Abstract
Background
Cell-culture-derived (CC) influenza vaccine production methods could provide benefits over classical embryonated-egg technology, including a higher production capacity and the faster creation of a supply that meets demand.
Methods
A CC-inactivated split-virus influenza A/Indonesia/5/2005(H5N1) vaccine derived from the EB66 cell line (hereafter, “CC-H5N1”) was investigated in a phase 1 randomized, blinded study. Healthy adults (n = 521) received 2 vaccine doses (days 0 and 21) of either investigational CC-H5N1 vaccine (1.9 µg or 3.75 µg of hemagglutinin antigen [HA] with the AS03 adjuvant system or 15 µg of plain HA), embryonated-egg-derived vaccines (3.75 µg of HA with AS03 or 15 µg of plain HA), or placebo. Assessment of the adjuvant effect and immunogenicity was performed using Center for Biologics Evaluation and Research acceptability criteria 21 days after dose 2. Safety was assessed until month 12.
Results
AS03-adjuvanted CC-H5N1 elicited a homologous hemagglutination inhibition antibody response that satisfied immunogenicity criteria 21 days after dose 2 and persisted at month 12. Adjuvant effect and immune response against a drift-variant strain were demonstrated. No vaccine-related serious adverse events were reported. The immunogenicity and safety of the CC-H5N1 formulation containing 3.75 µg of HA and AS03 appeared to be similar to those for the licensed egg-derived AS03-adjuvanted control vaccine.
Conclusions
The feasibility of the EB66 cell line to produce an immunogenic influenza vaccine with acceptable safety profile was demonstrated. Antigen sparing was achieved through combination with AS03 adjuvant. This CC-H5N1 might contribute to the rapid access of vaccine in the event of an influenza A(H5N1) pandemic.
Clinical Trials Registration
Keywords: influenza A(H5N1), pandemic influenza vaccine, AS03, cell culture
Influenza pandemics occur when a large proportion of the global population is immunologically naive to an emerging, transmissible influenza virus strain and may result in great loss of life [1]. While the impact of influenza pandemics could be attenuated by the prompt and widespread use of effective vaccines [2], vaccine production capacity is heavily constrained by current technology. Traditionally, influenza vaccines licensed in the United States have been manufactured using embryonated hen eggs, the limitations of which were highlighted by the 2009 influenza A(H1N1) pandemic: at least 6 months are needed for development of a new egg-based vaccine, by which time the peak of a pandemic may have already been reached, and vaccine supply is not able to meet global demand [3]. Consequently, several manufacturers have developed or are investigating production of influenza vaccines in cell culture (CC). Potential benefits of CC-derived influenza vaccines over manufacturing processes using eggs include independence from the need for a readily available supply of high-quality eggs, increased manufacturing flexibility, existing cell banks allowing immediate start of production, and the possibility of a simpler, more streamlined scale-up [4, 5].
The influenza A(H5N1) strain is a highly pathogenic avian influenza virus with pandemic potential [2]. Influenza A(H5N1) vaccines are poorly immunogenic without adjuvant, and the influenza A(H5N1) hemagglutinin antigen (HA) dose required to induce acceptable levels of humoral immunity in adults is 6 times that typically administered in seasonal influenza vaccines [6]. Egg-derived inactivated split-virion recombinant influenza A(H5N1) vaccines with the proprietary AS03 adjuvant system induced broad clade and subclade cross-reactivity and improved immunogenicity, compared with unadjuvanted formulations, allowing administration of lower HA doses [7–12]. GlaxoSmithKline's (GSK's) egg-derived influenza A(H5N1)–AS03 vaccines are licensed for use in adults aged ≥18 years in the European Union, the United States, and elsewhere.
To allow a rapid and flexible response in the event of an influenza A(H5N1) pandemic, GSK Vaccines has developed a CC-inactivated split-virus influenza A/Indonesia/5/2005(H5N1) vaccine (hereafter, “CC-H5N1”), using the EB66 cell line. The safety and immunogenicity of different formulations of CC-H5N1 and the benefit of the AS03 adjuvant were evaluated in a phase 1 study conducted in healthy adults.
METHODS
Study Design and Objectives
The study (clinical trials registration NCT01236040) was conducted according to good clinical practice and the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by a commercial independent institutional review board (Chesapeake Research Review; Columbia, Maryland). Written informed consent was obtained from participants prior to enrollment. This observer-blinded, randomized, controlled study was conducted in 4 US centers between 29 November 2010 and 20 August 2012.
The coprimary immunogenicity objectives were to demonstrate the adjuvant effect of AS03 and the immunogenicity of AS03A-adjuvanted CC-H5N1 containing 3.75 µg of HA 21 days after dose 2, using Center for Biologics Evaluation and Research (CBER) acceptance criteria for pandemic influenza vaccine candidates [13]. Secondary immunogenicity objectives were to characterize immunogenicity in terms of hemagglutinin inhibition (HI) and neutralizing antibodies (NAbs), antibody persistence to month 12, and the immune response to a drift variant strain and to contrast the immunogenicity of CC-H5N1 to that of an egg-derived vaccine (Q-H5N1; at similar antigen doses and with or without adjuvant).
The coprimary safety objective was to evaluate the reactogenicity of CC-H5N1 in terms of grade 3 injection site pain ≤7 days after any vaccination. Reactogenicity of the study vaccines and safety up to 12 months after vaccination were assessed as secondary objectives.
Enrollment was conducted in 2 waves to allow refinement of dose selection on the basis of wave 1 data. Recruitment was staggered to allow review of accrued safety data by an independent safety review committee.
Randomization was performed at GSK Vaccines, using MATEX in SAS (Cary, North Carolina). A randomization blocking scheme ensured that balance between vaccine groups was maintained. Treatment allocation at the investigator site was performed using an Internet-based randomization system, with study center and history of previous seasonal or pandemic A(H1N1)pdm09 influenza vaccination within the last 3 years as minimization factors. Owing to the difference in appearance of the study vaccines, the study was conducted with blinding of observers, meaning that study staff who administered the vaccines were not involved in assessment of study outcomes.
Study Subjects
Participants were healthy adults (age, 18–49 years) who satisfied baseline medical assessment by history, physical examination, and assessment of laboratory parameters. Participants were excluded if they had a history of influenza A(H5N1) infection, were at risk of occupational influenza A(H5N1) exposure, or if they had received previous vaccination with any influenza A(H5N1) vaccine or oil-in-water adjuvanted influenza vaccine. Other exclusion criteria included significant acute or chronic, uncontrolled illness; coagulation disorders; evidence of current substance abuse; cancer; suspected immunodeficiency; receipt of systemic glucocorticoids within 1 month or any other cytotoxic or immunosuppressive drug within 6 months of enrollment; receipt of immunoglobulins/blood products within 3 months of enrollment; or a history of Guillain-Barré syndrome. Participants who received inactivated seasonal influenza vaccination within 14 days before the first study dose or had a history of allergy or hypersensitivity to any vaccine component were also excluded. Female participants of childbearing potential had a negative pregnancy test result at vaccination and practiced adequate contraception from 30 days prior to vaccination until 2 months after completion of the vaccination. Female participants who were pregnant or lactating were excluded.
Vaccines
All subjects received 2 doses of vaccine administered 21 days apart. Wave 1 participants were equally assigned to one of 4 study groups: CC-3.75 µgHA + AS03A (a cell-culture-derived formulation with 3.75 µg of HA plus AS03A), CC-15 µg PlainHA (a cell-culture-derived formulation with 15 µg of HA without adjuvant), Q-15 µgPlainHA (an egg-derived formulation with 15 µg of HA without adjuvant), or placebo (saline). Wave 2 participants were equally assigned to one of 4 study groups: CC-3.75 µgHA + AS03A, CC-1.9 µgHA + AS03B (a cell-culture-derived formulation with 1.9 µg of HA plus AS03B), Q-3.75 µgHA + AS03A (an egg-derived formulation with 3.75 µg of HA plus AS03A), or placebo. All vaccines were given intramuscularly into the deltoid muscle, alternating sides for each dose.
All vaccines were manufactured by GSK Vaccines. The split virion vaccines were produced using an inactivated A/Indonesia/05/2005 (IBCDC-RG2) clade 2.1 strain (Centers for Disease Control and Prevention, Atlanta, Georgia). The CC-H5N1 candidates were manufactured in Belgium using EB66 as substrate. Egg-derived vaccines were produced in Quebec, Canada.
AS03A and AS03B are GSK Vaccines' proprietary Adjuvant Systems, manufactured in Rixensart, Belgium, and contain α-tocopherol (11.86 mg and 5.93 mg, respectively) and squalene in an oil-in-water emulsion.
Immunogenicity Assessment
Humoral immunity was assessed on days 0, 21, and 42 and 6 and 12 months after vaccination. HI antibodies to A/Indonesia/5/2005 (all samples) and to clade 1 strain A/Vietnam/1194/2004 (subset of samples) were measured as previously described but using horse erythrocytes rather than avian erythrocytes [14–17]. The titration end point was the highest dilution that completely inhibited hemagglutination. The lowest dilution tested was 1:10. HI antibody titers of ≥1:40 were considered seroprotective.
NAbs to A/Indonesia/05/2005 and A/Vietnam/1194/2004 were measured in a subset of samples, using methods described previously [14, 16]. In brief, a standardized amount of the reverse-genetics-derived virus was mixed with serial 2-fold dilutions of serum samples to allow antibody/virus binding. The mixture containing bound antibody was added to Madin-Darby canine kidney cell (Novartis) cultures and incubated for seven days at 33°C. Viral replication was visualized as hemagglutination of chicken red blood cells. The 50% neutralization titer of a serum specimen was calculated by the method of Reed and Muench [18]. The assay cutoff was 1:28. Results with a titer of <1:28 were coded as 1:14 for calculation of the geometric mean titers (GMTs).
Fifty samples in each treatment group were selected for the immunogenicity subsets: a random selection of 25 participants from each wave in the CC-3.75 µgHA + AS03A and placebo groups, all wave 1 participants in the CC-15 µgPlainHA and Q-15 µgPlainHA groups, and a random selection of 50 wave 2 participants in the CC-1.9 µgHA + AS03B and Q-3.75 µgHA +AS03A groups.
Safety and Reactogenicity Assessment
Injection site (pain, redness, and swelling) and general symptoms (body temperature, headache, fatigue, joint pain, muscle aches, shivering, increased sweating, and gastrointestinal symptoms) were recorded for 7 days after each dose, and any other adverse events (AEs) until 21 days after dose 2, on diary cards. Serious AEs (SAEs), potential immune-mediated diseases (pIMDs), and medically attended AEs (MAEs: defined as hospitalization, an emergency department visit, or a visit to or from medical personnel for any reason) were recorded throughout the study until the month 12 contact. AEs were graded on a 3-point scale, in which 0 was defined as absent; 1, as mild; 2, as moderate; and 3, as severe. All injection site symptoms were considered to be vaccine related. Potential causal relationships between vaccination and other symptoms or events were determined by the site investigator.
Hematological and biochemical analyses were performed on blood samples collected on days 0, 7, 21, 28, and 42 and month 6.
Statistical Methods
The primary cohort for the assessment of immunogenicity was the per-protocol cohort, which included all eligible participants who complied with protocol-defined procedures, received 2 doses of study vaccine, and had baseline vaccine-homologous HI results. Adjuvant effect was demonstrated in Wave 1 if, 21 days after dose 2 the lower limit of the 95% confidence intervals (CIs) for the GMT ratio for the CC-3.75 µgHA + AS03A group over the CC-15 µgPlainHA group was >1.0 and if the lower limit of the 95% CI for the difference in seroconversion rate (SCR) between groups was >0. Antigen sparing was demonstrated if the CC-3.75 µgHA +AS03A formulation induced a vaccine-homologous HI response that met CBER acceptance targets 21 days after the second dose in wave 1 (ie, the lower limit of the 95% CI for the SCR was ≥40%, and the lower limit of the 95% CI for the seroprotection rate [SPR] was ≥70%).
The notional SPR was defined as the percentage of participants with a vaccine-homologous HI antibody titer of ≥1:40. The SCR was defined as the percentage of initially seronegative participants with a postvaccination titer of ≥1:40 or a ≥4-fold increase in titer in initially seropositive participants. A vaccine response for serum NAb titers was defined as a ≥4-fold increase in the postvaccination titer relative to day 0.
An analysis performed using day 42 HI immunogenicity and safety data after wave 1 was used to guide dose selection for wave 2. Where applicable, results show the final analysis from both waves.
With 48 evaluable participants in the CC-3.75 µgHA +AS03A group during wave 1, the study had ≥93% power to meet the coprimary immunogenicity objectives, assuming a SPR and SCR each of 90.8%. With 50 enrolled subjects in the CC-3.75 µgHA + AS03A group during wave 1, the study had 80.0% probability of detecting at least 1 adverse event (grade 3 pain) if the true occurrence rate was 3.2%. Analyses were performed using SAS software, version 9.1.
RESULTS
Study Subjects
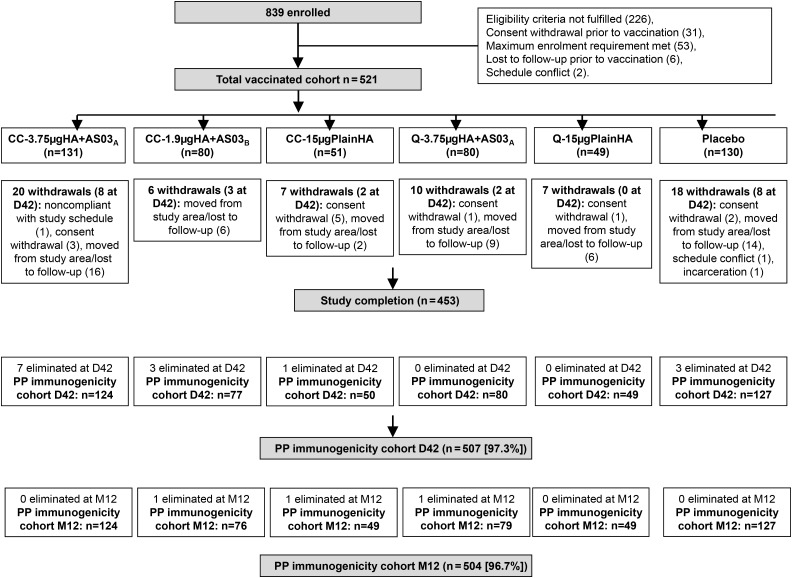
There were 839 adults screened, of whom 318 were not allocated to a treatment group (Figure 1). There were 521 vaccinated participants. No subject withdrew from the study because of an AE. In each treatment group, the mean age was 31.1–33.4 years, and there were more female than male participants (Table 1).
Figure 1.
Subject flow through the study. Subjects were eliminated at day 42 (D42) after the first dose for the following reasons (1 subject could have several elimination codes): protocol violation (n = 1), received protocol-forbidden medication (n = 1), underlying medical condition (n = 1), noncompliance with vaccination or blood sampling schedule (n = 11), and invalid results or insufficient serum quantity (n = 8). Subjects were eliminated at month 12 (M12) after the first dose for the following reasons (1 subject could have several elimination codes): protocol violation (n = 1), received protocol-forbidden medication (n = 2), underlying medical condition (n = 1), noncompliance with vaccination or blood sampling schedule (n = 5), invalid results or insufficient serum quantity (n = 3), and incomplete vaccination schedule and eliminated from the D42 analysis (n = 7). CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; HA, hemagglutinin antigen; PP, per protocol; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant.
Table 1.
Demographic Characteristics, by Study Group—Total Vaccinated Cohort
| Characteristic | CC-3.75 µgHA + A03A (n = 131) | CC-1.9 µgHA + AS03B (n = 80) | CC-15 µgPlainHA (n = 51) | Q-3.75 µgHA + AS03A (n = 80) | Q-15 µgPlainHA (n = 49) | Placebo (Saline) (n = 130) |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Mean ± SD | 31.5 ± 9.56 | 33.4 ± 9.50 | 31.1 ± 9.05 | 33.1 ± 10.10 | 32.1 ± 10.47 | 31.8 ± 9.29 |
| Range | 18–49 | 18–49 | 18–47 | 18–49 | 18–49 | 18–49 |
| Sex, no. (%) | ||||||
| Female | 75 (57.3) | 45 (56.3) | 33 (64.7) | 52 (65.0) | 27 (55.1) | 79 (60.8) |
| Male | 56 (42.7) | 35 (43.8) | 18 (35.3) | 28 (35.0) | 22 (44.9) | 51 (39.2) |
| Geographic ancestry, no. (%) | ||||||
| African/African American | 35 (26.7) | 19 (23.8) | 13 (25.5) | 24 (30.0) | 11 (22.4) | 27 (20.8) |
| American Indian/Alaskan native | 0 | 0 | 2 (3.9) | 0 | 0 | 1 (0.8) |
| Central/South Asian | 1 (0.8) | 0 | 0 | 2 (2.5) | 0 | 0 |
| East Asian | 2 (1.5) | 0 | 1 (2.0) | 1 (1.3) | 0 | 2 (1.5) |
| Southeast Asian | 3 (2.3) | 0 | 0 | 3 (3.8) | 0 | 0 |
| Caucasian | 87 (66.4) | 61 (76.3) | 34 (66.7) | 48 (60.0) | 38 (77.6) | 96 (73.8) |
| Othera | 3 (2.3) | 0 | 1 (2.0) | 2 (2.5) | 0 | 4 (3.1) |
Abbreviations: CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; HA, hemagglutinin antigen; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant; SD, standard deviation.
a Includes Japanese and Hawaiian.
Coprimary Immunogenicity Objectives
The criteria for confirmation of the adjuvant effect of AS03A (wave 1) were met. The HI-adjusted GMT ratio (calculated as the GMT for the CC-3.75 µgHA + AS03A group divided by the GMT for the CC-15 µgPlainHA group) at day 42 was 24.3 (95% CI, 17.5–33.6), and the difference in the SCR (calculated as the SCR for the CC-3.75 µgHA + AS03A group minus the SCR for the CC-15 µgPlainHA group) was 71.34 percentage points (95% CI, 56.4–82.3).
Antigen sparing was demonstrated. The vaccine-homologous HI response induced by CC-3.75 µgHA + AS03A met CBER criteria. At day 42 (wave 1), the SCR was 97.9% (95% CI, 88.7%–99.9%) and the SPR was 100% (95% CI, 92.5%–100%).
In a post-hoc analysis, the criteria for adjuvant effect and antigen sparing were also demonstrated for the CC-1.9 µgHA +AS03B group: the HI-adjusted GMT ratio (calculated as the GMT for the CC-1.9 µgHA + AS03B group divided by the GMT for the CC-15 µgPlainHA group) at day 42 was 13.7 (95% CI, 9.9–18.9), and the SCR difference was 62.8 percentage points (95% CI, 46.9–75.0). CBER criteria were met in terms of the SCR and SPR at day 42 (Table 2).
Table 2.
Hemagglutination Inhibition Antibodies to Vaccine-Homologous A/Indonesia/5/2005 and Vaccine-Heterologous A/Vietnam/1194/2004 After Vaccination—Per-Protocol Cohorts for Immunogenicity and Persistence
| Group, Time Pointa | A/Indonesia/5/2005 |
A/Vietnam/1194/2004 |
||||
|---|---|---|---|---|---|---|
| No. | SCR, % (95% CI) | SPR, % (95% CI) | No. | SCR, % (95% CI) | SPR, % (95% CI) | |
| CC-3.75 µgHA + AS03A | ||||||
| Day 0b | 124 | … | 6.5 (2.8–12.3) | 48 | … | 12.5 (4.7–25.2) |
| Day 21 | 124 | 48.4 (39.3–57.5) | 58.1 (48.9–66.9) | 48 | 18.8 (8.9–32.6) | 31.3 (18.7–46.3) |
| Day 42 | 119 | 97.5 (92.8–99.5) | 100 (96.9–100) | 46 | 73.9 (58.9–85.7) | 84.8 (71.1–93.7) |
| Month 6 | 112 | 56.3 (46.6–65.6) | 62.5 (52.9–71.5) | 43 | 14.0 (5.3–27.9) | 27.9 (15.3–43.7) |
| Month 12 | 106 | 29.2 (20.8–38.9) | 32.1 (23.3–41.8) | 40 | 7.5 (1.6–20.4) | 17.5 (7.3–32.8) |
| CC-1.9 µgHA + AS03B | ||||||
| Day 0b | 77 | … | 15.6 (8.3–25.6) | 52 | … | 19.2 (9.6–32.5) |
| Day 21 | 77 | 35.1 (24.5–46.8) | 50.6 (39.0–62.2) | 52 | 13.5 (5.6–25.8) | 36.5 (23.6–51.0) |
| Day 42 | 75 | 89.3 (80.1–95.3) | 97.3 (90.7–99.7) | 50 | 60.0 (45.2–73.6) | 88.0 (75.7–95.5) |
| Month 6 | 73 | 34.2 (23.5–46.3) | 46.6 (34.8–58.6) | 50 | 4.0 (.5–13.7) | 14.0 (5.8–26.7) |
| Month 12 | 71 | 11.3 (5.0–21.0) | 18.3 (10.1–29.3) | 49 | 4.1 (.5–14.0) | 6.1 (1.3–16.9) |
| CC-15 µgPlainHA | ||||||
| Day 0b | 50 | … | 4.0 (0.5–13.7) | 50 | … | 0.0 (.0–7.1) |
| Day 21 | 50 | 4.0 (.5–13.7) | 4.0 (.5–13.7) | 50 | 8.0 (2.2–19.2) | 10.0 (3.3–21.8) |
| Day 42 | 49 | 26.5 (14.9–41.1) | 28.6 (16.6–43.3) | 49 | 12.2 (4.6–24.8) | 16.3 (7.3–29.7) |
| Month 6 | 45 | 2.2 (.1–11.8) | 4.4 (.5–15.1) | 45 | 6.7 (1.4–18.3) | 6.7 (1.4–18.3) |
| Month 12 | 43 | 0.0 (.0–8.2) | 2.3 (.1–12.3) | 43 | 2.3 (.1–12.3) | 4.7 (.6–15.8) |
| Q-3.75 µgHA + AS03A | ||||||
| Day 0b | 80 | … | 17.5 (9.9–27.6) | 53 | … | 24.5 (13.8–38.3) |
| Day 21 | 80 | 46.3 (35.0–57.8) | 63.8 (52.2–74.2) | 53 | 15.1 (6.7–27.6) | 35.8 (23.1–50.2) |
| Day 42 | 78 | 94.9 (87.4–98.6) | 97.4 (91.0–99.7) | 52 | 53.8 (39.5–67.8) | 78.8 (65.3–88.9) |
| Month 6 | 73 | 47.9 (36.1–60.0) | 58.9 (46.8–70.3) | 49 | 8.2 (2.3–19.6) | 12.2 (4.6–24.8) |
| Month 12 | 69 | 24.6 (15.1–36.5) | 31.9 (21.2–44.2) | 46 | 4.3 (.5–14.8) | 6.5 (1.4–17.9) |
| Q-15 µgPlainHA | ||||||
| Day 0b | 49 | … | 2.0 (.1–10.9) | 49 | … | 4.1 (.5–14.0) |
| Day 21 | 49 | 6.1 (1.3–16.9) | 8.2 (2.3–19.6) | 49 | 6.1 (1.3–16.9) | 10.2 (3.4–22.2) |
| Day 42 | 49 | 51.0 (36.3–65.6) | 53.1 (38.3–67.5) | 49 | 8.2 (2.3–19.6) | 12.2 (4.6–24.8) |
| Month 6 | 45 | 6.7 (1.4–18.3) | 8.9 (2.5–21.2) | 45 | 2.2 (.1–11.8) | 11.1 (3.7–24.1) |
| Month 12 | 42 | 0.0 (.0–8.4) | 2.4 (.1–12.6) | 42 | 0.0 (.0–8.4) | 4.8 (.6–16.2) |
| Placebo (saline) | ||||||
| Day 0b | 127 | … | 11.8 (6.8–18.7) | 50 | … | 10.0 (3.3–21.8) |
| Day 21 | 127 | 1.6 (.2–5.6) | 9.4 (5.0–15.9) | 50 | 4.0 (.5–13.7) | 16.0 (7.2–29.1) |
| Day 42 | 120 | 4.2 (1.4–9.5) | 10.0 (5.3–16.8) | 48 | 0.0 (.0–7.4) | 8.3 (2.3–20.0) |
| Month 6 | 116 | 0.9 (.0–4.7) | 0.9 (.0–4.7) | 47 | 0.0 (.0–7.5) | 0.0 (.0–7.5) |
| Month 12 | 110 | 0.0 (.0–3.3) | 0.9 (.0–5.0) | 44 | 0.0 (.0–8.0) | 2.3 (.1–12.0) |
Abbreviations: CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; CI, confidence interval; HA, hemagglutinin antigen; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant; SCR, seroconversion rate; SPR, seroprotection rate.
a After receipt of the first dose of vaccine or placebo, unless otherwise indicated.
b Before receipt of the first dose of vaccine or placebo.
Homologous Immune Response: A/Indonesia/5/2005 Strain
Immunogenicity, Using the HI Assay
Prevaccination SPRs ranged between 2.0% and 17.5% across the study groups, with overlapping 95% CIs (Table 2). Prevaccination GMTs were low in all groups, but they were lower in groups receiving plain HA (Supplementary Figure 1). Two doses of CC-3.75 µgHA + AS03A or CC-1.9 µgHA + AS03B induced a robust immune response, with seroconversion for HI antibodies for the vaccine strain in at least 89.3% of participants and notional seroprotection in at least 97.3% at day 42 (Table 2). At day 42, both of the AS03-adjuvanted CC-H5N1 formulations met CBER criteria for the SCR and SPR. The day 42 HI GMT was 465.9 in the CC-3.75 µgHA + AS03A group and 324.4 in the CC-1.9 µgHA + AS03B group (Supplementary Figure 1). In the group immunized with Q-3.75 µgHA + AS03A, the day 42 SCR was 94.9%, the SPR was 97.4% (Table 2), and the GMT was 415.9 (Supplementary Figure 1). By contrast, the day 42 SCRs and SPRs in the groups receiving plain antigen were ≤53.1% (Table 2), and the GMTs at day 42 were ≤36.4 (Supplementary Figure 1). By month 12, the SPR was 32.1% in the CC-3.75 µgHA + AS03A group and 18.3% in the CC-1.9 µgHA + AS03B group, compared with 31.9% in the group that received Q-3.75 µgHA + AS03A and ≤2.4% in the groups that received plain antigen (Table 2).
Immunogenicity, Using the Microneutralization Assay
Vaccine-homologous NAb responses (defined as ≥4-fold increases) were detected on day 42 in 88.9% of CC-3.75 µgHA +AS03A recipients, 95.9% of CC-1.9 µgHA + AS03B recipients, and 96.2% of Q-3.75 µgHA + AS03A recipients, compared with ≤43.8% of participants who received 15 µg plain HA (Table 3). NAbs were detectable until month 12 in 100%, 89.6%, and 97.8% of participants, respectively, compared with 55.8% who received CC-15 µgPlainHA and 73.8% who received Q-15 µgPlainHA. After the peak at day 42, NAb titers decreased over time but remained higher than prevaccination levels at month 12 (Supplementary Figure 2).
Table 3.
Microneutralization Responses to Vaccine-Homologous A/Indonesia/5/2005 and Vaccine-Heterologous A/Vietnam/1194/2004 Strains After Vaccination—Per-Protocol Cohorts for Immunogenicity and Persistence
| Group, Time Pointa | A/Indonesia/5/2005 |
A/Vietnam/1194/2004 |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | NAb Titer ≥1:28, % (95% CI) | No. | VRR,b % (95% CI) | No. | NAb Titer ≥1:28, % (95% CI) | No. | VRR,b % (95% CI) | |
| CC-3.75 µgHA + AS03A | ||||||||
| Day 0c | 47 | 17.0 (7.6–30.8) | … | … | 48 | 33.3 (20.4–48.4) | … | … |
| Day 21 | 47 | 89.4 (76.9–96.5) | 47 | 10.6 (3.5–23.1) | 48 | 81.3 (67.4–91.1) | 48 | 6.3 (1.3–17.2) |
| Day 42 | 46 | 100 (92.3–100) | 45 | 88.9 (75.9–96.3) | 46 | 97.8 (88.5–99.9) | 46 | 23.9 (12.6–38.8) |
| Month 6 | 42 | 100 (91.6–100) | 42 | 66.7 (50.5–80.4) | 42 | 90.5 (77.4–97.3) | 42 | 23.8 (12.1–39.5) |
| Month 12 | 39 | 100 (91.0–100) | 39 | 61.5 (44.6–76.6) | 39 | 89.7 (75.8–97.1) | 39 | 23.1 (11.1–39.3) |
| CC-1.9 µgHA + AS03B | ||||||||
| Day 0c | 51 | 7.8 (2.2–18.9) | … | … | 52 | 34.6 (22.0–49.1) | … | … |
| Day 21 | 51 | 86.3 (73.7–94.3) | 51 | 7.8 (2.2–18.9) | 52 | 80.8 (67.5–90.4) | 52 | 3.8 (.5–13.2) |
| Day 42 | 49 | 100 (92.7–100) | 49 | 95.9 (86.0–99.5) | 50 | 100 (92.9–100) | 50 | 40.0 (26.4–54.8) |
| Month 6 | 49 | 100 (92.7–100) | 49 | 73.5 (58.9–85.1) | 48 | 89.6 (77.3–96.5) | 48 | 22.9 (12.0–37.3) |
| Month 12 | 48 | 89.6 (77.3–96.5) | 48 | 52.1 (37.2–66.7) | 47 | 78.7 (64.3–89.3) | 47 | 25.5 (13.9–40.3) |
| CC-15 µgPlainHA | ||||||||
| Day 0c | 49 | 4.1 (.5–14.0) | … | … | 48 | 31.3 (18.7–46.3) | … | … |
| Day 21 | 50 | 40.0 (26.4–54.8) | 49 | 6.1 (1.3–16.9) | 49 | 59.2 (44.2–73.0) | 48 | 6.3 (1.3–17.2) |
| Day 42 | 48 | 87.5 (74.8–95.3) | 48 | 8.3 (2.3–20.0) | 47 | 68.1 (52.9–80.9) | 47 | 6.4 (1.3–17.5) |
| Month 6 | 44 | 81.8 (67.3–91.8) | 43 | 16.3 (6.8–30.7) | 40 | 67.5 (50.9–81.4) | 40 | 12.5 (4.2–26.8) |
| Month 12 | 43 | 55.8 (39.9–70.9) | 42 | 11.9 (4.0–25.6) | 39 | 56.4 (39.6–72.2) | 39 | 12.8 (4.3–27.4) |
| Q-3.75 µgHA + AS03A | ||||||||
| Day 0c | 53 | 3.8 (.5–13.0) | … | … | 51 | 25.5 (14.3–39.6) | … | … |
| Day 21 | 53 | 94.3 (84.3–98.8) | 53 | 35.8 (23.1–50.2) | 51 | 94.1 (83.8–98.8) | 51 | 13.7 (5.7–26.3) |
| Day 42 | 52 | 100 (93.2–100) | 52 | 96.2 (86.8–99.5) | 50 | 100 (92.9–100) | 50 | 24.0 (13.1–38.2) |
| Month 6 | 48 | 100 (92.6–100) | 48 | 79.2 (65.0–89.5) | 46 | 84.8 (71.1–93.7) | 45 | 24.4 (12.9–39.5) |
| Month 12 | 46 | 97.8 (88.5–99.9) | 46 | 58.7 (43.2–73.0) | 44 | 84.1 (69.9–93.4) | 43 | 14.0 (5.3–27.9) |
| Q-15 µgPlainHA | ||||||||
| Day 0c | 49 | 16.3 (7.3–29.7) | … | … | 48 | 29.2 (17.0–44.1) | … | … |
| Day 21 | 49 | 55.1 (40.2–69.3) | 49 | 8.2 (2.3–19.6) | 49 | 73.5 (58.9–85.1) | 48 | 8.3 (2.3–20.0) |
| Day 42 | 48 | 91.7 (80.0–97.7) | 48 | 43.8 (29.5–58.8) | 47 | 78.7 (64.3–89.3) | 47 | 8.5 (2.4–20.4) |
| Month 6 | 45 | 84.4 (70.5–93.5) | 45 | 20.0 (9.6–34.6) | 45 | 73.3 (58.1–85.4) | 45 | 15.6 (6.5–29.5) |
| Month 12 | 42 | 73.8 (58.0–86.1) | 42 | 14.3 (5.4–28.5) | 42 | 73.8 (58.0–86.1) | 42 | 9.5 (2.7–22.6) |
| Placebo (saline) | ||||||||
| Day 0c | 50 | 16.0 (7.2–29.1) | … | … | 48 | 33.3 (20.4–48.4) | … | … |
| Day 21 | 49 | 10.2 (3.4–22.2) | 49 | 0.0 (.0–7.3) | 49 | 40.8 (27.0–55.8) | 48 | 0.0 (.0–7.4) |
| Day 42 | 47 | 10.6 (3.5–23.1) | 47 | 0.0 (.0–7.5) | 47 | 38.3 (24.5–53.6) | 47 | 0.0 (.0–7.5) |
| Month 6 | 46 | 13.0 (4.9–26.3) | 46 | 0.0 (.0–7.7) | 46 | 56.5 (41.1–71.1) | 46 | 4.3 (.5–14.8) |
| Month 12 | 43 | 14.0 (5.3–27.9) | 43 | 0.0 (.0–8.2) | 43 | 58.1 (42.1–73.0) | 43 | 4.7 (.6–15.8) |
Abbreviations: CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; CI, confidence interval; HA, hemagglutinin antigen; NAb, neutralizing antibody; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant; VRR, vaccine response rate.
a After receipt of the first dose of vaccine or placebo, unless otherwise indicated.
b Defined as the percentage of vaccinees with a minimum 4-fold increase in NAb titer after vaccination.
c Before receipt of the first dose of vaccine or placebo.
Drift Variant Immune Response: A/Vietnam/1194/2004 Strain
Immunogenicity, Using the HI Assay
Prevaccination SPRs ranged between 0.0% and 24.5% across the study groups (Table 2). Observed prevaccination GMTs were low in all groups, but they were lower in groups receiving plain HA (Supplementary Figure 1). Two doses of CC-3.75 µgHA + AS03A or CC-1.9 µgHA + AS03B induced seroconversion for heterologous HI antibodies at day 42 in 73.9% and 60.0% of participants, respectively (Table 2). The SPR was 84.8% and 88.0%, respectively, and the day 42 HI GMT was 73.0 and 69.6, respectively (Supplementary Figure 1). The day 42 SCR in the group immunized with Q-3.75 µgHA +AS03A was 53.8%, the SPR was 78.8%, and the GMT was 62.9 (Supplementary Figure 1). By contrast, the day 42 SCRs and SPRs in the plain antigen groups were ≤16.3% (Table 2), and the HI GMTs were ≤15.3 (Supplementary Figure 1). By month 12, the SPR for the heterologous strain was 17.5% in the CC-3.75 µgHA + AS03A group and lower in the other groups (Table 2).
Immunogenicity, Using the Microneutralization Assay
NAbs response (defined as a ≥4-fold increase) to the heterologous A/Vietnam/1194/2004 strain were detected at day 42 in 23.9% of CC-3.75 µgHA + AS03A recipients, 40.0% of CC-1.9 µgHA + AS03B recipients, and 24.0% of Q-3.75 µgHA + AS03A recipients, compared with ≤8.5% who received 15 µgPlainHA (Table 3). NAbs were detected until month 12 in 89.7% of CC-3.75 µgHA + AS03A recipients, 78.7% of CC-1.9 µgHA +AS03B recipients, and 84.1% of Q-3.75 µgHA + AS03A recipients, compared with 56.4% who received CC-15 µgPlainHA and 73.8% who received Q-15 µgPlainHA. By month 12, Nab titers had reduced over time but remained higher than prevaccination levels (Supplementary Figure 2).
Reactogenicity and Safety
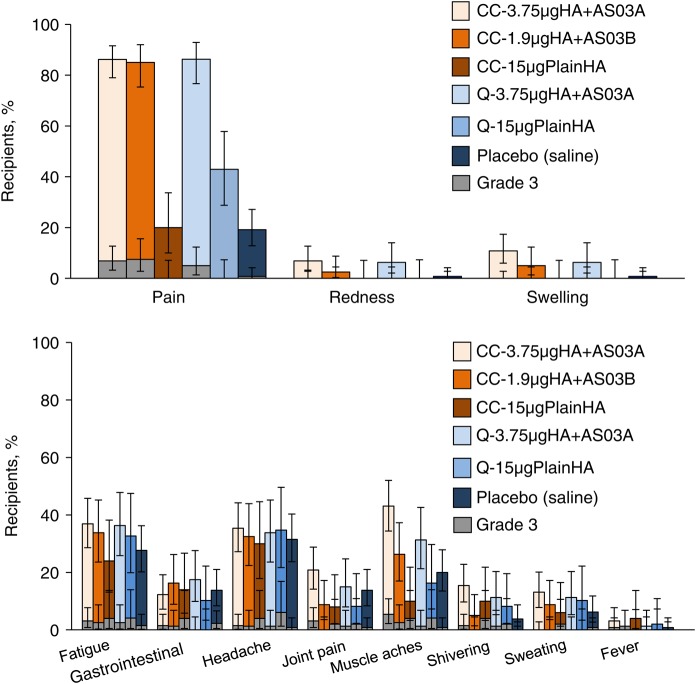
Pain was the most frequently reported solicited injection site symptom after vaccination and was reported more frequently in groups that received an AS03-adjuvanted vaccine (Figure 2). Grade 3 injection site pain was reported by 6.9% of CC-3.75 µgHA + AS03A recipients, 7.5% of CC-1.9 µgHA + AS03B recipients, 5.0% of Q-3.75 µgHA + AS03A recipients, and 0.8% of placebo recipients and was not reported by participants in groups that received plain HA antigen. Redness and swelling were reported by ≤10.8% of participants in each group. No grade 3 redness or swelling was reported during the study.
Figure 2.
Percentage of subjects reporting solicited injection site or general symptoms within 7 days of either vaccine dose—total vaccinated cohort. Vertical lines indicate 95% confidence intervals. Grade 3 is defined as redness and swelling (diameter, >100 mm), a temperature of ≥39.0°C (any route), and, for all other symptoms, as symptoms with sufficient severity to prevent normal activities (eg inability to attend work/school). CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; HA, hemagglutinin antigen; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant.
Fatigue, headache and muscle aches were the most frequently reported solicited general symptoms in groups receiving an AS03-adjuvanted vaccine (Figure 2), although reports of headache were similar across treatment groups including placebo recipients. Fever was reported by ≤4.0% of participants in any treatment group.
Any (unsolicited) AEs within 21 days after any dose were reported by 20.0%–33.6% of participants (Table 4). The most frequently reported unsolicited AEs were upper respiratory tract infection, cough, oropharyngeal pain, and back pain. Grade 3 unsolicited AEs were reported by 2.3%–5.3% of participants. Only 2 subjects reported grade 3 AEs that were considered to be vaccine related (cough and nasal congestion in the Q-3.75 µgHA + AS03A group and pain in extremity in the placebo group).
Table 4.
Global Summary of Adverse Events (AEs) Reported After Vaccination—Total Vaccinated Cohort
| Follow up Period,a Outcome | CC-3.75 µgHA + AS03A Recipients (n = 131) |
CC-1.9 µgHA + AS03B Recipients (n = 80) |
CC-15 µgPlainHA Recipients (n = 51) |
Q-3.75 µgHA + AS03A Recipients (n = 80) |
Q-15 µgPlainHA Recipients (n = 49) |
Placebo (Saline) Recipients (n = 130) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage (95% CI) | No. | Percentage (95% CI) | No. | Percentage (95% CI) | No. | Percentage (95% CI) | No. | Percentage (95% CI) | No. | Percentage (95% CI) | |
| Day 42b | ||||||||||||
| Any unsolicited AE | 44 | 33.6 (25.6–42.4) | 21 | 26.3 (17.0–37.3) | 15 | 29.4 (17.5–43.8) | 16 | 20.0 (11.9–30.4) | 15 | 30.6 (18.3–45.4) | 35 | 26.9 (19.5–35.4) |
| Any grade 3 unsolicited AE | 7 | 5.3 (2.2–10.7) | 3 | 3.8 (.8–10.6) | 2 | 3.9 (.5–13.5) | 2 | 2.5 (.3–8.7) | 2 | 4.1 (.5–14.0) | 3 | 2.3 (.5–6.6) |
| Month 12c | ||||||||||||
| Any medically attended AE | 21 | 16.0 (10.2–23.5) | 17 | 21.3 (12.9–31.8) | 4 | 7.8 (2.2–18.9) | 12 | 15.0 (8.0–24.7) | 9 | 18.4 (8.8–32.0) | 16 | 12.3 (7.2–19.2) |
| Any pIMD | 0 | … | 1 | 1.3 (.0–6.8) | 0 | … | 0 | … | 0 | … | 0 | … |
| Any severe AE | 3 | 2.3 (.5–6.5) | 3 | 3.8 (.8–10.6) | 0 | … | 2 | 2.5 (.3–8.7) | 0 | … | 1 | 0.8 (.0–4.2) |
Abbreviations: CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; CI, confidence interval; HA, hemagglutinin antigen; pIMD, potential immune-mediated disease; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant.
a After receipt of the first dose of vaccine or placebo.
b 21 day follow-up after each vaccination.
c 12 months follow-up after vaccination schedule.
Nine subjects reported SAEs during the entire study period until month 12 (Table 4); none were considered to be vaccine related. All but 2 participants (one with benign intracranial hypertension and one with noncardiac chest pain) recovered. During the same period, MAEs were reported by 7.8%–21.3% of participants, of which only ovarian cyst, back pain, procedural pain, bronchitis, sinusitis, and ear infection were reported more than once in any group. A radiculopathy secondary to cervical nerve compression was reported by a female subject in the CC-1.9 µgHA + AS03B group, with onset 141 days after dose 2. This event was not considered to be vaccine related and resolved after surgery; it was classified by the sponsor as a pIMD according to a predefined list of terms. No pregnancies were reported during the study.
DISCUSSION
We investigated the immunogenicity and safety of AS03-adjuvanted CC-H5N1 formulations in young healthy adults. The adjuvant effect of AS03 for both CC-H5N1 formulations was demonstrated, with HI immunogenicity in relation to the unadjuvanted CC-15 µgPlainHA formulation exceeding CBER guidance targets for adjuvant benefit of pandemic vaccines. The low immunogenicity of unadjuvanted vaccine formulations is consistent with other reports of unadjuvanted vaccines, where up to 90 µg of HA is needed to elicit an immune response in 50%–75% of subjects [6, 19]. The capacity of AS03-adjuvanted vaccines for antigen sparing has potentially important ramifications in the event of a pandemic, during which high numbers of vaccines may be required for rapid deployment.
Both of AS03-adjuvanted CC-H5N1 formulations were immunogenic for the homologous and drift variant A/Vietnam/1194/2004 strains in terms of HI and NAbs and met CBER criteria for immunogenicity of pandemic vaccines for both strains. The potential for cross-clade immunogenicity is fundamental to priming a population in a prepandemic or early pandemic setting, providing priming or protection until a pandemic strain-matched vaccine becomes available [10, 20, 21].
The kinetic profile of HI antibodies after vaccination with the AS03-adjuvanted CC-H5N1 formulations showed a peak in titer 21 days after dose 2, which decreased over time until month 12 but remained higher at month 12 than prior to vaccination. A similar profile was observed for NAbs, although the month 12 plateau was higher than for HI titers: at least 89.7% of participants in the CC-3.75 µgHA + AS03A group continued to have detectable NAbs after 1 year. The higher persistence of NAbs than HI antibodies at 6 and 12 months after vaccination may reflect the greater sensitivity of the neutralization test than the HI test to reveal affinity maturation of the antibody response. The immunogenicity of the GSK's EB66 CC-derived AS03-adjuvanted vaccine appeared to be similar to the GSK's egg-derived AS03-adjuvanted vaccine (Q-3.75 µgHA + AS03A) [7, 10, 22]. Although the magnitude of the HI and NAb responses tended to be lower in the group receiving half the HA dose (CC-1.9 µgHA + AS03B) versus a full dose (CC-3.75 µgHA +AS03A), immunogenicity regulatory acceptance criteria were met. This dose could be appropriate for a young adult or a pediatric population, pending further evaluation. Half an adult dose of GSK's egg-derived vaccine has shown acceptable immunogenicity and antibody persistence to 12 months after vaccination in a pediatric population [23].
In this randomized trial, we observed lower prevaccination HI antibody GMTs in groups who received plain antigen; these groups were recruited in wave 1 and included fewer participants than the study groups receiving adjuvanted vaccine or placebo. Because prevaccination GMTs were low in all groups, this observation is unlikely to impact on the study conclusions.
The reactogenicity profile of the CC-H5N1 formulations was acceptable, with few grade 3 AEs reported within 7 days of vaccination and no related SAEs reported up to 12 months after vaccination. The reactogenicity and safety profile of the adjuvanted and unadjuvanted CC-H5N1 formulations resembled that of the corresponding egg-derived vaccine formulations.
Cell lines investigated and licensed for use in the production of influenza vaccines include the Per.C6 cell line (Crucell), Vero cells (used by Baxter), and Madin-Darby canine kidney cells [24]. The EB66 cell line derives from duck embryonic stem cells and achieves high cell densities with rapid growth [25]. EB66 has been investigated as a means of producing monoclonal antibodies [26]. Here we confirm the feasibility of using the EB66 cell line for the production of an inactivated influenza vaccine for humans.
Antigen-sparing properties of the AS03-adjuvanted CC-H5N1 candidate were demonstrated in a phase 1 study in adults: 21 days after a 2-dose series, the AS03-adjuvanted CC-H5N1 candidate with antigen doses as low as 1.9 µg of HA elicited homologous and heterologous HI antibody responses that exceeded CBER guidance targets for pandemic influenza vaccines. The study demonstrated the feasibility of using the EB66 cell line to produce an immunogenic, inactivated split virus influenza vaccine with an acceptable safety profile. This vaccine candidate warrants further investigation in larger trials in adults and children.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the participating study volunteers, clinicians, nurses, and laboratory technicians at the study sites, as well as the sponsor's project staff, particularly Casey Johnson, Laurence Chu, and Peter Winkle, for their support and contributions throughout the study; members of all teams at GSK Vaccines, especially Stephanie Sharp (Veristat, on behalf of GSK Vaccines) and Janine Linden, for preparation of the study report, Kimberly Cerenze Short (global study manager) and Dawn Hall (senior study manager), for study management, Miguel Madariaga (medical monitor), Roger Bernhard (laboratory manager) and Philippe Boutet (global vaccine clinical laboratories project manager), for laboratory project management, Rosalia Calamera (clinical data coordinator), and Katalin Abraham (regulatory affairs representative); Joanne Wolter (independent medical writer on behalf of GSK Vaccines), for providing medical writing services; and Jennifer Dorts and Bruno Baudoux (Business and Decision Life Sciences, on behalf of GSK Vaccines), for editorial assistance and manuscript coordination.
All authors participated in the implementation of the study, including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in the development of this manuscript, had full access to the data, and gave final approval before submission.
GlaxoSmithKline Biologicals SA was involved in all stages of study conduct, including analysis of the data (clinical trials registration NCT01236040). GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Financial support. This work was supported by the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary of Preparedness and Response, Department of Health and Human Services (contract HHS O100200600011C); and GlaxoSmithKline Biologicals SA.
Potential conflicts of interest. N. S. received a grant from the GSK group of companies for the present study. A. S., M. D., and B. L. I. are employees of the GSK group of companies. A. S. and B. L. I. report ownership of stock and/or stock options in the GSK group of companies.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Taubenberger JK, Morens DM. Influenza: the once and future pandemic. Public Health Rep Wash DC 1974 2010; 125(suppl 3):S16–26. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). WHO activities in avian influenza and pandemic influenza preparedness. Geneva: WHO, 2007. http://www.who.int/influenza/resources/documents/WHO_CDS_EPR_GIP_2006_6.pdf Accessed 19 April 2014. [Google Scholar]
- 3.Rebmann T, Zelicoff A. Vaccination against influenza: role and limitations in pandemic intervention plans. Expert Rev Vaccines 2012; 11:1009–19. [DOI] [PubMed] [Google Scholar]
- 4.Perdue ML, Arnold F, Li S, et al. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines 2011; 10:1183–94. [DOI] [PubMed] [Google Scholar]
- 5.Montomoli E, Khadang B, Piccirella S, et al. Cell culture-derived influenza vaccines from Vero cells: a new horizon for vaccine production. Expert Rev Vaccines 2012; 11:587–94. [DOI] [PubMed] [Google Scholar]
- 6.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–51. [DOI] [PubMed] [Google Scholar]
- 7.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 2010; 201:1644–53. [DOI] [PubMed] [Google Scholar]
- 8.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580–9. [DOI] [PubMed] [Google Scholar]
- 9.Chu DW-S, Hwang S-J, Lim FS, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 2009; 27:7428–35. [DOI] [PubMed] [Google Scholar]
- 10.Langley JM, Risi G, Caldwell M, et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis 2011; 203:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díez-Domingo J, Garcés-Sanchez M, Baldó J-M, et al. Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J 2010; 29:e35–46. [DOI] [PubMed] [Google Scholar]
- 12.Nolan T, Izurieta P, Lee B-W, et al. Heterologous prime-boost vaccination using an H5N1-AS03-adjuvanted influenza vaccine in infants and children under 3 years of age. J Infect Dis 2014; 210:1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Center for Biologics Evaluation and Research. Vaccines guidance. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm Accessed 02 January 2014.
- 14.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res 2004; 103:163–71. [DOI] [PubMed] [Google Scholar]
- 15.Kendal A, Pereira M, Skehel J. Hemagglutination inhibition. Concepts and procedures for laboratory-based influenza surveillance. Atlanta, GA: Centers for Disease Control and Prevention and Pan-American Health Organization, 1985. [Google Scholar]
- 16.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004; 103:91–5. [DOI] [PubMed] [Google Scholar]
- 18.Reed L, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938; 27:493–7. [Google Scholar]
- 19.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001; 19:1732–7. [DOI] [PubMed] [Google Scholar]
- 20.Jennings LC, Monto AS, Chan PKS, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis 2008; 8:650–8. [DOI] [PubMed] [Google Scholar]
- 21.Lasko B, Reich D, Madan A, Roman F, Li P, Vaughn D. Rapid immunization against H5N1: a randomized trial evaluating homologous and cross-reactive immune responses to AS03(A)-adjuvanted vaccination in adults. J Infect Dis 2011; 204:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risi G, Frenette L, Langley JM, et al. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: results of a randomised single heterologous booster dose study at 15 months. Vaccine 2011; 29:6408–18. [DOI] [PubMed] [Google Scholar]
- 23.Kosalaraksa P, Jeanfreau R, Frenette L, et al. AS03B-adjuvanted H5N1 influenza vaccine in children 6 months through 17 years of age: a phase II/III randomized, placebo-controlled, observer-blind trial. J Infect Dis 2015; 211:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng S-Z, Jiao P-R, Qi W-B, Fan H-Y, Liao M. Development and strategies of cell-culture technology for influenza vaccine. Appl Microbiol Biotechnol 2011; 89:893–902. [DOI] [PubMed] [Google Scholar]
- 25.Brown SW, Mehtali M. The avian EB66(R) cell line, application to vaccines, and therapeutic protein production. PDA J Pharm Sci Technol PDA 2010; 64:419–25. [PubMed] [Google Scholar]
- 26.Olivier S, Jacoby M, Brillon C, et al. EB66 cell line, a duck embryonic stem cell-derived substrate for the industrial production of therapeutic monoclonal antibodies with enhanced ADCC activity. MAbs 2010; 2:405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.