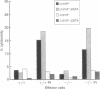
Abstract
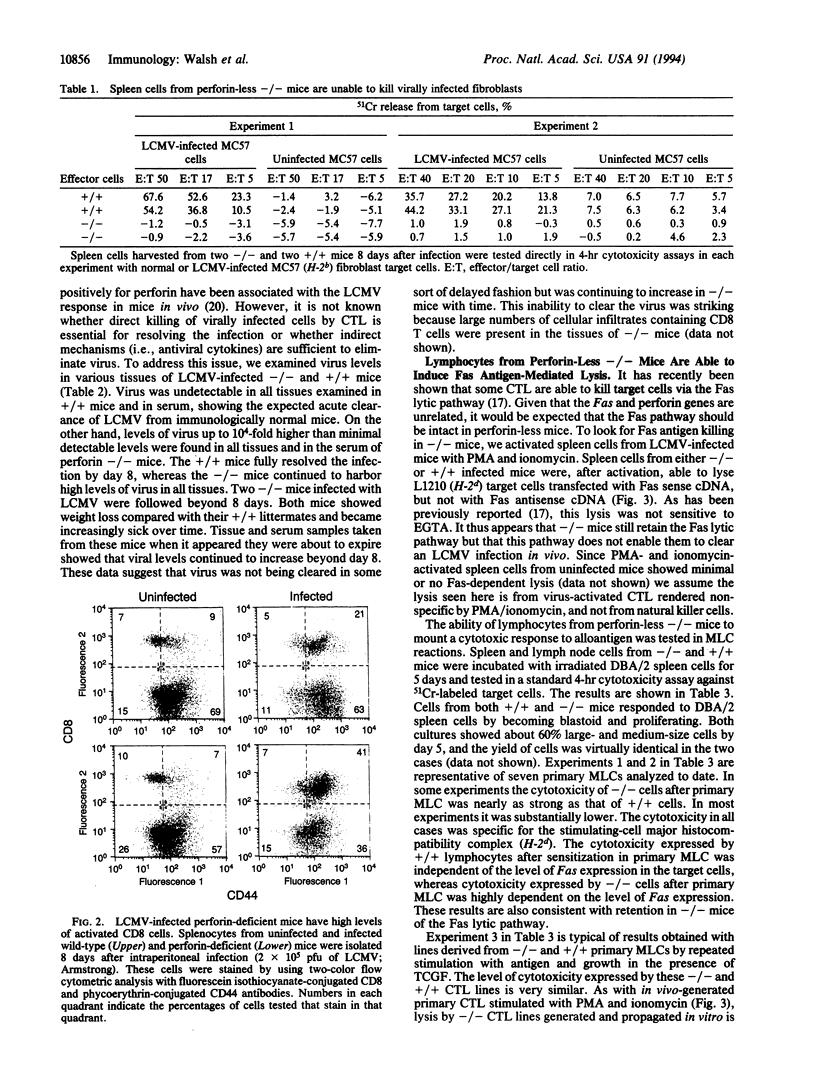
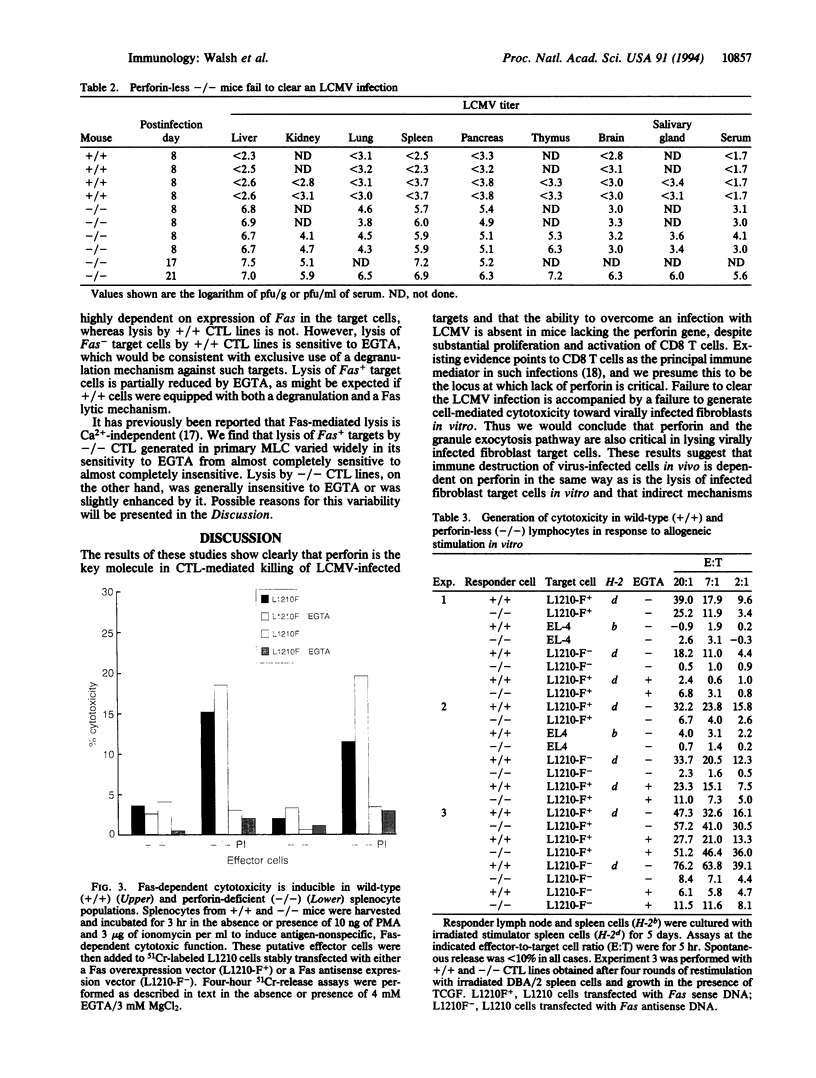
Mice lacking the perforin gene were generated by using targeted gene disruption in embryonal stem cells. When infected with lymphocytic choriomeningitis virus (LCMV), perforin-less (-/-) mice showed clear signs of having mounted an immune response based on activation of CD8 T cells but were unable to clear the LCMV infection. This failure to eliminate virus was accompanied by a failure to generate spleen cells capable of lysing LCMV-infected fibroblasts in vitro. Spleen cells from LCMV-infected -/- mice were able to lyse hematopoietic target cells after exposure to phorbol 12-myristate 13-acetate and ionomycin, provided the target cells expressed the Fas antigen. Spleen cells from -/- mice also responded to alloantigen in mixed leukocyte culture by blastogenesis and proliferation. The resulting cells were able to lyse hematopoietic target cells, although not as well as spleen cells from +/+ littermates sensitized in the same manner. However, lysis by -/- cells was again seen only if the target cells expressed Fas antigen. We conclude that perforin-less -/- mice retain and express the Fas lytic pathway as expressed in vitro but that this pathway is insufficient to clear an LCMV infection in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berke G., Rosen D., Ronen D. Mechanism of lymphocyte-mediated cytolysis: functional cytolytic T cells lacking perforin and granzymes. Immunology. 1993 Jan;78(1):105–112. [PMC free article] [PubMed] [Google Scholar]
- Blakely A., Gorman K., Ostergaard H., Svoboda K., Liu C. C., Young J. D., Clark W. R. Resistance of cloned cytotoxic T lymphocytes to cell-mediated cytotoxicity. J Exp Med. 1987 Oct 1;166(4):1070–1083. doi: 10.1084/jem.166.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Kurahara C., Young F., Kuo C. C., Xu Y., Loring J. F., Alt F. W., Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993 Mar;12(3):821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Chang C., Bradley A. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol Cell Biol. 1991 Sep;11(9):4509–4517. doi: 10.1128/mcb.11.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason C. D., Prendergast J. A., Berke G., Bleackley R. C. Peritoneal exudate lymphocyte and mixed lymphocyte culture hybridomas are cytolytic in the absence of cytotoxic cell proteinases and perforin. Eur J Immunol. 1992 Dec;22(12):3187–3190. doi: 10.1002/eji.1830221225. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Kojima H., Shinohara N., Hanaoka S., Someya-Shirota Y., Takagaki Y., Ohno H., Saito T., Katayama T., Yagita H., Okumura K. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994 Aug;1(5):357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Lancki D. W., Kaper B. P., Fitch F. W. The requirements for triggering of lysis by cytolytic T lymphocyte clones. II. Cyclosporin A inhibits TCR-mediated exocytosis by only selectively inhibits TCR-mediated lytic activity by cloned CTL. J Immunol. 1989 Jan 15;142(2):416–424. [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Kolhekar S. R., Somasundaram T., Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993 Dec;67(12):7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Kane K. P., Mescher M. F., Clark W. R. Cytotoxic T lymphocyte mediated lysis without release of serine esterase. Nature. 1987 Nov 5;330(6143):71–72. doi: 10.1038/330071a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H. Phorbol-ester stimulated lysis of weak and nonspecific target cells by cytotoxic T lymphocytes. J Immunol. 1986 Jan;136(1):23–27. [PubMed] [Google Scholar]
- Trapani J. A., Kwon B. S., Kozak C. A., Chintamaneni C., Young J. D., Dupont B. Genomic organization of the mouse pore-forming protein (perforin) gene and localization to chromosome 10. Similarities to and differences from C9. J Exp Med. 1990 Feb 1;171(2):545–557. doi: 10.1084/jem.171.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenn G., Takayama H., Sitkovsky M. V. Exocytosis of cytolytic granules may not be required for target cell lysis by cytotoxic T-lymphocytes. Nature. 1987 Nov 5;330(6143):72–74. doi: 10.1038/330072a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]
- Young L. H., Klavinskis L. S., Oldstone M. B., Young J. D. In vivo expression of perforin by CD8+ lymphocytes during an acute viral infection. J Exp Med. 1989 Jun 1;169(6):2159–2171. doi: 10.1084/jem.169.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanovello P., Rosato A., Bronte V., Cerundolo V., Treves S., Di Virgilio F., Pozzan T., Biasi G., Collavo D. Interaction of lymphokine-activated killer cells with susceptible targets does not induce second messenger generation and cytolytic granule exocytosis. J Exp Med. 1989 Sep 1;170(3):665–677. doi: 10.1084/jem.170.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Moskophidis D., Kündig T., Oehen S., Pircher H., Hengartner H. Effector T-cell induction and T-cell memory versus peripheral deletion of T cells. Immunol Rev. 1993 Jun;133:199–223. doi: 10.1111/j.1600-065x.1993.tb01517.x. [DOI] [PubMed] [Google Scholar]