Abstract
Background
Studies have demonstrated that self-testing for human immunodeficiency virus (HIV) is highly acceptable among individuals and could allow cost savings, compared with provider-delivered HIV testing and counseling (PHTC), although the longer-term population-level effects are uncertain. We evaluated the cost-effectiveness of introducing self-testing in 2015 over a 20-year time frame in a country such as Zimbabwe.
Methods
The HIV synthesis model was used. Two scenarios were considered. In the reference scenario, self-testing is not available, and the rate of first-time and repeat PHTC is assumed to increase from 2015 onward, in line with past trends. In the intervention scenario, self-testing is introduced at a unit cost of $3.
Results
We predict that the introduction of self-testing would lead to modest savings in healthcare costs of $75 million, while averting around 7000 disability-adjusted life-years over 20 years. Findings were robust to most variations in assumptions; however, higher cost of self-testing, lower linkage to care for people whose diagnosis is a consequence of a positive self-test result, and lower threshold for antiretroviral therapy eligibility criteria could lead to situations in which self-testing is not cost-effective.
Conclusions
This analysis suggests that introducing self-testing offers some health benefits and may well save costs.
Keywords: HIV, self-testing, cost-effectiveness, mathematical modeling, diagnostic
(See the editorial commentary by Linas on pages 513–5.)
In recent years, the scale up of antiretroviral therapy (ART) in low-income countries has been dramatic, helping transform human immunodeficiency virus (HIV) infection from a terminal illness to a chronic condition. Nevertheless, more than 50% of people living with HIV remain unaware of their HIV status [1]. They are therefore unable to benefit from HIV care and ART and to take action to reduce their risks of transmitting HIV. There are many reasons some people decide not to actively seek an HIV test, including fear of stigma discrimination, perceived lack of confidentiality, inconvenience and costs associated with accessing testing [2]. Some of these barriers relate particularly to current provider-based models of testing (eg, standard voluntary counseling and testing [VCT] and provider-initiated testing and counseling) and may be at least partially overcome through use of self-testing for HIV [3] if this were available as an alternative.
Self-testing involves individuals collecting and testing their own samples (typically saliva) without the involvement of a healthcare professional. Possible advantages of self-testing over more-standard provider-delivered HIV testing and counseling (PHTC) include greater convenience, confidentiality, and empowerment for users. Self-testing has potential to reach people who, for a variety of reasons, have chosen not to have an HIV test, using the PHTC option. Its availability may also lead to an increase in the frequency at which individuals choose to test for HIV.
A number of self-test kits remain under development by manufacturers. The costs of the kits themselves are likely to be slightly higher than for equivalent kits used in clinical settings, mainly because of the need for packaging and associated marketing. However, overall, the cost per test of self-testing is expected to be less than with PHTC, owing to a lack of healthcare worker involvement at the time of screening [4]. This needs to be balanced against possible disadvantages of self-testing, including a sensitivity that is likely to be lower than that of PHTC [5], the need for a confirmatory PHTC for a definitive HIV-positive diagnosis, the potential for linkage to posttest care to be less likely or less timely, as well as possible adverse psychological impacts due to receiving a HIV-positive result without the direct support of a trained counselor.
In 2012, the US Food and Drug Administration (FDA) approved the OraQuick In-Home HIV Test as the first HIV self-test kit. The kit is sold over the counter in the United States for $40. In low-income countries, regulated self-test kits are generally not available. Donors and stakeholders are evaluating whether investments should be made to support the development, promotion, and marketing of self-tests in low-income countries. Preliminary research conducted in Kenya and Malawi showed that acceptability and uptake is high, that accuracy is relatively good, and that there are interventions that appear to be effective in linking self-testers to HIV care [6–10].
Mathematical models offer a framework for evaluating the balance of the advantages and disadvantages of self-testing. Hence, they can provide insights into the potential net costs and health effects of self-testing at a population level over the longer term and can help determine whether introducing free or subsidized self-testing would be cost-effective. The aim of this study is to assess the potential cost-effectiveness and examine the main drivers of introducing self-testing in the general population over 20 years in a low-income countries such as Zimbabwe.
METHODS
HIV Synthesis Transmission Model
We used an updated version of the HIV Synthesis Transmission Model, an individual-based stochastic model of HIV transmission and infection progression and treatment among heterosexuals that has been previously described [11–13]. All variables in the model are updated in 3-month intervals. The model includes an age structure, and sexual risk behavior is modeled as the number of short-term partners with whom sex is condomless and the presence of a long-term partner with whom sex is condomless in each period. In any given period, the probability of an HIV-negative person having condomless sex with a partner who is HIV-positive depends on the number of sex partners of the HIV-negative partner and on the prevalence of HIV among the sex partners, accounting for patterns of age mixing. Given exposure to an HIV-positive sex partner, the probability of transmission depends on the viral load of the partner (obtained by sampling from the distribution of viral loads in partnerships formed by HIV-positive people, accounting for gender and age), on the estimated risk of transmission at that viral load, on the presence of a concurrent sexually transmitted infection, and on gender. For people who have become infected with HIV, the variables modeled include their viral load and CD4+ T-cell count, whether resistance mutations are present in their viral population, their risk of AIDS and death, whether they have tested positive for HIV, whether they are linked to care, whether they are remaining in care, and whether they are receiving treatment, if eligible. The model of progression of HIV and the effect of ART has been shown to provide a generally close fit to observed data relating to the natural progression of HIV infection and the effect of ART [12, 14]. So that observed trends in testing and the proportion of infected individuals receiving a diagnosis can be mimicked, people within the model who have not tested positive for HIV and who have access to HIV testing have an increasing chance over calendar years to be tested for HIV. In addition, pregnant women can access HIV testing through antenatal clinics. Updates to the model presented here, compared with the version of the model previously published, include age and gender-specific rates of first-time and repeat testing, including self-testing, and calibration to reflect HIV prevalence and age-specific and gender-specific levels of testing observed in Zimbabwe (Supplementary Materials) [15]. The model was programmed in SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
Scenarios Modeled
The HIV epidemic in Zimbabwe is simulated until 2015 on the basis of existing data on HIV prevalence and HIV testing [15]. From 2015, we compare two possible base case scenarios: a reference scenario, in which self-testing is not introduced and there is continued reliance on PHTC alone, and the self-testing scenario, in which self-testing is made available to the general population aged 15–65 years from 2015 onward. In the reference scenario, the rates of first-time and repeat testing increase linearly by 0.5% per year (the proportion tested for HIV in the last year is 39% in 2015, increasing up to 48% in 2035), and the rates of being linked to care, being retained in pre-ART care, and initiating ART, if eligible, remain the same as they were before 2015. One single simulation run was performed up to 2015, while several hundred runs were simulated for the two scenarios from 2015 through 2035, with means taken to limit stochastic effects (the extent of uncertainty due to stochastic effects is conveyed by providing 95% confidence limits).
The introduction of self-testing is assumed to have 3 main effects: (1) halving of the population unwilling to receive an HIV test (from 5% to 2.5%), (2) substituting 10% of first-time and 30% of repeat PHTC with self-tests, and (3) increasing the rate of first-time and repeat testing by 20%, owing to the availability of self-testing. The availability of self-testing is not assumed to affect PHTC in antenatal care settings. These assumptions are based on limited current evidence but, overall, are believed to be conservative in estimating the potential benefits of self-testing.
Additional Assumptions
The sensitivity and specificity of self-testing were assumed to be 92% and 99%, respectively, as observed in the studies leading to FDA approval of the OraQuick In-Home HIV Test [16]. Variability across studies has been observed in the sensitivity of the OraQuick In-Home HIV Test, mainly reflecting the models and approaches used for HIV self-testing and the population of individuals who tested for HIV. High accuracy (99.2%) has been found both in studies that offered supervised self-testing [6] and studies characterized by a lower level of supervision [10, 17, 18]. To our knowledge, only one study reported a lower accuracy than that identified in the OraQuick FDA studies [19], and this was due to the lack of information given on how to interpret a faint positive result and to the fact that people receiving ART were self-testing. It is known that ART may decrease the overall sensitivity of oral fluid-based self-testing [19, 20]. In this analysis, we assumed that only people who had an unknown HIV status (and were therefore not receiving ART) could use self-testing.
PHTC was considered more accurate, with a sensitivity estimated to be 98% [21] and a specificity of 100% (given confirmatory testing, when a person is found to be HIV positive, it is unlikely that a person receives a diagnosis of HIV infection if they are not infected).
Once a person receives a positive self-test result, a confirmatory PHTC was assumed to be necessary to consider a person as having a diagnosis of HIV infection, as well as the possibility of linking that person to care (ie, where they receive an ART-eligibility assessment) and eventually initiating ART. The probability of a confirmatory PHTC by 1 year following a positive self-test result was assumed to be 0.8, based on evidence that, in the context of supervised self-testing, most people disclose to their counselor [10]. Once the person receives a diagnosis (following a positive PHTC result), the probability of being linked to care was assumed to be 0.6 within 1 year after diagnosis [22], regardless of whether they initially underwent self-testing or PHTC. No change in the frequency of condomless sex among people who tested negative for HIV was assumed, whereas those who tested positive for HIV reduced the frequency of condomless sex with casual partners by 17% in the first 6 months (and by 9% afterward) and by 13% with long-term partners, regardless of the type of HIV test. In the context of VCT, there is evidence that this is the case [23], and in the base case we assume that this holds for self-testing, although we are assuming quite a small effect. People were assumed to be eligible for ART when their CD4+ T-cell count decreased to below 500 cells/mm3 in the base case, in line with guidelines in Zimbabwe [24] and from the WHO [25]. Implementation of prevention of mother-to-child-transmission option B+ (ie, provision of lifelong ART for pregnant and breast-feeding women) was not taken into account.
Economic Analysis
The two scenarios (the reference scenario and the self-testing scenario) were compared on the basis of their costs and health outcomes, which are both discounted to present values at 3% per annum, from a healthcare perspective [26]. Costs were estimated on the basis of resource use (eg, number of tests and number of clinic visits) and associated unit costs. The fully loaded cost of a PHTC was assumed to be $9 for a negative test result and $25 for a positive test result or a confirmatory test following a positive self-test result, so that the average cost was around $10 [27]. The fully loaded cost of self-testing was assumed to be $3 per unit. Health outcomes were summarized in the form of disability-adjusted life-years (DALYs) averted, which captures changes in both mortality and morbidity, including the impact on onward HIV transmission (Supplementary Materials) [28].
Results are presented across a range of cost-effectiveness thresholds (CETs) from $0 (an extreme case, implying a health system would only be concerned with reducing costs) to $10 000 (a relatively high threshold only likely to be relevant in well-financed health systems with full coverage of interventions offering health gains at less than this amount).
Costs and health outcomes were rescaled to provide figures relevant to the entire adult population (15–65 years old) of Zimbabwe. Because of the stochastic variation inherent in the model, a high number of simulations were required, so figures are presented on a discrete scale, rather than a continuous scale.
Sensitivity Analyses
The following parameters were varied in univariate sensitivity analyses: the cost of self-testing, the sensitivity of self-testing, the probability of PHTC as a direct consequence of a positive self-test, the probability of being linked to care by 1 year after a positive PHTC result (whether prompted by a self-testing or not), and the reduction in the frequency of condomless sex for those receiving a diagnosis on the basis of a positive self-test result, eligibility criteria to initiate ART, the magnitude of the increase in the rates of first and repeat testing due to the introduction of self-testing, and the level of substitution of self-testing for PHTC. The most determinant parameters of cost-effectiveness were also varied in multivariate analyses.
RESULTS
Introduction of self-testing, as assumed in the base case scenario, led in 2035 to a 7% higher proportion tested for HIV in the past year, compared with the reference scenario (57% vs 50%; Table 1). The total discounted costs over 20 years for HIV testing and resulting HIV care and ART under the reference scenario base case assumptions is estimated to be almost $3.1 billion (Figure 1). Of this, 40% is attributable to the cost of antiretroviral drugs, around a quarter to the cost of clinic visits, and 24% to the cost of PHTC. Over a 20-year period, the self-testing scenario is predicted to lead to savings of $75 million (95% confidence interval [CI], $73 million–$77 million), for a cost reduction of 2.6%, due primarily to lower testing costs. The introduction of self-testing would also result in health gains, with approximately 7000 DALYs averted (95% CI, 700–13 000 DALYs averted; Table 2).
Table 1.
Predictions Over Time in the Reference Scenario and the Self-Testing Scenario
| Variable | 2011 DHS Dataa | Model Estimate |
||||
|---|---|---|---|---|---|---|
| Baseline | Reference Scenario |
Self-Testing Scenario |
||||
| 2015 | 2025 | 2035 | 2025 | 2035 | ||
| HIV prevalence, % | 15 | 13 | 10 | 7 | 10 | 7 |
| Ever tested for HIV, % | 50 | 66 | 77 | 79 | 80 | 83 |
| Tested for HIV in the past year, % | 28 | 39 | 48 | 50 | 54 | 57 |
| Received HIV infection diagnosis, % | … | 85 | 91 | 93 | 92 | 93 |
| HIV-positive patients receiving ART, % | … | 56 | 66 | 69 | 66 | 69 |
Model estimates are median values of all simulations.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
a Demographic and Health Surveys (DHS) [15].
Figure 1.
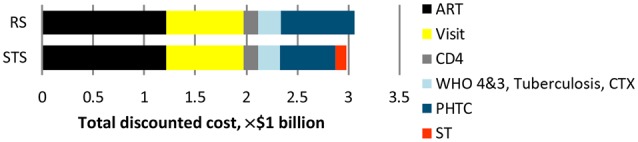
Total discounted cost over 20 years. Abbreviations: ART, antiretroviral therapy; CD4, CD4+ T-cell count; CTX, cotrimoxazole; PHTC, provider-delivered human immunodeficiency virus testing and counseling; RS, reference scenario; ST, self-testing; STS, self-testing scenario; WHO 3/4, treatment of World Health Organization stage 3 and 4 human immunodeficiency virus disease [29].
Table 2.
Cost-effectiveness for the Self-Testing Scenario (STS) and Reference Scenario (RS) Under Base Case Assumptions and Alternative Assumptions and According to Cost-effectiveness Threshold
| Variable | Cost-effectiveness Threshold, $ |
Total Discounted Change in Costs, × $1 Million, Compared With RS (95% CI) | Discounted DALYs Averted, ×1000, Compared With RS (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 500 | 1000 | 5000 | 10 000 | |||
| Base casea | STS | STS | STS | STS | STS | −75 (−77 to −73) | 7 (.7–13) |
| Cost of ST, $9 (base case: $3) | RS | RS | RS | RS | RS | 136 (134–137) | 7 (.7–13) |
| Sensitivity of ST, 0.55 (base case: 0.92) | STS | STS | STS | STS | RS | −81 (−84 to −79) | −11 (−22 to −.5) |
| Probability of PHTC as a direct consequence of a positive ST, 0.37 (base case: 0.8) | STS | STS | STS | STS | STS | −87 (−90 to −84) | 0.1 (−11 to 11) |
| Linkage to care following diagnosis for those who had a ST of 0.4 by 1 y (base case: 0.6) | STS | STS | STS | RS | RS | −105 (−112 to −97) | −19 (−32 to −6) |
| ART initiation at CD4 <350 cells/mm3 (base case: CD4 <350 cells/mm3 without introduction of ST) | STS | STS | STS | RS | RS | −69 (−74 to −64) | −21 (−40 to −2) |
| No reduction in condomless sex following a positive ST (base case: as PHTC) | STS | STS | STS | STS | STS | −74 (−77;−70) | 19 (6–33) |
| Increase in rate of first test due to ST (base case: 20%) | |||||||
| 2.5% | STS | STS | STS | STS | STS | −82 (−85 to −79) | 10 (−.6 to 20) |
| 7.5% | STS | STS | STS | STS | STS | −81 (−85 to −78) | 13 (−.6 to 26) |
| Increase in rate of repeat test due to ST (base case: 20%) | |||||||
| 2.5% | STS | STS | STS | STS | STS | −102 (−105 to −99 | 4 (−6 to 14) |
| 7.5% | STS | STS | STS | STS | STS | −82 (−86 to −78) | 13 (−1 to 26) |
| Substitution (base case: 30% for repeat test, 10% for first test) | |||||||
| 5% for repeat test, 2% for first test | RS | RS | STS | STS | STS | 38 (34–41) | 39 (20–57) |
| 15% for repeat test, 5% for first test | STS | STS | STS | STS | STS | −12 (−17 to −7) | 22 (5–39) |
| 25% for repeat test, 8% for first test | STS | STS | STS | STS | STS | −52 (−57 to −47) | 20 (.5–39) |
The discounted total cost of the RS over 20 years from 2015 is $3168 million.
Abbreviations: ART, antiretroviral therapy; CD4, CD4+ T-cell count; CI, confidence interval; DALY, disability-adjusted life-year; PHTC, provider-delivered human immunodeficiency virus testing and counseling; ST, self-testing.
a See the “Scenarios Modeled” subsection in “Methods” section.
Table 2 summarizes increments in total discounted costs and total discounted DALYs averted with the self-testing scenario, compared with the reference scenario, along with the cost-effectiveness of self-testing, under the base case and alternative assumptions in sensitivity analyses. This is provided for different CETs. In general, for any scenario in which DALYs are averted, the probability that self-testing is cost-effective will increase with increasing CETs, because at some point the cost of the DALYs averted becomes affordable. In contrast, for scenarios that lead to a loss in DALYs, self-testing is less likely to be cost-effective with increasing CETs because at some point the money saved from the self-testing scenario is not worth the loss in DALYs. The results are particularly driven by the cost of self-testing itself. If the cost of self-testing is the same as that of a negative result of a PHTC-associated test ($9), it is not cost-effective at a CET of $10 000 per DALY averted or less because it results in higher costs ($136 million more than under the reference scenario) but relatively small health gains. If, however, self-testing were to cost $4 or less, then the self-testing scenario would be cost-effective even at a CET of $500, keeping other base case assumptions fixed (Figures 3 and 4).
Figure 3.
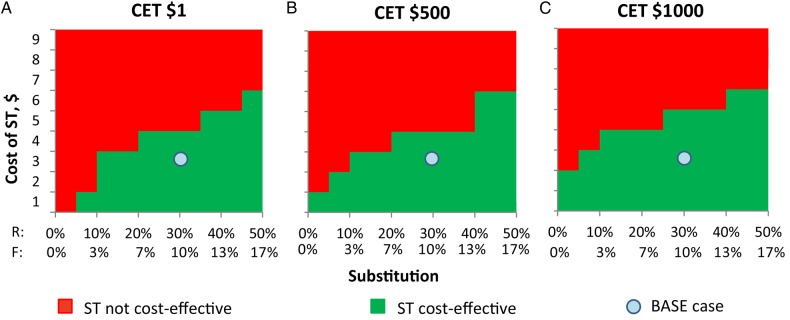
Cost-effective scenario under different assumptions of substitution and cost of self-testing (ST). Abbreviations: CET, cost-effectiveness threshold; F, substitution of provider-delivered human immunodeficiency virus testing and counseling with self-testing during first test; R, substitution of provider-delivered human immunodeficiency virus testing and counseling with ST during repeat test.
Figure 4.
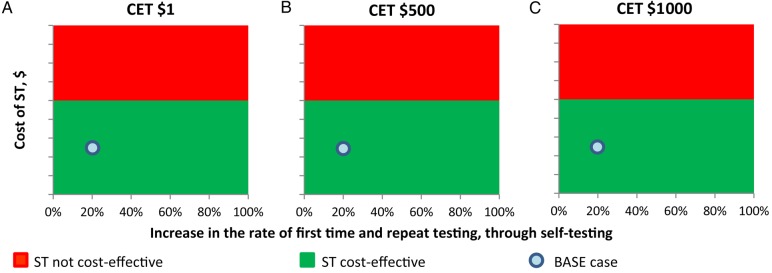
Cost-effective scenario under different assumed increases in the rates of first test and repeat test and cost of self-testing (ST). Abbreviation: CET, cost-effectiveness threshold.
The level of substitution of PHTC with self-testing affects the results in a slightly counterintuitive way (Figure 2). At all CETs considered ($1–$1000), the lower cost self-testing is preferred even when the probability of PHTC following a positive self-test result (and therefore the chance of receiving a diagnosis) is low; this is the case even at relatively high CETs, because even if the probability of PHTC as a direct consequence of a positive self-test is low and the level of substitution high, the cost savings outweighs the loss in health. Self-testing is clearly more likely to be cost-effective as the cost of self-testing decreases (Figures 3 and 4).
Figure 2.
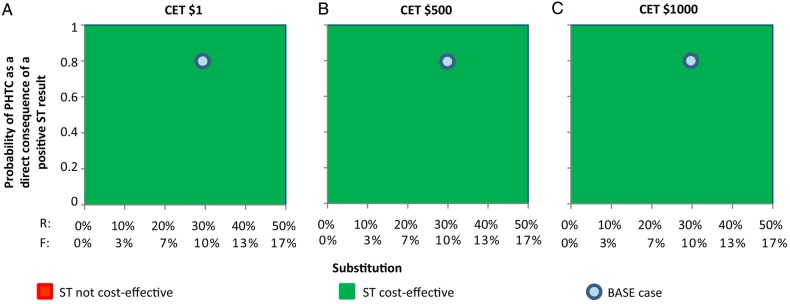
Cost-effective scenario under different assumptions of substitution and probability of provider-delivered human immunodeficiency virus testing and counseling (PHTC) as a direct consequence of a positive self-testing (ST). Abbreviations: CET, cost-effectiveness threshold; F, substitution of PHTC with self-testing during first test; R, substitution of PHTC with self-testing during repeat test.
Regarding the levels of increase in the frequencies of undergoing an initial test and a repeat test due to the availability of self-testing (Figure 4), we found that the greater the levels of increase, the greater the total cost; however, this is counterbalanced by the dramatic cost savings due to the partial substitution of PHTC with self-testing.
DISCUSSION
Self-testing holds the promise of expanding the reach of HIV testing. It may enable some people to test who would not otherwise choose PHTC because of stigma, confidentiality, cost, or other barriers to access. It may also increase the overall frequency of testing in the population, owing to greater convenience. As the basis for affirming HIV status, leading to linkage to care and receipt of ART for those eligible, as well as prompting individuals who learn they are HIV-positive to reduce the risks of onward HIV transmission to others, the potential health gains from self-testing are clear. What is more, self-testing may also reduce costs, enabling savings in HIV testing programs that can be reinvested in further expansion of testing or in delivery of other HIV- and healthcare-related interventions.
Overall, under our base case assumptions, we estimate that the introduction of self-testing will allow savings of around $75 million over 20 years in Zimbabwe, with a low number of DALYs averted (7000, in the context of an adult population of 7.5 million in mid-2013). If these assumptions hold in other settings, self-testing should always be introduced, regardless of how poorly or well resourced a health system is, because it leads to a win-win situation involving better health outcomes at reduced cost. If we assume a CET of $500 (similar to Zimbabwe's gross domestic product per capita in 2012), the $75 million savings could be used to avert at least 150 000 DALYs by introduction of interventions with incremental cost-effectiveness ratios of $500 or less per DALY averted. The monetary value of introducing self-testing to Zimbabwe (ie, the net monetary benefit, calculated as follows: [health gains × CET] + cost savings [30]) would be $78.5 million over 20 years, indicating huge benefits associated with supporting the availability of self-testing in the country.
However, the population costs and health effects of self-testing depend on a range of complex and interacting factors, and there are plausible scenarios with characteristics such as an inadequate cascade of care following self-testing that would result in worse outcomes than those of the reference scenario.
Our sensitivity analyses provide insight into what determines the magnitude of population health gains and suggest what programmatic recommendations may support optimal implementation of self-testing. First, the cost of self-testing relative to that of PHTC appears to be the most important factor determining cost-effectiveness, particularly at lower CETs. The OraQuick HIV test kit designed for clinical use was sold for $4 in low-income countries in 2005 [31]. Lower-cost alternatives to self-testing would lead to additional gains if these were to become available. The cost of distributing self-tests cannot be ignored, but it is expected to be significantly lower than the cost of providing PHTC. To the best of our knowledge, data on the cost of rolling out self-testing are not currently available, and the only study conducted in sub-Saharan Africa evaluating the uptake of self-testing distributed the kit through community counselors [8]. Second, we evaluated the impact of introducing self-testing in addition to PHTC in a setting such as Zimbabwe, with a high level of HIV diagnosis and ART coverage; in 2015, before the introduction of self-testing, an estimated 85% of people with HIV receive a diagnosis. It is likely that the impact of introducing self-testing would be even greater as the proportion of people with HIV infection that is undiagnosed increases. Third, in some circumstances, self-testing may lead to improved health outcomes but also to higher costs, compared with the reference scenario, if the overall level of testing (ie, the increase in the rate of first-time and repeat testing) increases dramatically because of the availability of self-testing. The few studies conducted so far on uptake of self-testing have found it to be extremely acceptable [7–10]. Studies in Malawi, for example, have shown that 76% of adults avail themselves of self-testing by 12 months following its introduction [10]. In settings with higher CETs, the potential for health improvement will usually outweigh higher costs. Appropriate targeting of self-testing, for example, to populations afflicted by a higher HIV prevalence or that are known to be less likely to test for HIV, such as men in many countries, is likely to be necessary in such situations. There is also a risk in some circumstances that self-testing may lead to worse health outcomes, such as at CETs of $5000 or more, if ART initiation occurs at a CD4+ T-cell count of <350 cells/μL or if the linkage to care following a diagnosis prompted by a positive self-test is low. This suggests that, to be translated into health gains, self-testing needs to be accompanied by proactive interventions to support a continuum of care following diagnosis. A study in Malawi [8] investigated one scheme that could provide complementary benefits—offering ART initiation at home among people who self-test—and found that this significantly increased the probability of initiating ART within 6 months. Finally, there is always potential for unintended adverse consequences associated with any public health intervention, so countries should closely monitor their experiences with introducing self-testing. Linkage to care, promoting counseling services, and reporting of test outcomes need to be strengthened in support of such public health interventions.
In conclusion, our results suggest that the introduction of self-testing may not only be cost-effective, but also may be cost saving under the assumptions described in the base case scenario. Under these assumptions, self-testing should be made available even in settings where resource-constraints are greatest. Notably, in some circumstances, it may be necessary to target self-testing to individuals who have certain risk factors.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the University College London Legion High Performance Computing Facility (Legion@UCL) and associated support services, for assistance in the completion of this work; and Cheryl Johnson, Stefano Bertozzi, Michael Borowitz, Charlene Brown, Augustine Choko, Liz Corbett, Cari Courtenay-Quirk, Frances Cowan, Geoff Garnett, Hendy Maheswaran, Jennifer Osborne, Christine Rousseau, and Mickey Urdea, for insightful comments on the analysis plan and preliminary results and useful discussion on this topic.
Disclaimer. The views expressed are not necessarily those of the UK Department for International Development.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (global health grant OPP1064862) and the UK Department for International Development (via the Lablite Project, to D. F. and P. R.).
Potential conflicts of interest. A. P. has received research funds from Bristol Myers Squibb and the World Health Organization and has received payment for consulting work from Gilead Sciences and GSK Biologicals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Staveteig S, Wang S, Head SK, Bradley SEK, Nybro E. Demographic patterns of HIV testing uptake in Sub-Saharan Africa. DHS Comparative Reports 30 Calverton, MD: ICF International, 2013. [Google Scholar]
- 2.WHO/UNAIDS. Guidance on provider-initiated HIV testing and counselling in health facilities. Geneva: World Health Organization, 2007. http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf. Accessed 12 October 2013. [Google Scholar]
- 3.Report on the first international symposium on self-testing for HIV: the legal, ethical, gender, human rights and public health implications of HIV self-testing scale-up. Geneva: World Health Organization, 2013. [Google Scholar]
- 4.Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis 2013; 57:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med 2013; 10:e1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choko AT, Desmond N, Webb EL, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med 2011; 8:e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett EL. Uptake, accuracy and linkage into care following access to community-based self-testing for HIV in Malawi. Presented at: The legal, ethical, gender, human rights and public health implications of self-testing scale-up, Geneva, Switzerland, 8–9 April 2013. [Google Scholar]
- 8.MacPherson P, Lalloo D, Choko A, et al. Home assessment and initiation of ART following HIV self-testing: a cluster-randomised trial to improve linkage to ART in Blantyre, Malawi. 2013.
- 9.Kalibala S, Tun W, Muraah W, Cherutich P, Oweya E, Oluoch P. Knowing myself first’: feasibility of self-testing among health workers in Kenya. Nairobi, Kenya: Population Council, 2011. http://www.popcouncil.org/pdfs/2011HIV_KenyaHWSelfTesting.pdf. Accessed 5 April 2013. [Google Scholar]
- 10.Choko AT, MacPherson P, Webb EL, et al. One year outcomes following community-based HIV self-testing: a prospective study in Malawi. Presented at: 21st Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 3–6 March 2014. [Google Scholar]
- 11.Cambiano V, Bertagnolio S, Jordan MR, Lundgren JD, Phillips A. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J Infect Dis 2013; 207(suppl 2):S57–62. [DOI] [PubMed] [Google Scholar]
- 12.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet 2008; 371:1443–51. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Pillay D, Garnett G, et al. Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS 2011; 25:843–50. [DOI] [PubMed] [Google Scholar]
- 14.Phillips AN, Sabin C, Pillay D, Lundgren JD. HIV in the UK 1980–2006: reconstruction using a model of HIV infection and the effect of antiretroviral therapy. HIV Med 2007; 8:536–46. [DOI] [PubMed] [Google Scholar]
- 15.Zimbabwe National Statistics Agency, MEASURE DHS, ICF Macro. Zimbabwe Demographic and Health Survey 2010–11: preliminary report. Harare, Zimbabwe: Zimbabwe national Statistics Agency, 2011. [Google Scholar]
- 16.US Food and Drug administration. First rapid home-use HIV kit approved for self-testing, 2012. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm310545.htm. Accessed 5 March 2013.
- 17.Asiimwe S, Oloya J, Song X, Whalen CC. Accuracy of un-supervised versus provider-supervised self-administered HIV testing in Uganda: a randomized implementation trial. AIDS Behav 2014; 18:2477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaydos CA, Hsieh YH, Harvey L, et al. Will patients "opt in" to perform their own rapid HIV test in the emergency department? Ann Emerg Med 2011; 58(1 suppl 1):S74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pant Pai N, Behlim T, Abrahams L, et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS One 2013; 8:e79772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaspard M, Le MG, Saberan-Roncato M, et al. Finger-stick whole blood HIV-1/-2 home-use tests are more sensitive than oral fluid-based in-home HIV tests. PLoS One 2014; 9:e101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pant Pai N, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:373–80. [DOI] [PubMed] [Google Scholar]
- 22.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonner VA, Denison J, Kennedy CE, O'Reilly K, Sweat M. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev 2012; 9:CD001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Medicine and Therapeutics Policy Advisory Committee (NMTPAC) and The AIDS and TB Directorate Ministry of Health and Child Care, Zimbabwe. Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe. Harare, Zimbabwe: NMTPAC, 2013. [Google Scholar]
- 25.World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO, 2013. http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html Accessed 15 July 2013. [PubMed] [Google Scholar]
- 26.Edejer T-T, Baltussen R, Hutubessy HR, Acharya A, Evans DB, Murray CJL. Making choices in health: WHO guide to cost effectiveness analysis. Geneva: World Health Organization, 2003. http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 15 October 2013. [Google Scholar]
- 27.Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Global Health 2014; 2:e23–34. [DOI] [PubMed] [Google Scholar]
- 28.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012; 380:2129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO). WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: WHO, 2007. http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf Accessed 8 July 2013. [Google Scholar]
- 30.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford, United Kingdom: Oxford University Press, 2005. [Google Scholar]
- 31.UNICEF, United Nations Programme on HIV/AIDS, WHO, MSF. Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS. 76th ed. Geneva: WHO, 2005. http://www.unaids.org/en/media/unaids/contentassets/dataimport/publications/irc-pub02/jc645-sources_prices_en.pdf. Accessed 25 October 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


