Abstract
Purpose
Survivin inhibits apoptosis and enables tumor cells to escape from therapy-induced senescence. High expression of survivin is associated with bladder cancer aggressiveness and recurrence. The present study evaluated if survivin expression is reduced by siRNA and if survivin silencing enhances the activity of mitomycin C (MMC), in human RT4 bladder transitional cell tumors in vitro and in vivo.
Materials and Methods
The effectiveness of siRNA therapy was evaluated using two newly developed pegylated cationic liposome carriers (PCat, PPCat). Both carries used a fusogenic lipid to destabilize the endosomal membrane. One carrier (PPCat) further contained paclitaxel to enhance the in vivo delivery and transfection of survivin siRNA (siSurvivin). In vitro antitumor activity was evaluated using short and long term cytotoxicity assays (microtetrazolium and clonogenicity). In vivo intravenous therapy was evaluated in mice bearing subcutaneous tumors.
Results
The nontarget-siRNA had no antitumor activity in vitro or in vivo. Treatment of cultured cells with MMC at 50% cytotoxic concentration enhanced survivin mRNA and protein levels; addition of PPCat or PCat containing siSurvivin reversed the survivin induction and enhanced the MMC activity (p<0.05). In tumor-bearing mice, single agent MMC delayed tumor growth and nearly tripled the survivin protein level in residual tumors, whereas addition of PPCat-siSurvivin, which by itself yielded a minor survivin reduction (<10%), completely reversed the MMC-induced survivin and enhanced the MMC activity (p<0.05).
Conclusions
The results indicate effective in vivo survivin silencing and synergism between MMC and PPCat-siSurvivin. This combination represents a potentially useful chemo-gene therapy for bladder cancer.
Keywords: Bladder cancer, survivin, siRNA silencing, mitomycin C, chemo-gene therapy
INTRODUCTION
At presentation, more than 80% of bladder tumors are organ-confined, among which 70–80% are nonmuscle-invading that include Ta tumors located in the urothelium, T1 tumors in the lamina propria, and carcinoma in situ. This group is managed by transurethral tumor resection with or without fulguration, plus neoadjuvant or adjuvant intravesical immunotherapy or chemotherapy. Intravesical therapy involves instilling a drug solution into the bladder through a catheter, typically for 2 hr and repeated weekly for 6 weeks or longer. Recurrence occurs in 40 to 80% of patients, and between 10 to 20% of recurrences are accompanied by grade and/or stage progression (including the more fatal metastatic disease)1.
Through a series of preclinical and clinical studies2,3, our group has established that the efficacy of intravesical chemotherapeutics is limited by two factors: inadequate drug delivery to tumors and low chemosensitivity (especially the more aggressive tumors). We then identified a method that uses pharmacokinetic interventions to maximize the MMC delivery to nonmuscle-invading tumors. This method was tested in a multi-center, randomized phase III trial; the results confirm our hypothesis that improving drug delivery significantly improves the 5-yr recurrence-free rate of high risk patients (from 23.5% to 42.6%)2.
As a part of our ongoing efforts to enhance the bladder tumor sensitivity to MMC, the present study evaluated the potential usefulness of silencing survivin, a chemotherapy-induced anti-apoptotic gene. Survivin is highly and selectively expressed in a majority of human cancers including bladder cancer4,5, and is a marker/predictor of bladder cancer aggressiveness and recurrence6–8. In addition, high survivin expression is correlated with chemo/radio-resistance in multiple tumor types whereas low expression enhances cell death9,10. Inhibition of survivin by antisense, siRNA or shRNA enhances the sensitivity of tumor cells to chemotherapy including MMC10–12.
siRNA produces post-transcriptional gene silencing and represents a promising gene therapy approach. We have shown that intraperitoneal administration of siRNA reverses chemotherapy-induced survivin expression in pancreatic xenograft tumors13. The potential utility of survivin silencing in bladder cancer was shown in a recent study where intratumoral injection of siRNA-containing nanoparticles reduced survivin expression and growth of subcutaneous xenograft tumors14. However, the feasibility of intravenous siRNA therapy is not known because these earlier studies used locoregional injections and because systemic siRNA therapy is impeded by inadequate delivery and transfection15. We recently reported that tumor priming using apoptosis-inducing chemotherapy enhances the in vivo effectiveness of siRNA therapy13. This earlier study used paclitaxel as the tumor priming agent. In the current study, MMC was used as the chemotherapy and, due to its ability to induce apoptosis16, also as the priming agent. For the siRNA carriers, we evaluated two pegylated cationic liposomes. One carrier was identical to the PCat carrier used in our earlier study13, whereas the second carrier PPCat contained, in addition, paclitaxel. This study shows that both carriers yielded survivin silencing and enhanced MMC activity, in vitro and in vivo, in human bladder RT4 xenograft tumors.
MATERIALS AND METHODS
Chemicals and Supplies
MMC was purchased from A.G. Scientific (San Diego, CA), paclitaxel from Bioxel (Quebec, Canada), lipids (1,2-dioleoyl-3-trimethylammoniumpropane or DOTAP, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine or DOPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] or DSPE-PEG, cholesterol) from Avanti Polar Lipids (Alabaster, AL), and all cell culture supplies from Life Technologies (Grand Island, NY). Survivin siRNA (siSurvivin, human-specific, #6351), nontarget siRNA (NT-siRNA) and primer probe sets were purchased from IDT (San Diego, CA), rabbit monoclonal HLA-A antibody (EP1395Y) from Abcam (Cambridge, MA), survivin monoclonal antibody (71G4B7E) from Cell Signaling Technology (Danvers, MA), Ki67 antibody from Novocastra Lab (UK), and In Situ Cell Death Detection Kit (TUNEL) from Roche Diagnostics (Indianapolis, IN). All chemicals and reagents were used as received.
Preparation of liposomes and lipoplexes
Cationic liposomes form lipoplexes with anionic siRNA, which improves the siRNA stability and internalization15,17. Both PCat and PPCat comprised cationic lipid (DOTAP), neutral lipids (cholesterol, DOPE) and pegylated lipid (DSPE-PEG2000), at a ratio of 50:30:19:1. The fusogenic lipid DOPE destabilizes the endosomal membrane. PCat was prepared as previously described13, whereas the PPCat preparation used an extra step of adding paclitaxel dissolved in methanol at 0.0406 weight % of total lipids. PCat or PPCat were gently mixed with an aqueous siRNA solution (10 µM) at room temperature, in a 1:4 siRNA-to-DOTAP charge ratio. Particle size distribution and zeta potential were measured using Zetasizer Nano ZS90.
Measurement of survivin mRNA and protein levels
mRNA and protein levels were respectively measured using real time (RT)-PCR and Western blotting as previously described13. Briefly, total RNA was extracted using the Rneasy Plus Mini Kit (Qiagen, Valencia, CA), and reversed transcribed to cDNA using qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). RT-PCR (triplicate samples, 5 µL cDNA per reaction) was performed with PerfeCTa® MultiPlex qPCR SuperMix (Quanta) in CFX96™ RT-PCR Detection Systems (BioRad, Hercules, CA). The prime probe sets were: survivin, forward: 5’- CAACCGGACGAATGCTTTT-3’; reverse: 5’-AAGAACTGGCCCTTCTTGGA-3’; probe: 5’-/5HEX/CCAGATGAC/ZEN/GACCCCATAGAGGAA/3IABkFQ/-3’; GAPDH, forward: 5’- AATCCCATCACCATCTTCCAG-3’; reverse: 5’-AAATGAGCCCCAGCCTTC-3’; probe: 5’-/5Cy5/CCAGCATCGCCCCACTTGATTTT/3IAbRQSp/-3’. The multiplex thermal reaction program was: 3 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 61°C. Survivin mRNA expression relative to GAPDH expression was calculated using the ΔΔCt-method18. For protein analysis, cells were lysed and analyzed for protein concentrations using BCA kit (ThermoFisher, Rockford, IL). Samples (1.5 µg protein/µl) were run on Bolt™ 10% Bis-TrisPlus Gel (Life technologies, Carlsbad, CA). The gels were transferred to a nitrocellulose membrane using Trans-blot Turbo Transfer System (BioRad). Blots were probed with survivin and β-actin primary antibodies (Cell Signaling). Chemi-luminescence was detected and the band intensity analyzed using BioRad Molecular Imager. Protein levels were normalized to loading control (β-actin for cultured cell lysates and HLA-A for animal tumor lysates). HLA-A is the major histocompatibility complex specific to humans; it was used to eliminate ambiguity due to the presence of mouse tissues.
In vitro studies
Human bladder transitional RT4 cancer cells (ATCC, Manassaas, VA) were maintained in complete medium comprising Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids, 100 µg/ml penicillin and 100 µg/ml streptomycin, in 5% CO2 at 37°C.
Antitumor activity was measured using the short term microtetrazolium (MTT) cytotoxicity assay and the long term clonogenic assay. All cytotoxicity studies used 9 groups, including two control groups (untreated or treated with nontarget siRNA or siNT), and groups treated with siSurvivin, two concentrations of MMC (10% and 50% cytotoxicity, or IC10 and IC50), and combinations of MMC and siNT or siSurvivin (i.e., IC10+siNT, IC10+siSurvivin, IC50+siNT, IC50+siSurvivin). The transfection medium was the complete medium minus FBS and antibiotics. The siRNA concentration was 100 nM. The MMT assay used PPCat carrier, whereas the clonogenic assay used PCat in order to avoid potential complications due to the delayed cytotoxicity of paclitaxel embedded in PPCat19. For both assays, cells were treated with MMC (2 hr), PPCat-siSurvivin (4 hr), or their combinations (in the sequence of siSurvivin followed by MMC). For the MTT assay, cells were seeded in 96-well plates (5,000–15,000 cells/well), allowed to attach to the growth surface for 24 hr, treated, and the numbers of metabolically active cells remaining at 48 hr post-treatment were measured. For the clonogenic assay, cells were seeded in culture dishes (100 mm2) for 24 hr, treated, harvested by trypsinization, re-plated (1,000 cells per dish), incubated with medium change every 4 days, and stained with the violet crystal dye on day 23. Pictures were analyzed using ImageJ (NIH, Bethesda, MD); the numbers of colonies with a size >0.1 mm2 (>100 cells) were counted.
The effect of MMC and siRNA treatments on survivin expression was studied using two concentrations of MMC (0.1 and 3 µM) and one concentration of siRNA (100 nM in PPCat). Cells were collected 48 hr after treatment, and analyzed for survivin mRNA and protein levels.
In vivo studies
Female athymic nude mice (NCI, Frederick, MD), 5 to 6 weeks old, were cared for according to Institutional Animal Care and Use Committee-approved protocols. Subconfluent RT4 cells were harvested and implanted subcutaneously into the left and right flanks (3 million cells per side). Treatments were initiated after 28 days when tumors reached at least 3 mm in width. MMC solution (1 mg/ml physiological saline) was prepared immediately before treatment. Mice were randomized according to initial tumor size and body weight, to six treatment groups to receive intravenous injections of physiological saline, single agents (4mg/kg MMC per dose, 1 nmole PPCat-siNT or PPCat-siSurvivin per dose), or their combinations (MMC plus PPCat-siNT, MMC plus PPCat-siSurvivin). MMC was given every 4 days for a total of 3 doses and siRNA was given 2 days after each MMC treatment.
Antitumor activities were monitored using two sets of pharmacodynamic endpoints. The macroscopic endpoint was change in tumor size (calculated as 0.5 × (width)2 × (length)). The microscopic cellular and molecular endpoints were survivin protein level, apoptosis and antiproliferation, as described previously13. Briefly, two days after the last treatment, tumors were excised from anesthesized mice and divided into two halves. One half was stored frozen at −80°C, and later lysed with M-PER and the lysates analyzed for survivin protein level. The second-half was fixed with 10% formalin, embedded in paraffin, cut into 10 µm histologic sections, and processed for immunostaining. Micrographs were taken with a Leica SP8 confocal microscope and images were analyzed for survivin-, Ki67-, and TUNEL-positive cells. We determined the number of labeled and unlabeled tumor cells in randomly selected microscopic fields at 200× magnification, and counted, on average per animal, >500 cells per data point.
Statistical Analysis
For comparison of in vivo antitumor activity, differences between treatment groups were analyzed using analysis of variances (ANOVA with repeated measures). p values of less than 5% were considered statistically significant. The concentration-response curves were analyzed using GraphPad Prism (La Jolla, CA).
RESULTS
Characterization of siRNA carriers
Blank PCat and PPCat liposomes (i.e., no siRNA cargo) were stable at 4°C, with no significant changes in particle size and zeta potential after 18 months. Upon mixing with siRNA, the respective particle size increased from 110±4 to 170±3 nm and from 110±1 to 164±2 nm, and the respective zeta potential reduced from 62±1 to 34±1 mV and from 59±1 to 33.5±1mV (mean±SD, n=3 experiments).
Effect of MMC and siSurvivin on survivin expression in cultured RT4 cells
Pilot studies showed that MMC transiently elevated the mRNA and protein levels of survivin, which reached maximal levels on day 2 and returned to baseline levels on day 4, whereas PPCat-siSurvivin (100 nM) showed the opposite profiles (maximum knockdown on day 2 and full recovery on day 4). Subsequent studies on survivin expression used the day 2 time point; the results are summarized in Figure 1 and Table 1A.
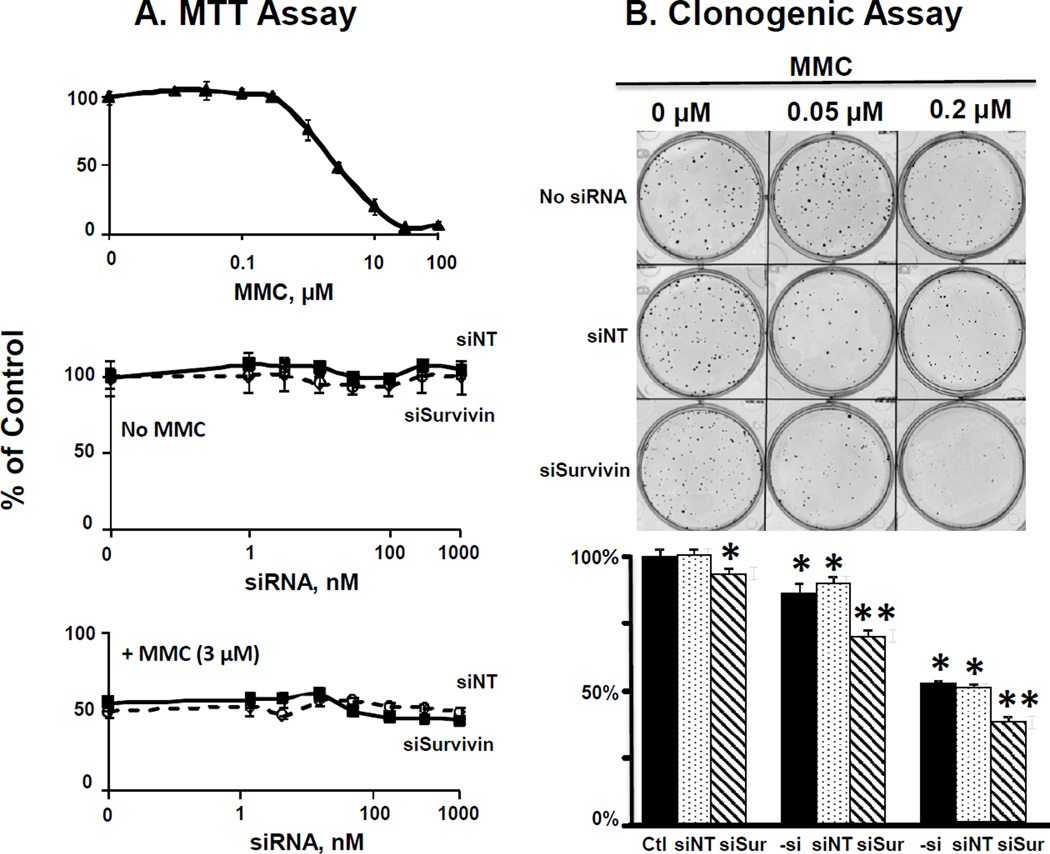
Figure 1. Effect of MMC and siRNA on survivin expression in vitro.
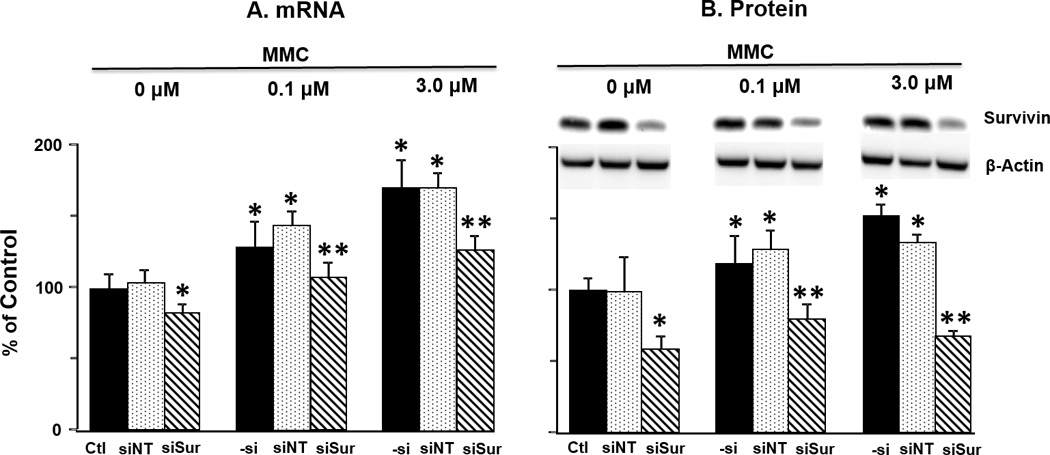
RT4 cells were incubated for 4 hr in transfection medium (control), with PPCat-siNT (100 nM) or PPCat-siSurvivin (100 nM), followed by 2 hr treatment with complete medium or MMC (0.1 and 3 µM). At 48 hr post-treatment, cells were collected and analyzed for survivin mRNA and protein levels. Ctl: control. –si: no siRNA, siNT: nontarget siRNA, siSur: survivin siRNA. Data for mRNA levels are mean+1 SD (n=3 experiments, with triplicate samples per experiment). Data for protein levels are mean+1 SD (n=3 experiments, single sample per experiment). *p<0.05 compared to control. **p<0.05 compared to MMC and MMC+PPCat-siNT.
Table 1. Survivin silencing enhances MMC activity in vitro and in vivo.
Cultured RT4 cells and mice bearing RT4 xenograft tumors were treated with MMC, nontarget siRNA and survivin siRNA (siRNA were delivered in PPCat), as described in the legends of Figures 1 and 3. For the in vitro studies, the MMC concentration was 3 µM and the siRNA concentration was 100 nM. For the in vivo studies, the MMC dose was 3 mg/kg given every 4 days for a total of 3 doses and the siRNA dose was 1 nmole given 2 days after each MMC treatment.
| Group | A. In Vitro results | B. In Vivo results | ||||
|---|---|---|---|---|---|---|
| Survivin expression mean±SD (3 experiments, 3 samples each) |
Time for 50% tumor size increase, median (range), days |
Survivin protein level, % of control (n=3–5) |
% Ki67- positive cells (n=3–5) |
% TUNEL- positive cells (n=3–5) |
||
| mRNA | Protein | |||||
| Control | 1.00 ± 0.10 | 1.00 ± 0.09 | 8 (8-12, n=5) | 100 ± 21 | 66 ± 5 | 4 ± 1 |
| PPCat-siNT | 1.08 ± 0.05 | 0.99 ± 0.25 | 12 (8-12, n=5) | 90 ± 23 | 65 ± 7 | 5 ± 4 |
| PPCat-siSurvivin | 0.82 ± 0.02* | 0.58 ± 0.10* | 12 (8-12, n=5) | 55 ± 15* | 60 ± 6 | 4 ± 2 |
| MMC | 1.47 ±0.34* | 1.52 ± 0.08* | 37 (23-44, n=10)* | 302 ± 18* | 38 ± 6* | 31 ± 4* |
| MMC + PPCat-siNT | 1.53 ± 0.24* | 1.33 ± 0.06* | 37 (23-44, n=5)* | 240 ± 45* | 41 ± 8* | 33 ± 7* |
| MMC + PPCat-siSurvivin | 1.13 ± 0.17** | 0.90 ± 0.04** | 44 (37-58, n=10)*** | 90 ± 28** | 16 ± 5*** | 59 ± 6*** |
p<0.05 vs. the untreated control and PPCat-siNT control.
p<0.05 vs. MMC and MMC+PPCat-siNT, but not different from untreated control and PPCat-siNT control.
p<0.05 vs. all other groups.
MMC induced survivin expression in a dose-dependent manner; MMC treatment at 0.1 and 3 µM (equivalent to the IC10 and IC50 in MTT assay) yielded, respectively, about 30% and 70% increases in mRNA levels and about 20% and 50% increases in protein levels, compared to untreated controls (p<0.05). Single agent PPCat-siSurvivin (100 nM) significantly reduced the survivin mRNA and protein levels. PPCat-siSurvivin also reversed the survivin induction by MMC, with complete/partial reversal of mRNA induction and more-than-complete reversal of protein induction (i.e., below the level in untreated control) (p<0.05 compared to MMC alone).
Activity of MMC and siSurvivin in cultured cells
Figures 2A and 2B show the results of the short term cytotoxicity MTT assay (measured at 48 hr post-treatment) and the long term clonogenic assay (measured on day 23 post-treatment), respectively.
Figure 2. Survivin silencing enhances antitumor activity of MMC in vitro.
RT4 cells were incubated for 4 hr in transfection medium (control), with PPCat-siNT (100 nM) or PPCat-siSurvivin (100 nM), followed by 2 hr treatment without or with MMC in complete medium, and then processed for short term MTT assay (cytotoxicity measured at 48 hr post-treatment, Panel A), or the long term clonogenic assay (cytotoxicity measured at 23 days post treatment, Panel B). For Panel A, data are mean+1 SD (n=3 experiments, with triplicate samples per experiment). Some SD values are smaller than the symbols. For Panel B, data are mean+1 SD (n=3 experiments, triplicate samples per experiment). Ctl: control. –si: no siRNA, siNT: nontarget siRNA, siSur: survivin siRNA. *p<0.05 compared to untreated control (i.e., no MMC, no siRNA). **p<0.05 compared to untreated control as well as single agent MMC at same MMC concentration (i.e., no siRNA or MMC+siNT).
PCat- and PPCat-siRNA lipoplexes loaded with siNT (i.e., no siSurvivin), at up to 1,000 nM siRNA, were nontoxic, as shown by the lack of cytotoxicity in the two assays. MMC showed dose-dependent cytotoxicity in MTT and clonogenic assays whereas siSurvivin produced different outcomes in the two assays. In MTT assay, PPCat-siSurvivin had no activity and did not alter the activity of MMC. In contrast, in the clonogenic assay, PCat-siSurvivin treatment yielded minor but significant growth inhibition (7±2%, p<0.05 compared to untreated control and siNT) as well as significantly enhanced the MMC activity (from 13±3% to 30±2% inhibition at 0.05 µM MMC and from 48±2% to 62±2% inhibition at 0.2 µM MMC, p<0.05 in both cases). Furthermore, the average sizes of colonies in groups treated with PCat-siSurvivin (with or without MMC) were slightly but significantly smaller compared to without PCat-siSurvivin treatment (0.18 vs. 0.16 mm2 and 0.12 vs. 0.11 mm2, respectively, p<0.05). Collectively, these results indicate siSurvivin had long term cytotoxicity but no short term cytotoxicity.
In vivo antitumor activity
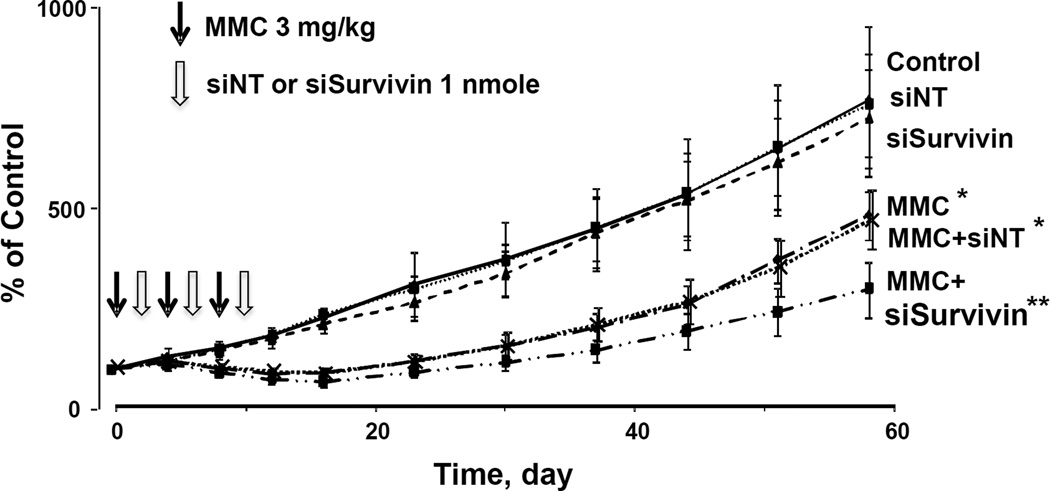
Figure 3 shows the tumor size changes over time. The untreated control showed continuous tumor growth, reaching ~700% of the initial size on day 58 (day 0 is day of first treatment). Tumor growth was not affected by PPCat-siSurvivin (p>0.8 compared to untreated control), indicating the lack of activity of PPCat carrier and siSurvivin siRNA. In contrast, single agent MMC (3 mg/kg per dose×3 doses) caused tumor regression (maximal 16% size reduction on day 12) and reduced tumor growth (to <430% of initial size on day 58) (p<0.05 compared to untreated control and the two single agent siRNA groups). Addition of PPCat-siSurvivin (1 nmole per dose × 3 doses) to MMC further improved the MMC activity (i.e., maximal 32% tumor regression and <300% tumor growth, p<0.05 compared to single agent MMC). The times for tumor size to increase by 50% were similarly extended by MMC and PPCat-siSurvivin (Table 1B).
Figure 3. Survivin silencing enhances antitumor activity of MMC in vivo: Tumor growth.
Antitumor activity was evaluated in immunodeficient mice bearing subcutaneous RT4 human bladder xenograft tumors. All treatments (indicated by arrows) were administered by intravenous injections. Day 0 represents the day of treatment initiation, which corresponded to 28 days post-tumor implantation. The MMC dose was 3 mg/kg, given every 4 days for a total of 3 doses. The PPCat-siSurvivin dose was 1 nmole, given 2 days after each MMC treatment. Animals were maintained for 58 days post treatment, with tumor size measurements taken every 4 days for the first 16 days and once a week afterwards. From top to bottom: control (filled diamonds, n=5), siNT (open squares, n=5), siSurvivin (filled triangles, n=5), MMC (open diamonds, n=10), MMC+siNT (crosses, n=5), MMC+siSurvivin (filled squares, n=10). *p<0.05 vs. control and single agent siNT and siSurvivin. **p<0.05 vs. all other groups.
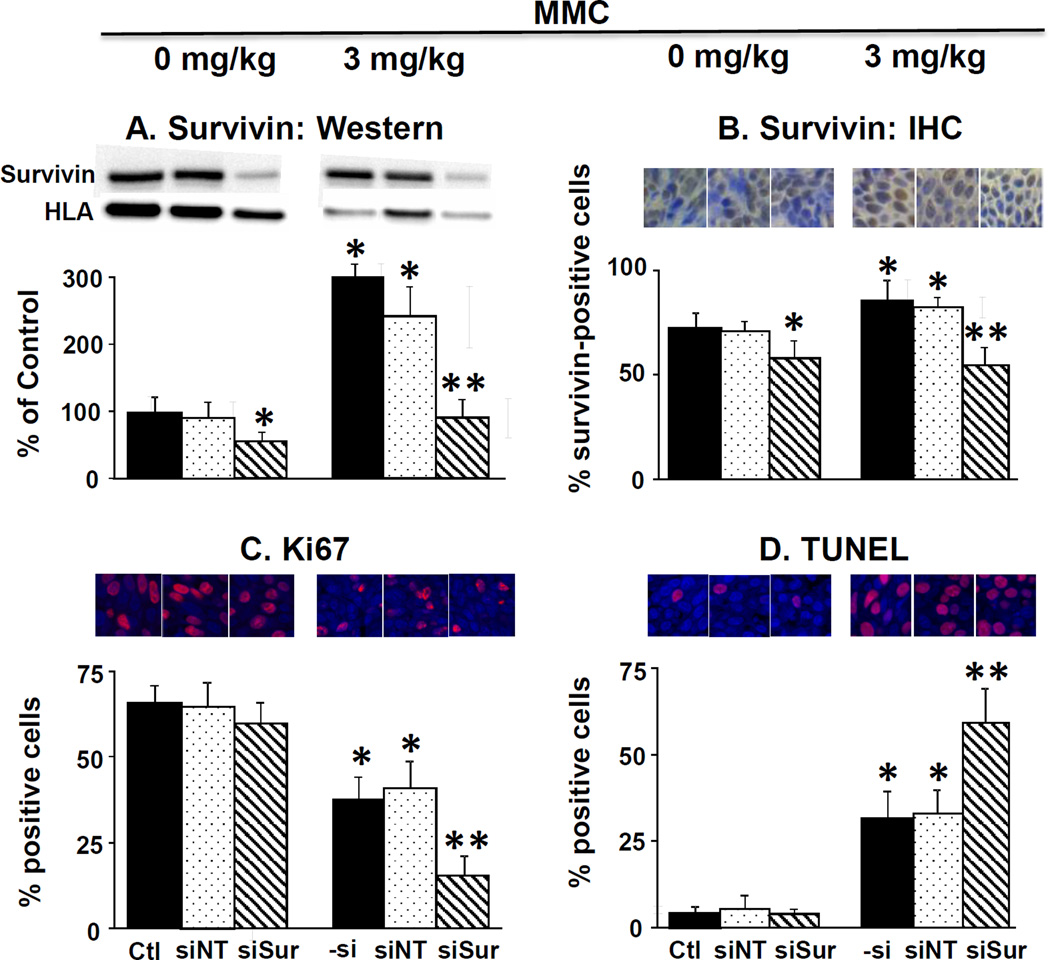
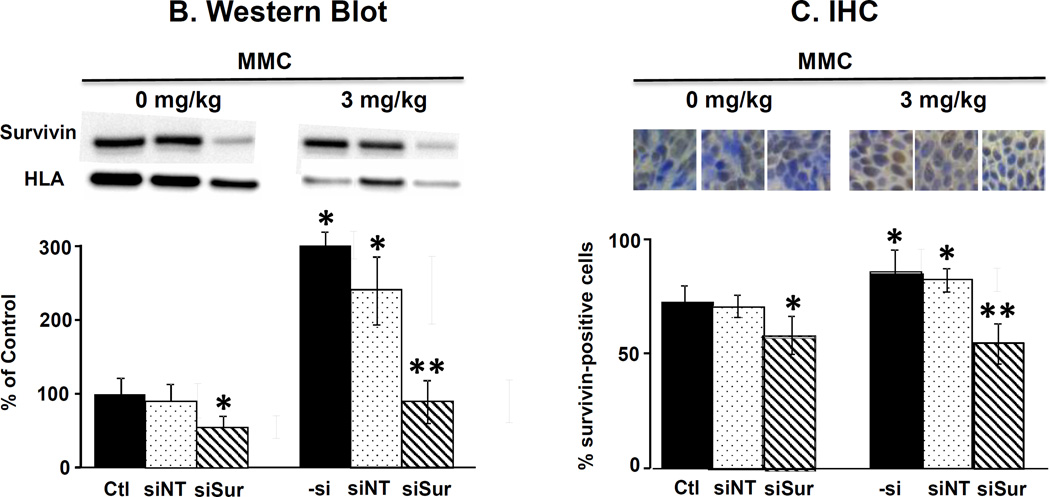
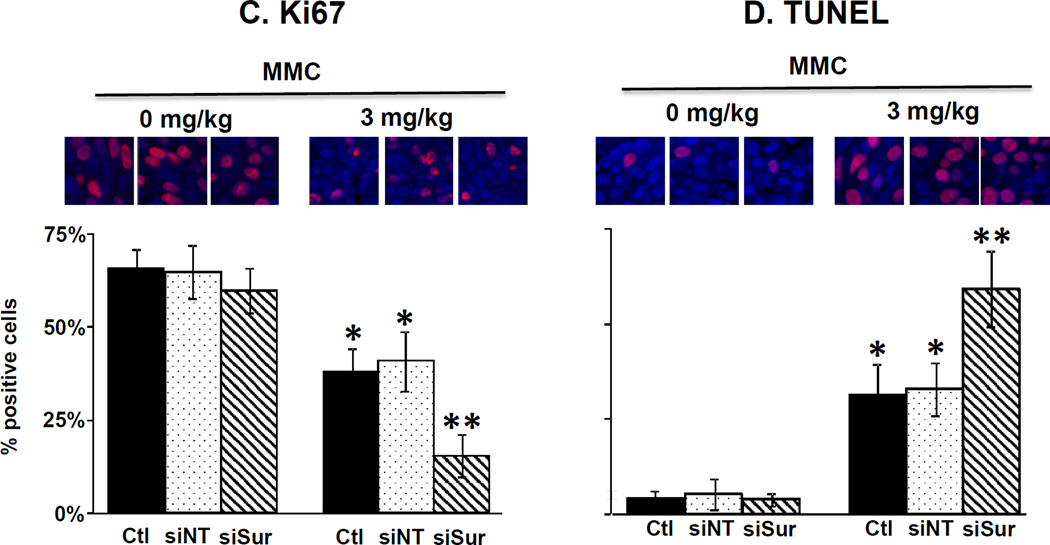
Figure 4 and Table 1B show the results on several cellular and molecular pharmacodynamic endpoints in tumors (i.e., survivin expression, proliferating and apoptotic cell fractions). MMC treatment tripled the protein level whereas PPCat-siSurvivin significantly reduced the protein level, compared to untreated control. Adding PPCat-siSurvivin to MMC more than reversed the survivin induction by MMC (i.e., below the level in untreated control). These findings were confirmed by the immunohistochemical results; MMC significantly increased the survivin-positive cell fraction whereas PPCat-siSurvivin significantly reduced the fraction. Consistent with the in vitro data in cultured cells, PPCat-siSurvivin yielded more-than-complete reversal of the MMC induction in animal tumors. MMC significantly reduced the Ki67-positive proliferating cell fraction and increased the TUNEL-positive apoptotic cells (p<0.05). Although PPCat-siSurvivin had no effects as single agent, its addition enhanced the MMC effects, i.e., significantly lower proliferating fraction and greater apoptotic fraction compared to MMC alone (p<0.05).
Figure 4. Survivin silencing enhances antitumor activity of MMC in vivo: Cellular and molecular pharmacodynamic endpoints.
Animals were treated as described in the legend of Figure 3, with the exception that animals were anesthesized 2 days after the last treatment and their tumors excised and processed for Western blotting and for immunohistochemical staining and quantitative imaging analysis. (A) Survivin protein levels in tumors detected by Western blotting. Results were normalized by HLA. (B) Survivin expression detected by immunohistochemical staining (IHC, stained brown). Blue: nucleus dye DAPI. (C) Immunohistochemical staining of Ki67 (red). Blue: nucleus dye DAPI. Note the Ki67 stain overlapped with the nucleus stain (i.e., stained cells showed only red stain). (D) Immunohistochemical staining of apoptotic cells (TUNEL, red).Blue: nucleus dye DAPI. Note the TUNEL stain overlapped with the nucleus stain (i.e., stained cells showed only red stain). Ctl: control. –si: no siRNA, siNT: nontarget siRNA, siSur: survivin siRNA. Data are mean+1 SD (3–5 tumors). *p<0.05 vs. control groups.**p<0.05 vs. MMC and MMC+PPCat-siNT.
In contrast to siSurvivin which produced significant changes, the nontarget siRNA had no effects in vivo, i.e., it did not alter survivin expression or the proliferating/apoptotic cell fractions in tumors, and did not slow tumor growth or enhance the MMC activity.
DISCUSSION
The present study shows several interesting findings. First, MMC induced survivin expression, in a dose-dependent manner, in cultured RT4 cells and RT4 xenograft tumor-bearing animals; this is consistent with the literature results20. Second, PCat and PPCat were efficient siSurvivin carriers and produced survivin mRNA and protein knockdown in vitro. Third, survivin silencing produced different outcomes in short term MTT assay and long term clonogenic assay. Fourth, PCat- and PPCat-siSurvivin reversed the survivin induction by MMC, and enhanced the MMC cytotoxicity in cultured cells and animal tumors. In contrast, the nontarget siRNA had no activities, confirming the role of survivin silencing of MMC chemosensitization. Fifth, because single agent PPCat-siSurvivin was inactive in vivo, the enhanced activity for the combination of MMC and PPCat-siSurvivin indicates synergy. The potential clinical implications of these findings are as follows.
The expression and prognostic value of several anti-apoptotic genes, including survivin, XIAP, p53, bcl2 and bcl-xL, in bladder cancer have been compared6–8,21,22.The expression of survivin is the most frequently upregulated in tumors (65–100%) and the most highly correlated with poor prognosis, whereas the expression of the other genes has no or inferior correlations, indicating the more important role of survivin among the known anti-apoptotic genes. This is further supported by the finding in monolayer EJ28 and J82 bladder tumor cells that siRNA-mediated survivin silencing yielded greater antitumor effect compared to silencing of XIAP, bcl2 and bcl-xL10. Our finding that PCat-siSurvivin produced a minor but significant growth reduction only in the clonogenic assay and not in the MTT assay indicates slow onset cytotoxicity such as cytostasis instead of cell kill by survivin knockdown in RT4 cells.
The successful in vivo siRNA transfection using the PPCat carrier is noteworthy as the current study used intravenous administration, which subjects siRNA to multiple transport and elimination barriers that have limited the efficacy and development of siRNA therapeutics15. There have been very few successful gene silencing by intravenous RNAi. The first demonstration of RNAi in humans was in 2010 and was accomplished using pegylated cyclodextrin, transferrin-targeted nanoparticles (target gene was ribonucleotide reductase)23. A 2013 study used pegylated cationic liposomes in a phase I trial and detected VEGF and/or KSP siRNA in tumor biopsies of all 12 patients plus partial gene knockdown in 3 of 12 patients24. To our knowledge, the present study is the first to demonstrate survivin silencing in bladder tumors by intravenous RNAi. For example, the earlier study demonstrating the in vivo activity of survivin siRNA was accomplished using the intratumoral injection route14.
Survivin is universally upregulated by cytotoxic treatments, i.e., radiation and drugs used for intravesical or intravenous therapy of organ-confined or metastatic bladder cancer (e.g., doxorubicin, cisplatin, gemcitabine, methotrexate, vincristine, paclitaxel, docetaxel)9,25–30. Hence, the in vivo synergy between intravenous MMC and PPCat-siSurvivin observed in the current study, plus the in vivo synergy between intraperitoneal paclitaxel and PCat-siSurvivin observed in our earlier study13, indicate siSurvivin gene therapy can be used to overcome drug resistance in bladder cancer, in either the intravesical or the systemic therapy setting.
At present, there is limited information on the disposition of siRNA therapeutics, e.g., the dose fraction that is delivered intact to tumors, residence time, and duration of gene silencing. These data, however, are needed to determine the dose intensity and frequency. Another consideration for intravesical therapy is the ability of the siRNA carrier to reach the target site, as the urothelium is a well-known barrier to the absorption of intravesical therapeutics (e.g., <3% of the intravesical MMC dose reaches the locations of Ta and T1 tumors3). Additional pharmacokinetic and pharmacodynamic studies are needed to facilitate the successful translation of PCat/PPCat-siSurvivin to clinical application.
CONCLUSIONS
The synergy between intravenous MMC and PPCat-siSurvivin in vivo supports further development of chemo-survivin gene therapy for organ-confined and metastatic bladder cancer.
ACKNOWLEDGEMENTS
This research was supported in part by research grant R43TR000356 from the National Center for Advancing Translational Sciences and by research grant R01CA158300 from the National Cancer Institute, NIH, DHHS.
Abbreviations used
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammoniumpropane
- DSPE-PEG
1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-[methoxy(polyethylene glycol)-2000]
- FBS
fetal bovine serum
- MMC
mitomycin C
- MTT
microtetrazolium
- PCat
liposomes comprising DOTAP:cholesterol:DOPE:DSPE-PEG in 50:30:19:1 ratio
- PPCat
paclitaxel-containing PCat
- siNT
non-target siRNA
- siRNA
small interfering RNA
- siSurvivin
survivin siRNA
Footnotes
Publisher's Disclaimer: DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
REFERENCES
- 1.Lerner SP, Au JL. Risk-adapted use of intravesical chemotherapy. BJU Int. 2008;102:1247. doi: 10.1111/j.1464-410X.2008.07967.x. [DOI] [PubMed] [Google Scholar]
- 2.Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 3.Dalton JT, Wientjes MG, Badalament RA, et al. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. 1991;51:5144. [PubMed] [Google Scholar]
- 4.Akhtar M, Gallagher L, Rohan S. Survivin: role in diagnosis, prognosis, and treatment of bladder cancer. Adv Anat Pathol. 2006;13:122. doi: 10.1097/00125480-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Karam JA, Lotan Y, Ashfaq R, et al. Survivin expression in patients with non-muscle-invasive urothelial cell carcinoma of the bladder. Urology. 2007;70:482. doi: 10.1016/j.urology.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Hausladen DA, Wheeler MA, Altieri DC, et al. Effect of intravesical treatment of transitional cell carcinoma with bacillus Calmette-Guerin and mitomycin C on urinary survivin levels and outcome. J Urol. 2003;170:230. doi: 10.1097/01.ju.0000063685.29339.24. [DOI] [PubMed] [Google Scholar]
- 7.Jeon C, Kim M, Kwak C, et al. Prognostic role of survivin in bladder cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e76719. doi: 10.1371/journal.pone.0076719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz IJ, Kiemeney LA, Witjes JA, et al. Survivin mRNA expression is elevated in malignant urothelial cell carcinomas and predicts time to recurrence. Anticancer Res. 2003;23:3327. [PubMed] [Google Scholar]
- 9.Guan HT, Xue XH, Dai ZJ, et al. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12:2901. doi: 10.3748/wjg.v12.i18.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunze D, Erdmann K, Froehner M, et al. siRNA-mediated inhibition of antiapoptotic genes enhances chemotherapy efficacy in bladder cancer cells. Anticancer Res. 2012;32:4313. [PubMed] [Google Scholar]
- 11.Tian H, Liu S, Zhang J, et al. Enhancement of cisplatin sensitivity in lung cancer xenografts by liposome-mediated delivery of the plasmid expressing small hairpin RNA targeting Survivin. J Biomed Nanotechnol. 2012;8:633. doi: 10.1166/jbn.2012.1419. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Zheng JY, Shi H, et al. Survivin knockdown enhances gastric cancer cell sensitivity to radiation and chemotherapy in vitro and in nude mice. Am J Med Sci. 2012;344:52. doi: 10.1097/MAJ.0b013e318239c4ee. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Lu Z, Yeung BZ, et al. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors. J Control Release. 2014;178:79. doi: 10.1016/j.jconrel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin DT, Steinbach JM, Liu J, et al. Surface-modified nanoparticles enhance transurothelial penetration and delivery of survivin siRNA in treating bladder cancer. Mol Cancer Ther. 2014;13:71. doi: 10.1158/1535-7163.MCT-13-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Lu Z, Wientjes MG, et al. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010;12:492. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirnia F, Schneider E, Betticher DC, et al. Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ. 2002;9:905. doi: 10.1038/sj.cdd.4401062. [DOI] [PubMed] [Google Scholar]
- 17.Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Au JL, Li D, Gan Y, et al. Pharmacodynamics of immediate and delayed effects of paclitaxel: role of slow apoptosis and intracellular drug retention. Cancer Res. 1998;58:2141. [PubMed] [Google Scholar]
- 20.Verma A, Degrado J, Hittelman AB, et al. Effect of mitomycin C on concentrations of vascular endothelial growth factor and its receptors in bladder cancer cells and in bladders of rats intravesically instilled with mitomycin C. BJU Int. 2011;107:1154. doi: 10.1111/j.1464-410X.2010.09543.x. [DOI] [PubMed] [Google Scholar]
- 21.Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 22.Swana HS, Grossman D, Anthony JN, et al. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999;341:452. doi: 10.1056/NEJM199908053410614. [DOI] [PubMed] [Google Scholar]
- 23.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 25.Peng XH, Karna P, O'Regan RM, et al. Down-regulation of inhibitor of apoptosis proteins by deguelin selectively induces apoptosis in breast cancer cells. Mol Pharmacol. 2007;71:101. doi: 10.1124/mol.106.027367. [DOI] [PubMed] [Google Scholar]
- 26.Uehara S, Oue T, Kawatsu M, et al. Increased expression of survivin in hepatoblastoma after chemotherapy. Eur J Pediatr Surg. 2013;23:400. doi: 10.1055/s-0033-1333637. [DOI] [PubMed] [Google Scholar]
- 27.Yoon DH, Shin JS, Jin DH, et al. The survivin suppressant YM155 potentiates chemosensitivity to gemcitabine in the human pancreatic cancer cell line MiaPaCa-2. Anticancer Res. 2012;32:1681. [PubMed] [Google Scholar]
- 28.Penuelas S, Noe V, Ciudad CJ. Modulation of IMPDH2, survivin, topoisomerase I and vimentin increases sensitivity to methotrexate in HT29 human colon cancer cells. FEBS J. 2005;272:696. doi: 10.1111/j.1742-4658.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- 29.Souza PS, Vasconcelos FC, De Souza Reis FR, et al. P-glycoprotein and survivin simultaneously regulate vincristine-induced apoptosis in chronic myeloid leukemia cells. Int J Oncol. 2011;39:925. doi: 10.3892/ijo.2011.1103. [DOI] [PubMed] [Google Scholar]
- 30.Ling X, Bernacki RJ, Brattain MG, et al. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004;279:15196. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]