Abstract
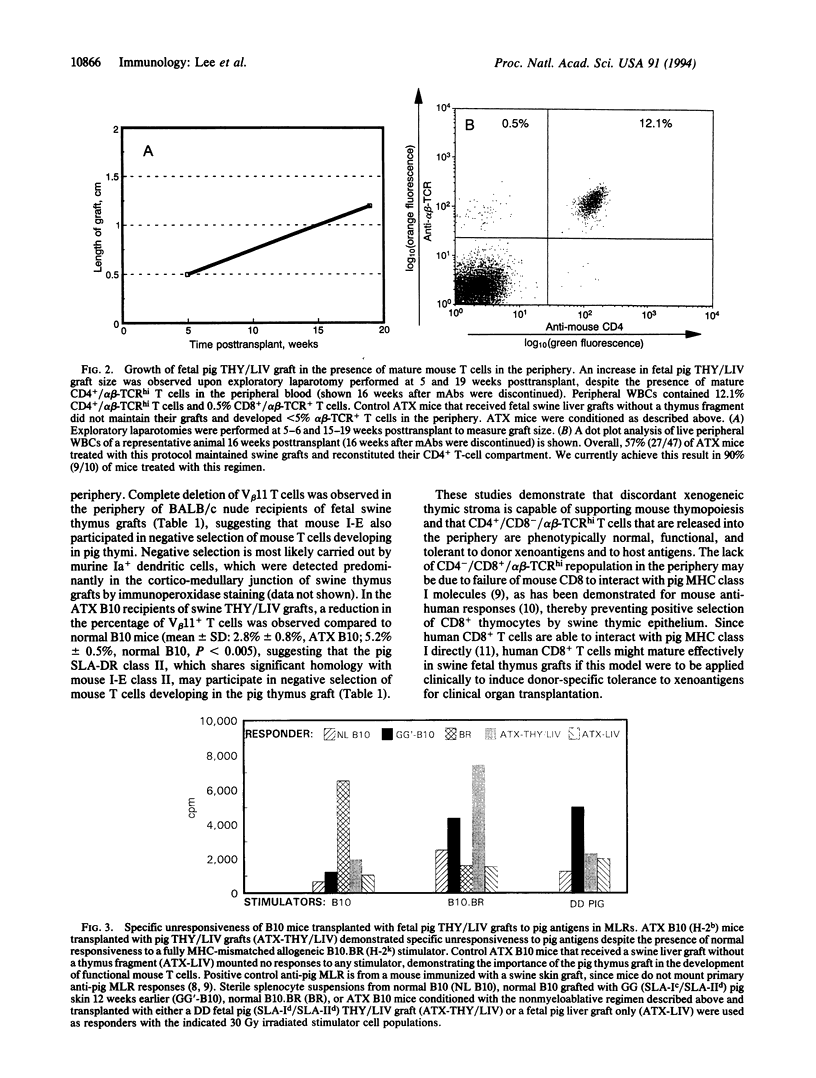
Successful induction of tolerance across disparate (discordant) species barriers could overcome the organ shortage that presently limits clinical transplantation. We demonstrate here that xenogeneic swine thymic transplants can induce tolerance to swine antigens in mice, while positively selecting functional host CD4+ T cells. Immunologically normal C57BL/10 mice were thymectomized and depleted of T and natural killer cells; then they received transplants of fetal pig thymus and liver fragments. Mature mouse CD4+ T cells developed in the pig thymus grafts and migrated to the periphery. Swine grafts grew markedly and no anti-pig IgG response was produced. Mixed lymphocyte reactions confirmed that the new T cells were functional and were tolerant to pig antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Sprent J. Strong T cell tolerance in parent----F1 bone marrow chimeras prepared with supralethal irradiation. Evidence for clonal deletion and anergy. J Exp Med. 1990 Apr 1;171(4):1101–1121. doi: 10.1084/jem.171.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsch H. A., Glaser R. M., Emery D. W., Lee L. A., Smith C. V., Sablinski T., Arn J. S., Sachs D. H., Sykes M. The importance of nonimmune factors in reconstitution by discordant xenogeneic hematopoietic cells. Transplantation. 1994 Mar 27;57(6):906–917. doi: 10.1097/00007890-199403270-00024. [DOI] [PubMed] [Google Scholar]
- Irwin M. J., Heath W. R., Sherman L. A. Species-restricted interactions between CD8 and the alpha 3 domain of class I influence the magnitude of the xenogeneic response. J Exp Med. 1989 Oct 1;170(4):1091–1101. doi: 10.1084/jem.170.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann T. R., Goldstein M. M., Goldstein H. The concurrent maturation of mouse and human thymocytes in human fetal thymus implanted in NIH-beige-nude-xid mice is associated with the reconstitution of the murine immune system. J Exp Med. 1993 Mar 1;177(3):821–832. doi: 10.1084/jem.177.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Sprent J. Tolerance of CD8+ T cells developing in parent-->F1 chimeras prepared with supralethal irradiation: step-wise induction of tolerance in the intrathymic and extrathymic environments. J Exp Med. 1993 Feb 1;177(2):367–378. doi: 10.1084/jem.177.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. J., Shearer G. M., Neudorf S., Gress R. E. The human antimurine xenogeneic cytotoxic response. I. Dependence on responder antigen-presenting cells. J Immunol. 1990 Jun 15;144(12):4548–4554. [PubMed] [Google Scholar]
- Moses R. D., Pierson R. N., 3rd, Winn H. J., Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. J Exp Med. 1990 Aug 1;172(2):567–575. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. D., Winn H. J., Auchincloss H., Jr Evidence that multiple defects in cell-surface molecule interactions across species differences are responsible for diminished xenogeneic T cell responses. Transplantation. 1992 Jan;53(1):203–209. doi: 10.1097/00007890-199201000-00039. [DOI] [PubMed] [Google Scholar]
- Salaün J., Bandeira A., Khazaal I., Calman F., Coltey M., Coutinho A., Le Douarin N. M. Thymic epithelium tolerizes for histocompatibility antigens. Science. 1990 Mar 23;247(4949 Pt 1):1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- Sharabi Y., Aksentijevich I., Sundt T. M., 3rd, Sachs D. H., Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990 Jul 1;172(1):195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D. E., Pircher H., Ohashi P. S., Kyburz D., Hengartner H., Zinkernagel R. M. Clonal deletion induced by either radioresistant thymic host cells or lymphohemopoietic donor cells at different stages of class I-restricted T cell ontogeny. J Exp Med. 1992 May 1;175(5):1277–1283. doi: 10.1084/jem.175.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]