice
Increased pro-inflammatory markers and glia activation in Hybrid
Reduced neuronal marker NeuN levels in ethanol-sensitive brain regions in Hybrid
Increased P2X7R levels in ethanol-sensitive brain regions in Hybrid
High fat diet contributed to the increase in P2X7R expression and glia activation
Acknowledgments
This work is dedicated to the late Dr. Ronald L. Alkana, who played a significant role in the development of this project. We would like to thank Raul Lazzaro and Ivetta Vorobyova from the Animal Core of the Southern California Research Center for ALPD and Cirrhosis for generation of mouse models, Eliza Asherian, Emer Bowne, Daniel Freire, for assistance with brain tissue harvesting, processing, Western blot and immunofluorescence analyses, Miriam Fine for RNA isolation and RT-PCR studies.
Sources of support: Zumberge Individual Research Fund (USC) and NIH/NIAAA AA017243 (to L.A.), NIH/NIAAA A022448 (to D.L.D.), HL08722 (to K.R.), NIH P50AA011999, Department of Veterans Affairs, 1I01BX001991 and senior research career scientist award (to H.T.).
Abbreviations
- CNS
central nervous system
- Hipp
hippocampus
- Striat
striatum
- MidBr
midbrain
- iG
intragastric
- EtOH
ethanol
- HCFD
high fat diet
- ATP
adenosine triphosphate
- NO
nitric oxide
- iNOS
inducible NO synthase
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- IL-10
interleukin 10
- TNFα
tumor necrosis factor alpha
- TGFβ
transforming growth factor beta
- MCP-1
monocyte chemoattractant protein 1
- CCL2 (same as MCP-1)
chemokine CC ligand 2
- TLR
toll-like receptor
- P2X7R
purinergic P2X7 receptor
- Iba1
ionized calcium binding adapter molecule 1
- GFAP
glial fibrillary acidic protein
- NeuN
neuronal nuclear antigen
- HRP
horseradish peroxidase
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cel Neurosci. 2014;8:216. doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1β release. J Neurochem. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Cho K, Shin S, Ko H, Kwon K, Shin C, Ko K. ATP induced microglial cell migration through non-transcriptional activation of matrix metalloproteinase-9. Arch Pharm Res. 2010;33:257–265. doi: 10.1007/s12272-010-0211-8. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TKY, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1β and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Front Biosci (Elite Ed) 2012a;4:1358–1367. doi: 10.2741/465. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcoholism, Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–357. doi: 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinol. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatol. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, Gonzalez FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K. Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetol. 2009;52:1579–1588. doi: 10.1007/s00125-009-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1[alpha][beta] knockout mice. Behav Brain Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. J Neuroinflam. 2013;10:66. doi: 10.1186/1742-2094-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108:115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav Immun Suppl. 2011;1:S13–20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PA. International union of pharmacology. XXIV Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, Xu J, Machida K, Tsukamoto H. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatol. 2015;61:129–140. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Matsumoto I. Alcoholism: protein expression profiles in a human hippocampal model. Expert Rev Proteomics. 2008;5:321–331. doi: 10.1586/14789450.5.2.321. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X7 receptor in Alzheimer Disease and amyloid-β peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65:1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. The Journal of neuroscience : the official journal of the Society for Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Monif M, Burnstock G, Williams DA. Microglia: Proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Storti M, Christova M, Tezzon F, Golaszewski S, Trinka E, Brigo F. Thiamine deficiency induced neurochemical, neuroanatomical, and neuropsychological alterations: a reappraisal. Sci World J. 2013;2013:309143. doi: 10.1155/2013/309143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism, Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s Disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes R, Pluzarev O, Hong JS, Crews F. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflam. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shin S, Cho K, Choi M, Lee S, Han S, Kang Y, Kim H, Cheong J, Shin C, Ko K. Urokinase-type plasminogen activator induces BV-2 microglial cell migration through activation of matrix metalloproteinase-9. Neurochem Res. 2010;35:976–985. doi: 10.1007/s11064-010-0141-3. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol. 2013;231:342–353. doi: 10.1002/path.4237. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and Release of Neuroprotective Tumor Necrosis Factor by P2X7 Receptor-Activated Microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Lippai D. Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol. 2014;118:359–380. doi: 10.1016/B978-0-12-801284-0.00011-7. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, Sato M, Hashimoto M, Kitani H. Lysophospholipids and ATP mutually suppress maturation and release of IL-1β in mouse microglial cells using a Rho-dependent pathway. J Immunol. 2008;180:7827–7839. doi: 10.4049/jimmunol.180.12.7827. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H, Hashimoto M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunolog Therap Exp. 2010;58:91–96. doi: 10.1007/s00005-010-0069-y. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 2008;447:33–48. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7:771–781. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Apolloni S, Carri MT, D’Ambrosi N. ALS: focus on purinergic signalling. Pharmacol Ther. 2011;132:111–122. doi: 10.1016/j.pharmthera.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]