Abstract
Defects in mitochondrial fission and cyclin dependent kinase 5 (CDK5) activation are early events that precede neuronal loss following NMDA-induced neuronal death. Here, we report that the cytoplasmic CDK5 tightly regulates mitochondrial morphology defects associated with NMDA-induced neuronal injury via regulation of the mitochondrial fission protein, dynamin-related protein 1 (DRP1). We show that DRP1 is a direct target of CDK5. CDK5-mediated phosphorylation of DRP1 at a conserved Serine residue, S585, is elevated at the mitochondria and is associated with increased mitochondrial fission. Ectopic expression of a cytoplasmic CDK5 or mutant DRP1-S585D results in increased mitochondrial fragmentation in primary neurons. Conversely, expression of a dominant negative form of cytoplasmic CDK5 or mutant DRP1-S585A results in elongated mitochondria. In addition, pharmacological inhibition of CDK5 by Roscovitine inhibits DRP1 phosphorylation and mitochondrial fission associated with NMDA-induced neuronal loss. Importantly, conditional deletion of CDK5 significantly attenuates DRP1 phosphorylation at S585 and rescues mitochondrial fission defects in neurons exposed to NMDA. Our studies delineate an important mechanism by which CDK5 regulates mitochondrial morphology defects associated with neuronal injury.
Introduction
Overactivation of NMDA receptors is a major cause of cell death following acute neuronal injury including stroke, trauma and neurodegenerative diseases (1–3). We have previously established that defects in mitochondrial morphology including excessive mitochondrial fission, cessation of mitochondrial fusion and cristae dilation precede neuronal loss upon NMDA-induced injury (4). However, the mechanism that leads to mitochondrial defects associated with NMDA-induced neuronal loss remains to be investigated.
Dynamin related protein 1 (DRP1), a cytoplasmic protein belonging to the family of large GTPases, is a key regulator of mitochondrial fission (5). DRP1 is recruited from the cytosol to the mitochondria at the site of scission to induce mitochondrial fission (6). DRP1 recruitment is tightly regulated by post-translational modifications. For example, DRP1 is sumoylated by small ubiquitin like modifier (SUMO) protein (7). Upon its sumoylation, DRP1 becomes stably associated with the mitochondrial membranes (8,9). Importantly, multiple kinases have been identified to regulate DRP1 phosphorylation at various sites (10–16) that impact mitochondrial integrity and cell fate in different cell systems.
Regulation of DRP1 activity is a critical event in the nervous system. In post-mitotic neurons, DRP1 is phosphorylated by the Ca++/Calmodulin-dependent protein kinase Iα (CamKIα) (13). CamKIα-mediated DRP1 phosphorylation induces mitochondrial fragmentation in response to calcium influx associated with neuronal activity. Expression of a dominant negative DRP1 (DRP1-K38A) in primary neurons reduces the content of dendritic mitochondria and results in the loss of synapses and dendritic spines (17). While DRP1 activity is required during synaptogenesis to regulate neuronal development and synaptic strength, excessive DRP1-mediated mitochondrial fission is linked to neuronal death (18). DRP1 downregulation confers neuroprotection against nitric oxide-induced neuronal loss (18). How DRP1 regulates mitochondrial morphology during neuronal injury is unclear.
In recent years, an intimate relationship between DRP1 and cyclin dependent kinases has been documented (14–16). During mitosis, DRP1 is phosphorylated by cyclin dependent kinase 1 (CDK1) at a conserved Serine residue (Serine585 rat/Serine 616 human) (14), which is suggested to contribute to mitochondrial segregation in cycling cells. In contrast, phosphorylation of DRP1 at the same Serine site in a CDK5 dependent manner has been suggested to regulate mitochondrial elongation during neuronal maturation (15). However, whether CDK5 directly phosphorylates DRP1 and the functional consequences on mitochondrial length is controversial (15,16). Cyclin dependent kinase 5 (CDK5) plays an important role in the regulation of CNS development and synaptic plasticity under steady state and during neuronal death in response to stress (19–24). In addition, CDK5 is reported to regulate mitochondrial morphology associated with neuronal death or during neuronal development (25,26). This raises questions of how CDK5 impacts mitochondrial morphology associated with NMDA-induced neuronal loss and whether there is a physical and functional interaction between DRP1 and CDK5 in this context.
In the present study, we have identified that DRP1 is a direct target of CDK5. CDK5-mediated phosphorylation of DRP1 at Serine 585 is elevated at the mitochondria and drives mitochondrial fission associated with NMDA-induced neuronal loss. Our studies identify a novel mechanism by which CDK5 contributes to NMDA-induced neuronal death by targeting the mitochondrial fission machinery.
Results
DRP1 is directly phosphorylated by CDK5 at a conserved Serine residue critical for regulation of mitochondrial length in neurons
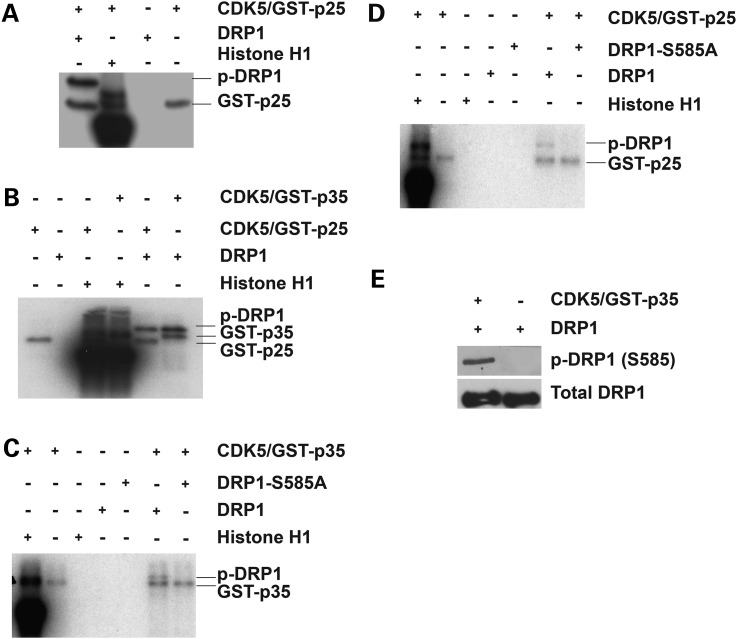
We first examined whether DRP1 is a direct target of CDK5. In vitro kinase assays were performed using bacterially-derived recombinant His-DRP1 protein and CDK5/GST-p25 or CDK5/GST-p35 active complexes in the presence of radiolabeled ATP (γ-32 P) ATP (Fig. 1A and B). Analyses of the in vitro kinase reactions revealed that both p35/CDK5 and p25/CDK5 directly phosphorylate DRP1 in vitro (Fig. 1A and B). In these reactions, p25/p35 is also phosphorylated by CDK5. We next determined whether the identified cyclin dependent kinase (CDK) site at Serine 585 in DRP1 (DRP1-S585) is a direct target of CDK5 in vitro. The recombinant wild type rat DRP1 (DRP1-WT) and a mutant DRP1 in which Serine-585 was mutated to Alanine (DRP1-S585A) were incubated in the presence of CDK5/GST-p35 or CDK5/GST-p25 complexes in the presence of radiolabeled ATP (γ-32 P) ATP (Fig. 1C and D). We found that while the active CDK5 complex phosphorylated DRP1-WT, no phosphorylation signal was detected in the presence of the DRP1-S585A mutant, suggesting that S585 is a direct CDK5 phosphorylation site. We performed parallel in vitro kinase assays using recombinant rat DRP1-WT in the presence or absence of CDK5 active complex and cold ATP. The in vitro kinase reactions were analyzed by immunoblotting using a specific phospho-DRP1 antibody which detects DRP1 phosphorylation at S585. A phosphorylated band corresponding to phosphorylation at S585 was detected in assays with active CDK5 complex while no band was detected in the absence of CDK5. These data established that S585 in DRP1 is a direct CDK5 target in vitro (Fig. 1C–E).
Figure 1.
DRP1 is a direct substrate of CDK5. (A and B) Recombinant His-tagged wild type rat DRP1 (WT-DRP1) was incubated with radiolabeled ATP (γ-32 P-ATP) in kinase assay buffer (30°C, 30 min) in the absence and presence of either of CDK5/GST-p25 active complex (A) or CDK5/GST-p35 and CDK5/GST-p25 active complexes (B). Histone H1 was used as a positive control. DRP1 alone was used as negative control. (C and D) In vitro kinase assays were performed on recombinant His-tagged WT-DRP1 and a His-tagged mutant DRP1 in which Ser585 was mutated to alanine (DRP1-S585A). The kinase assays were carried out as described in (A). (E) In vitro kinase assays were performed on wild type DRP1 with cold ATP in the absence and presence of CDK5/p35 complex and the kinase reactions were analyzed by immunoblotting using a specific antibody that recognizes DRP1 phosphorylation at Ser 585. Total DRP1 was also probed as control.
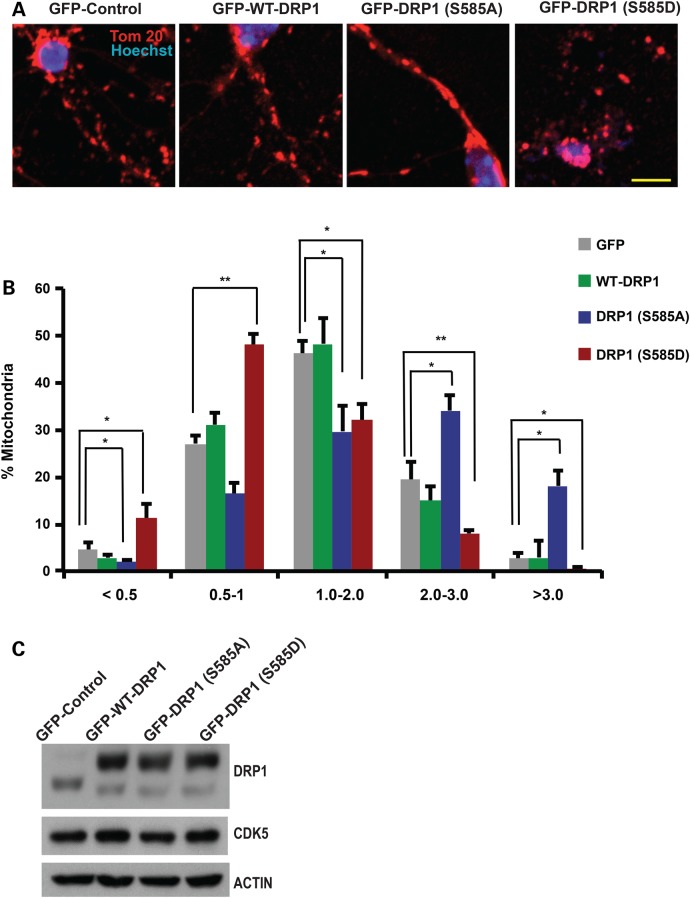
Next, we determined whether DRP1 phosphorylation at S585 regulates mitochondrial morphology in primary neurons. We expressed three AAV constructs of DRP1: DRP1-WT, DRP1-S585A (phosphorylation incompetent mutant) or DRP1-S585D (phosphomimic mutant) in primary granule neurons and examined mitochondrial length in each group using the mitochondrial marker Tom20 as described (27). Since mitochondria in primary neurons exhibit variable length, mitochondrial length was binned into different length categories of <0.5, 0.5–1.0, 1.0–2.0, 2.0–3.0 and >3.0 µm. Analyses of mitochondrial length showed that neurons expressing the S585A mutant construct exhibited an increase in mitochondria length relative to neurons expressing DRP1-WT (Fig. 2A and B). In contrast, neurons expressing the S585D active mutant exhibited an increase in mitochondrial fragmentation compared to neurons expressing DRP1-WT (Fig. 2A and B). Western blot analyses of primary granule neurons infected with either of DRP1-WT, DRP1-S585A or DRP1-S585D confirmed expression and demonstrated that neither of DRP1 constructs had an effect on CDK5 expression levels (Fig. 2C). These data support a model in which DRP1 phosphorylation at S585 increases mitochondrial fragmentation in post-mitotic primary neurons.
Figure 2.
DRP1 phosphorylation at Ser585 regulates mitochondrial morphology in cerebellar granule neurons. (A) Cerebellar granule neurons (CGNs) were infected with adeno-associated viruses for GFP-expressing wild type DRP1 (GFP-WT-DRP1), GFP-expressing DRP1 mutants [GFP-DRP1 (S585A) and GFP-DRP1 (S585D)] or a GFP control for 6 days. GFP was fused to DRP1 constructs at the N terminal on the same promoter. Neurons were fixed and stained with TOM-20 antibody to assess mitochondrial morphology. Nuclei were stained with Hoechst. Representative images of mitochondrial morphology under different treatment conditions are shown. (B) Mitochondrial length was measured and binned into different categories of <0.5, 1.0–2.0, 2.0–3.0 and >3 µm. Quantification of mitochondrial length are shown as percentages of total mitochondria. *P < 0.05; **P < 0.01; Mag bar, 10 µm. (C) Protein lysates of CGN infected with GFP, GFP-DRP-WT, S585A or S585E were analyzed by western blot analyses using DRP1 and CDK5 antibodies as indicated . Beta-ACTIN was used as loading control.
CDK5 regulates mitochondrial morphology in cerebellar granule neurons
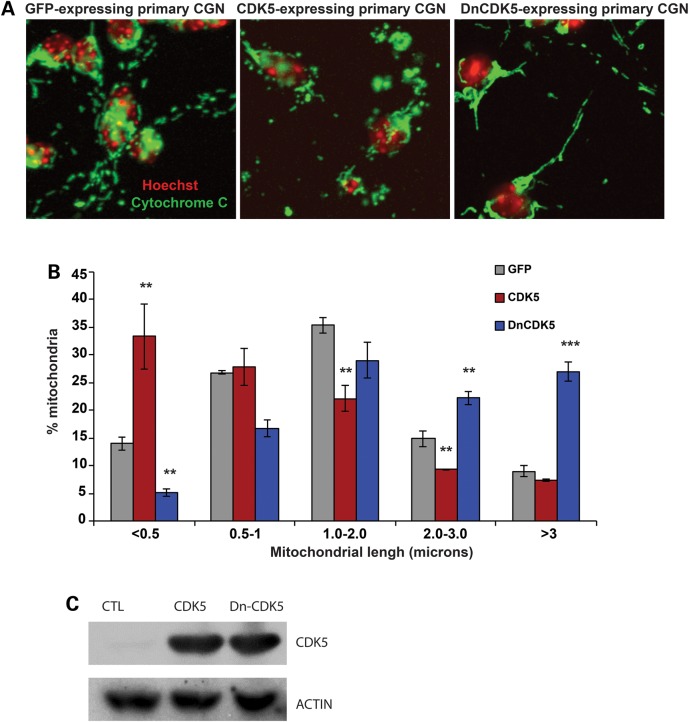
To examine the physiological relevance of CDK5-mediated DRP1 phosphorylation, we first studied mitochondrial length in neurons expressing either a wild type (wt) cytoplasmic CDK5 [CDK5-NES (nuclear exclusion factor)], a dominant negative mutant form of cytoplasmic CDK5 (DnCDK5-NES), or a GFP control (Fig. 3A–C). Use of CDK5-NES and DnCDK5-NES allowed us to more specifically examine role of cytoplasmic CDK5 on cytoplasmic DRP1. Importantly, these constructs allowed us to better exclude the CDK5 impact on cell death or cell cycle at the nuclear level (28,29). Mitochondrial length was measured in primary neurons expressing wtCDK5-NES, DnCDK5-NES and control GFP as described above. Representative images are shown in Figure 3A. Our analyses revealed that mitochondrial length was significantly shifted from elongated to a more fragmented phenotype in primary neurons expressing cytoplasmic CDK5. 33.34 ± 5.9% of mitochondria exhibited a length of less than 0.5 µm (fragmented) in CDK5-expressing neurons compared with control GFP-expressing neurons at 13.95 ± 1.15% (P < 0.001, n = 3, Fig. 3B). In contrast, in neurons expressing the DnCDK5-NES, 26.97 ± 1.7% of mitochondria maintained a length of greater than 3 µm (elongated) compared with control GFP-expressing neurons at 8.9 ± 0.97% (Fig. 3A and B). Expression of our Cdk5 constructs were confirmed as shown in Figure 3C. Together these studies demonstrate that CDK5 induces mitochondrial fragmentation in post-mitotic cerebellar granule neurons.
Figure 3.
Cytoplasmic CDK5 regulates mitochondrial morphology in cerebellar granule neurons. (A) Cerebellar granule neurons were infected with adenoviruses carrying expression cassettes for either of GFP-tagged cytoplasmic CDK5 (CDK5), a dominant negative cytoplasmic CDK5 (DN-CDK5), or a GFP control at the time of plating. Following 48 h in culture, neurons were fixed and mitochondrial morphology was assessed as described in Figure 2. Representative images of mitochondrial morphology in each group are shown. (B) Mitochondrial length was quantified and graphed. **P < 0.01; ***P < 0.001, Mag bar, 5 µm. (C) Protein lysates of CGN infected with GFP, CDK5-NES or Dn-CDK5-NES were analyzed by western blot analysis using antibodies to DRP1 and CDK5. Beta-ACTIN was used as loading control.
CDK5 results in increased DRP1 phosphorylation at the mitochondria
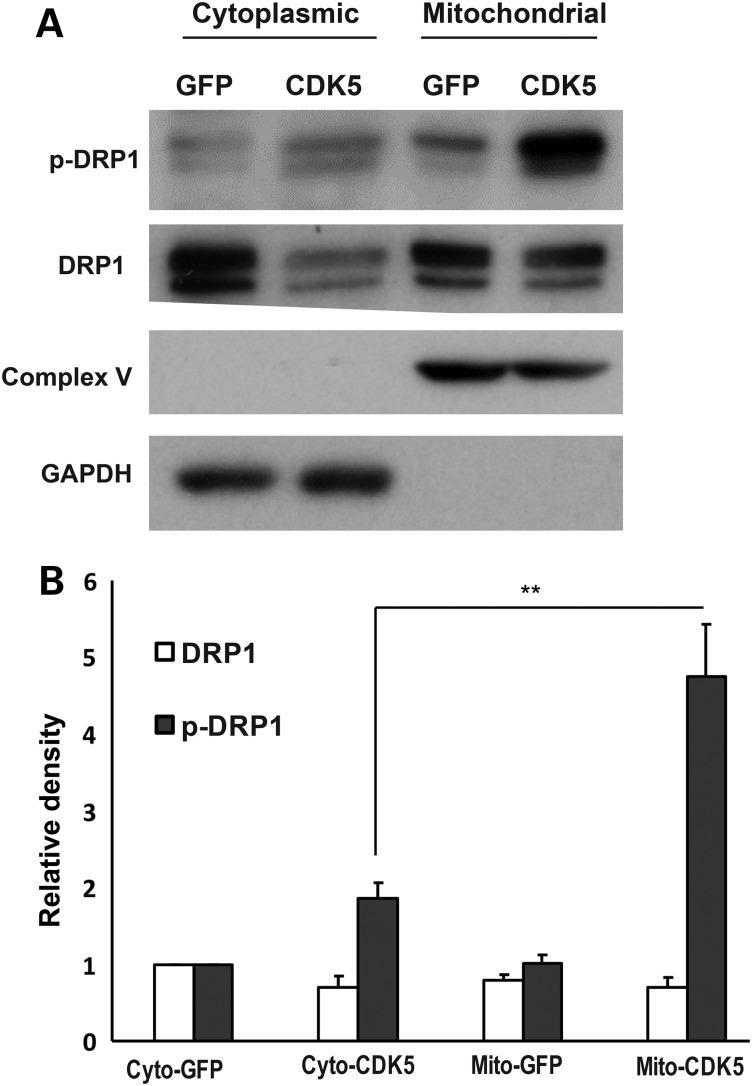
An early step in DRP1-mediated mitochondrial fission is DRP1 recruitment to the mitochondria and its assembly at the site of division (5). Accordingly, we examined whether CDK5 increases DRP1 phosphorylation at the mitochondria. We performed subcellular fractionation on primary neurons infected with adenovirus vectors carrying a wt-CDK5-NES or GFP control constructs. Mitochondrial and cytoplasmic fractions were analyzed using a pan DRP1 and phospho-DRP1 antibodies (Fig. 4A) and the relative density of either phospho-DRP1 or total DRP1 protein levels was quantified in each fraction (Fig. 4B). We found a significant increase of phospho-DRP1 protein in the mitochondria in wt-CDK5-NES group compared to control.
Figure 4.
CDK5 induces DRP1 recruitment from the cytosol to the mitochondria. (A) Cerebellar granule neurons were infected with adenoviruses carrying expression cassettes for either of GFP control or cytoplasmic CDK5 at 1 DIV. The protein lysates were collected at 3 DIV and were subjected to cell fractionation. Cytoplasmic and mitochondrial fractions were analyzed by immunoblotting using indicated antibodies. Complex V and GAPDH were used as control for mitochondrial and cytoplasmic fractions, respectively. (B) Quantification of the relative densitometric values of DRP1 and phospho-DRP1 is shown for neurons expressing GFP control or CDK5-NES. **P < 0.01.
CDK5 regulates mitochondrial fission associated with NMDA-induced neuronal death
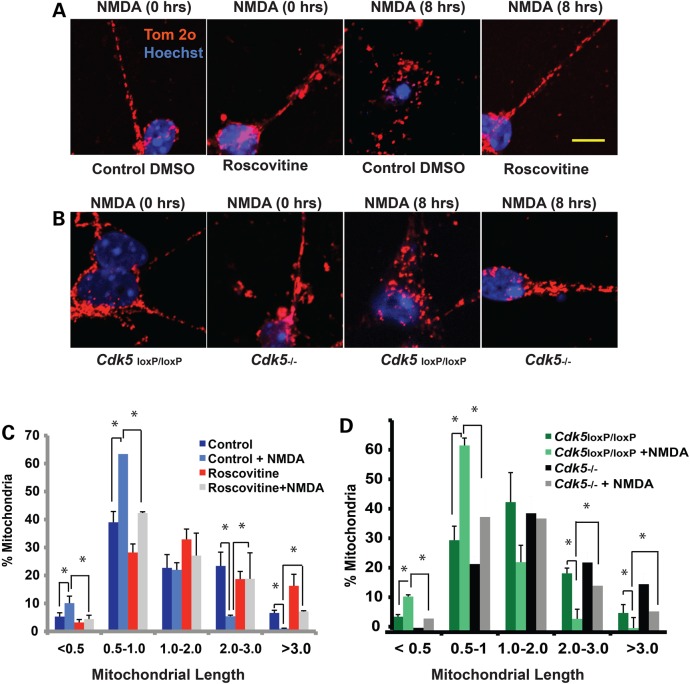
Given that CDK5 regulates DRP1 phosphorylation, we examined whether CDK5 regulates early mitochondrial morphology defects associated with NMDA-induced neuronal injury. Primary neurons were treated with Roscovitine, a pharmacological inhibitor of CDK5, or DMSO as control and exposed to NMDA. Following 8 h of NMDA treatment, neurons were fixed and mitochondrial lengths were analyzed as described previously. In the absence of Roscovitine, NMDA treatment induced an increase in mitochondrial fragmentation and the percentage of mitochondria exhibiting a length of less than 0.5 µm increased from 5.3 ± 1.4% in the control untreated neurons to 10.1 ± 2.5% in the NMDA-treated neurons (Fig. 5A and C). In contrast, neuronal cultures pre-treated with Roscovitine exhibited significantly less fragmentation upon addition of NMDA. 4.4 ± 1.4% of mitochondria exhibited a length of less than 0.5 µm in NMDA-treated neurons in the presence of Roscovitine, which is comparable to that of control untreated neurons at 3.2 ± 1.0% (Fig. 5A and C). These results indicate that pharmacological inhibition of CDK5 rescues mitochondrial fission associated with NMDA-induced neuronal loss (P < 0.05).
Figure 5.
Pharmacological inhibition of CDK5 or genetic deletion of CDK5 rescues mitochondrial fragmentation in NMDA-induced neuronal injury. (A) Cerebellar granule neurons were treated with 10 µm Roscovitine or DMSO control at 7 DIV for 3 h prior to exposure to NMDA (100 µm NMDA/10 µm Glycine). Following 8 h of treatment with NMDA, mitochondrial morphology was assessed using an antibody to Tom-20. Representative images of mitochondrial morphology under different treatment conditions are shown. (B). The cultured Cdk5−/− CGNs were obtained from Cdk5loxP/loxP cultures by addition of CRE adenoviruses at 5 DIV for 48 h. GFP adenoviruses were added to parallel cultures and used as control (referred to as Cdk5loxP/loxP). The neurons were treated with NMDA at 7 DIV as described in (A). Mitochondrial morphology was assessed using an antibody to Tom-20. Representative images of mitochondrial morphology under different treatment conditions are shown. (C and D) Mitochondrial length was measured and binned into different length categories and quantified as percentage. *P < 0.05; Mag bar, 10 µm.
To confirm our pharmacological results, we utilized a genetic model to examine whether germline deletion of CDK5 impacts mitochondrial morphological defects associated with NMDA-induced neuronal loss. Primary granule neurons obtained from the conditional Cdk5 knockout mice (Cdk5loxP/loxP) were cultured and Cdk5 was deleted by infection of cultures with the CRE adenovirus (Ad-CRE). Neuronal cultures obtained from Cdk5loxP/loxP mice were treated with a GFP adenovirus as a control. Mitochondrial morphology was evaluated by immunohistochemistry as described above. In control GFP-expressing neurons, 5.0 ± 0.7% and 11.5 ± 2.9% of mitochondria were fragmented with a length of less than 0.5 µm in the absence and presence of NMDA, respectively (Fig. 5B and D). In contrast, CRE expressing neurons in which Cdk5 was excised (Cdk5−/−) exhibited a significant reduction in percentage of fragmented mitochondria whereby 1.4 ± 0.6% and 4.4 ± 0.7% of mitochondria with a length of less than 0.5 µm were detected in the absence and presence of NMDA treatment, respectively (Fig. 5B and D). Together, our data confirm that either pharmacological inhibition of CDK5 or genetic deletion of Cdk5 rescues the mitochondrial fragmentation associated with NMDA-induced neuronal loss.
CDK5 drives NMDA-induced mitochondrial defects by targeting DRP1
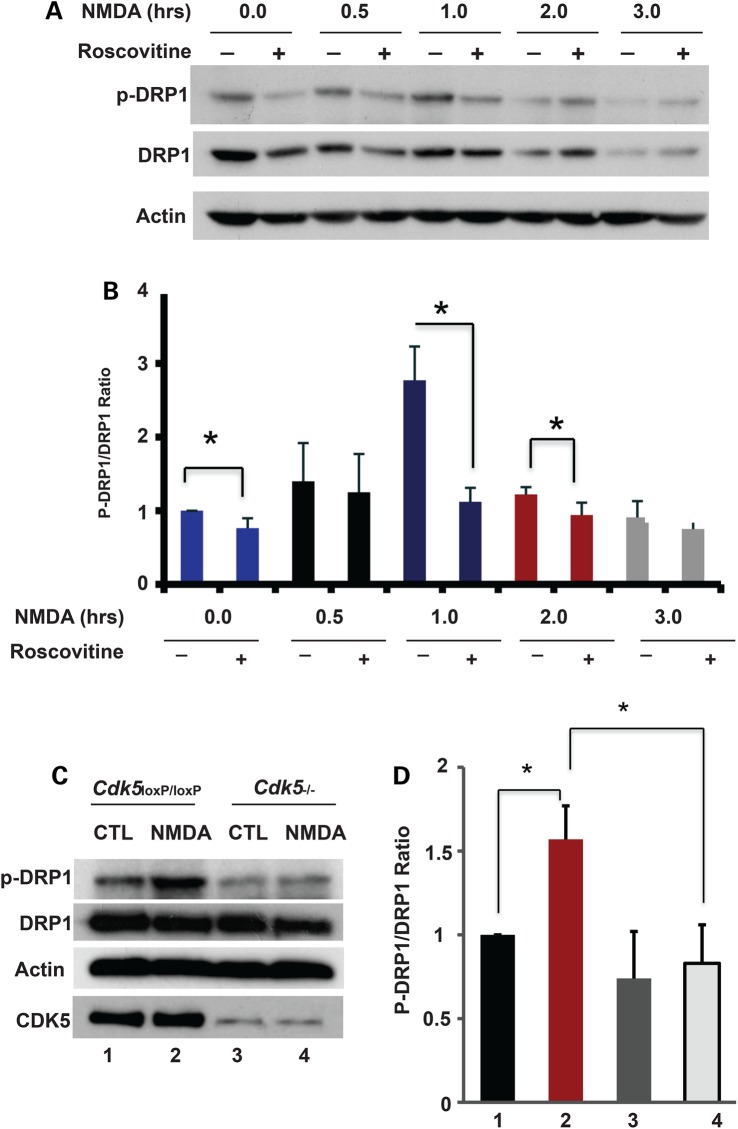
Given that CDK5 tightly regulates mitochondrial fragmentation following NMDA-induced neuronal death and that DRP1 is a direct target of CDK5 in vitro, we examined whether CDK5 regulates DRP1 phosphorylation following NMDA exposure. Primary neurons were first cultured and treated with NMDA in the absence and presence of Roscovitine. Protein lysates were analyzed by immunoblotting using phosphorylated-DRP1 (S585) and pan DRP1 antibodies. The ratio of phosphorylated DRP1 to total DRP1 was quantified (Fig. 6A and B). We found that phospho-DRP1 was upregulated and peaked 1 h following NMDA treatment in primary neurons in the absence of Roscovitine (Fig. 6A and B). Notably, the induction of phospho-DRP1 at S585 was blocked by the CDK5 inhibitor Roscovitine. We then examined DRP1 phosphorylation levels in NMDA-treated primary neurons in the presence of CDK5 (Cdk5loxP/loxP cultures infected with GFP control adenoviruses) or in Cdk5 knockout cultures (Cdk5loxP/loxP infected with CRE recombinase). The induction of phospho-DRP1 was blocked in the Cdk5 knockout cultures 1 h following NMDA treatment compared with control Cdk5loxP/loxP cultures (Fig. 6C and D). These data indicate that DRP1 phosphorylation at S585 is specifically mediated by CDK5 in primary neurons exposed to NMDA. Taken together our results reveal a mechanism whereby phosphorylation of DRP1 at S585 by CDK5 regulates NMDA-induced mitochondrial fission.
Figure 6.
CDK5 regulates DRP1 phosphorylation at S585 in NMDA-treated primary neurons. (A) Cerebellar granule neurons were treated with 10 µm Roscovitine or DMSO control at 7 DIV for 3 h followed by treatment with 100 µm NMDA/10 µm Glycine for 0, 0.5, 1, 2 or 3 h. Protein lysates were analyzed by immunoblotting using antibodies to phospho-DRP1 and pan-DRP1. Actin was used as loading control. (B) Quantification of the ratio of densitometric values of phospho-DRP1 to DRP1 in (A) is shown. *P < 0.05. (C) Cerebellar granule neurons were cultured from conditional Cdk5 knockout mice. Adenoviruses for CRE (Cdk5−/−) or GFP control (Cdk5loxP/loxP) were added to the cultures at 5 DIV for 48 h. Neurons were treated with 100 µm NMDA/10 μm Glycine for 0 and 1 h at 7 DIV. Protein lysates were subjected to immunoblotting analyses using antibodies to DRP1 and phospho-DRP1. Actin was used as loading control. (D) Quantification of the ratio of densitometric values of phospho-DRP1 to DRP1 is shown. *P < 0.05.
Discussion
In the present study, we describe a novel mechanism by which CDK5 mediates enhanced mitochondrial fission associated with excitotoxic neuronal injury by targeting the mitochondrial fission protein DRP1. CDK5 promotes mitochondrial fission following NMDA-induced neuronal injury through phosphorylation of DRP1 at Serine 585. Pharmacological inhibition of CDK5 by Roscovitine or genetic deletion of CDK5 rescues the mitochondrial morphological defects associated with excitotoxic injury and attenuates the DRP1 phosphorylation at Serine 585. We thereby establish that CDK5-mediated DRP1 phosphorylation on Serine 585 drives the mitochondrial morphological defects associated with excitotoxic neuronal cell death.
CDK5 plays an essential role in the regulation of neuronal death (30,31). Interestingly, while RNAi knockdown of Cdk5 was shown to rescue apoptosis-associated mitochondrial fission (25), the underlying molecular mechanism remained unclear. Excitotoxic neuronal injury is a distinct mode of cell death. Unlike classic apoptosis, excitotoxicity does not require BAX/BAK-mediated signaling (3). We previously showed that loss of mitochondrial integrity is an early event that precedes neuronal loss following NMDA-induced excitotoxicity, however the molecular mechanism leading to enhanced mitochondrial fragmentation in NMDA-treated neurons remained unclear.
In the present study, we show that CDK5 induces mitochondrial morphology defects associated with NMDA-induced excitotoxicity by directly targeting and phosphorylating Serine 585 on DRP1. Interestingly, CDK1 was shown to regulate DRP1 phosphorylation at the same site to enhance mitochondrial fission in mitotic cells (14). These two studies support that DRP1 phosphorylation on Serine 585 results in enhanced mitochondrial fission. This contrasts a recent study showing that in maturing neurons, CDK5-mediated phosphorylation of DRP1 at S585 inhibits mitochondrial fission (15). How the phosphorylation of the same site within DRP1 could yield opposing results in a different cellular context remains an interesting area for future research. However, it appears that while DRP1 phosphorylation at Serine 585 in mitotic cells and post-mitotic neurons results in enhanced mitochondrial fission, other post-translational modifications to DRP1 may produce a different outcome in maturing neurons. This hypothesis is supported by the findings that in maturing neurons direct phosphorylation of Drp1 by CDK5 on Serine 585 was not documented (15). In our study, we establish that DRP1 is a direct substrate of CDK5 and CDK5-mediated phosphorylation of DRP1 at Serine 585 rat/Serine 616 human results in its recruitment and subsequent mitochondrial fission associated with neuronal injury. Our findings also demonstrate that in post-mitotic neurons CDK5 phenocopies the CDK1-induced phenotype observed in mitotic cells.
The fact that DRP1 phosphorylation results in different physiological outcomes depending on the cell context is not surprising. The Serine 637 (human) and Serine 656 (rat) residue of DRP1 is phosphorylated by protein kinase A (PKA) (11,12). The PKA-mediated phosphorylation of DRP1 at Serine 637(human)/Serine 656(rat) resulted in an elongated mitochondrial phenotype. In further support of this study, calcineurin mediated dephosphorylation of DRP1 at Serine 637 (human)/Serine 656 (rat) was shown to induce its translocation from the cytoplasm to the mitochondria resulting in mitochondrial fission (10,12). In contrast, phosphorylation of DRP1-isoform-3 at Serine 600 (consensus site of Ser 637 at DRP1 isoform-1) by Ca2+/calmodulin-dependent protein kinase Iα (CamKIα) resulted in DRP1 translocation to the mitochondria and enhanced mitochondrial fission in neurons (13). Therefore, although the physiological context relevant to these multiple phosphorylation sites requires further study, our results together with the studies performed in non-neuronal cells clearly support an important link between DRP1 phosphorylation at Serine585 and mitochondrial fission associated with neuronal death. Our studies further suggest an important link between CDK5 and DRP1 function in regulation of excitotoxic injury.
Finally, our observation that either of p35- or p25- containing CDK5 complexes can directly phosphorylate DRP1, raises the possibility that CDK5 may control both steady state mitochondrial fission and excessive mitochondria fission associated with cell death. p35 is most often associated with physiological CDK5 function (24). However, p25 is generated through calpain-mediated cleavage of p35 following neuronal injury (32). Our findings suggest a model whereby the CDK5/p35 complex regulates physiological mitochondrial fission required for synaptogenesis in post-mitotic neurons, and the CDK5/p25 complex regulates excessive mitochondrial fission associated with neuronal death. This model may explain how CDK5 performs its dual role during synaptogenesis and cell death via regulation of mitochondrial morphology and function. In further support of these results, we recently showed that inhibition of calpain, the protease responsible for p35 cleavage into p25, rescues mitochondrial fragmentation associated with NMDA-induced neuronal death (4). Our study provides a potential explanation on how calpastatin can rescue excessive mitochondrial fission by inhibiting cleavage of p35 (4). In summary, our results show that DRP1 is a direct target of CDK5 and CDK5-mediated phosphorylation of DRP1 at Serine585 tightly regulates mitochondrial morphology during neuronal injury.
Materials and Methods
Animals
CD1 animals were purchased from Charles Rivers. The floxed Cdk5 (Cdk5loxP/loxP) mice have been described (33,34). The Cdk5 (Cdk5−/−) conditional knockout cell cultures were generated by adding CRE adenoviruses to primary cerebellar granule neurons obtained from Cdk5loxP/loxP mice as described below. GFP adenovirus were added to parallel cultures and used as control. Experiments involving animals conformed to the guidelines set forth by the Canadian Council for the Use and Care of Animals in Research (CCAC) and the Canadian Institutes for Health Research (CIHR) with approval from the University of Ottawa Animal Care Committee.
Primary cerebellar granule neurons cultures and virus constructions
Cerebellar granule neurons (CGNs) were cultured from either CD1 mice or Cdk5loxP/loxP mice at postnatal day 7 as described (35). Recombinant adenoviral vectors carrying CDK5 or DnCDK5 expression cassettes were prepared using AdEasy system, as described (36). Primary cerebellar granule neurons were infected with adenoviral vectors at 5 days in vitro (DIV) for two days with a ranging multiplicity of infection (MOI; 25–150). MOI of 100 was selected as optimum titer based on the efficiency of infection and minimum toxicity. To measure toxicity, the number of infected dead cells was compared to the total number of infected cells in the field. Recombinant adeno-associated virus (AAV) vectors were constructed by subcloning cDNA sequences of GFP-DRP1-WT, GFP-DRP1-585A and GFP-DRP1–585D mutants in the AM/CBA-pl-WPRE-bGH plasmid and the viruses were generated as described (37). The DRP1 constructs were fused with GFP at the N terminal on the same promoter. Primary cerebellar granule neurons cultures were infected with AAV vectors at the second day of plating for 6 days.
NMDA treatment
NMDA treatments were performed as described (4). Primary cerebellar granule neurons were treated with 100 μm NMDA and 10 μm Glycine at 7 DIV (1 h, 100 μm), after which they were switched to conditioned media from parallel cultures.
Immunoblotting
Western blotting was performed as previously described (19). The following antibodies were used as indicated on the blots: DRP1 (BD bioscience, Cat: 611112), P-DRP1 (S616, Cell Signaling, Cat: 3455S), CDK5 (Millipore, Cat: 05-364) and Actin (Sigma, Cat: A5316).
Immunofluorescence
Immunofluorescence was performed as previously described (27,38). At each time point, neurons were fixed for 30 min with ice cold 4% paraformaldehyde in 1X phosphate-buffered saline (1XPBS) and then rinsed twice with 1XPBS. Cells were permeabilized with 300 µl of ice-cold 0.4% Triton-X in 1XPBS for 10 min and stained with the primary antibodies to either Tom20 (Santa Cruz: sc-17764), or cytochrome C (BD Biosciences, Cat: 556432) in 10% normal goat Serum-0.4% Triton X/PBS for 1 h. Following three washes (5 min each) with ice-cold 1XPBS, cells were incubated with the secondary antibodies in 10% normal goat Serum-0.4% Triton X/PBS for 1 h. The cells were washed for 5 min and stained with Hoechst for 5 min. Following Hoechst staining, neurons were washed with 1X PBS for 3 × 5 min and mounted. Representative samples were photographed using a Zeiss 510 meta confocal microscope or a Zeiss Axiovert 100 (Oberkochen, Germany) fluorescence microscope equipped with a QiCam Digital camera (QImaging Corporation, Burnaby, Canada) and Northern Eclipse software (Empix Imaging Inc., Mississauga, ON, Canada).
Mitochondrial length measurement
Neurons were fixed and stained with an antibody against cytochrome c or Tom20 as described above. For each replicate, more than 1000 mitochondria were counted from 5 to 8 different random fields. Whole cell images were acquired by exciting at 488 nm with the GFP filter (Chroma Technology Corp., Rockingham, VT, USA). Mitochondrial length was measured by tracing the mitochondria using Northern Eclipse software. As mitochondrial length varied remarkably even in control neurons, mitochondria were classified into different categories with a length ranging from less than 0.5 µm, 0.5 to 1 µm, 1 to 2 µm, 2–3 µm, and greater than 3 µm for comparative purposes.
Analysis of DRP1 recruitment
Primary cerebellar granule neurons were infected with GFP or CDK5 adenoviral vectors at 1 DIV for 2 days. Neurons were harvested at 3 DIV and Mitochondria were isolated using subcellular fractionation as described previously (39). Mitochondria and cytoplasmic fractions were run on SDS–PAGE gel side by side and the membranes were blotted for DRP1 and Phospho-DRP1 antibodies. The efficiency of mitochondrial isolation were marked using mitochondrial complex V (Abcam; Cat: ab14748) and GAPDH (Millipore, Cat: MAB374). The relative densitometric values of DRP1 and phospho-DRP1 were measured in each fraction analyzed by Image J.
In vitro kinase assay
Recombinant His-tagged wild type or mutant DRP1 were expressed and purified by metal chelating resin and further purified by ion exchange chromatography using Q-fast flow (Pharmacia, Uppsala, Sweden). All GST-CDK5, GST-p35 and GST-p25 fusion proteins were expressed in E. coli and affinity purified using Glutathione Sepharose 4B (GE healthcare, Cat 17-0756-01) as per manufacturer's instruction. The in vitro kinase reaction with Cdk5 was analyzed as described previously (19,21,40).
Quantification and statistical analysis
The data represent the mean and standard deviation from three independent experiments (n = 3). Statistical analyses were performed using unpaired two-tailed Student's t-test. All histograms were presented as mean ± SEM. A P-value <0.05 was considered significant and was indicated on the graphs by an asterisk. A P-values of <0.01 was marked by two asterisks and a P-value of <0.001 was marked with three asterisk.
Funding
This work was supported by Heart Stroke Foundation, Canadian Institute of Heath Research (CIHR), Brain Canada/Krembil Foundation, Parkinsons Society Canada, Parkinsons Research Consortium, Eurpoean Union Joint Program- Neurodegenerative Disease Research, and Ontario Brain Institute to D.S.P.. D.S.P. is a Heart and Stroke Career Investigator. A.J.-A. was supported by a CIHR doctoral research award. A.J.-A. and I.I. are recipient of CIHR fellowship awards.
Acknowledgement
We like to thank Dr Vahab D. Soleimani at McGill University for critical help with generation of the recombinant proteins.
Conflict of Interest statement. None declared.
References
- 1.Beal M.F. (1992) Mechanisms of excitotoxicity in neurologic diseases. FASEB J., 6, 3338–3344. [PubMed] [Google Scholar]
- 2.Kalia L.V., Kalia S.K., Salter M.W. (2008) NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol., 7, 742–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahani-Asl A., Germain M., Slack R.S. Mitochondria: joining forces to thwart cell death. (2010) Biochim. Biophys. Acta, 1802, 162–166. [DOI] [PubMed] [Google Scholar]
- 4.Jahani-Asl A., Pilon-Larose K., Xu W., MacLaurin J.G., Park D.S., McBride H.M., Slack R.S. (2011) The mitochondrial inner membrane GTPase, optic atrophy 1 (Opa1), restores mitochondrial morphology and promotes neuronal survival following excitotoxicity. J. Biol. Chem., 286, 4772–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lackner L.L., Nunnari J.M. (2009) The molecular mechanism and cellular functions of mitochondrial division. Biochim. Biophy. Acta, 1792, 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soubannier V., McBride H.M. (2009) Positioning mitochondrial plasticity within cellular signaling cascades. Biochim. Biophy. Acta, 1793, 154–170. [DOI] [PubMed] [Google Scholar]
- 7.Harder Z., Zunino R., McBride H. (2004) Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr. Biol., 14, 340–345. [DOI] [PubMed] [Google Scholar]
- 8.Wasiak S., Zunino R., McBride H.M. (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol., 177, 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zunino R., Schauss A., Rippstein P., Andrade-Navarro M., McBride H.M. (2007) The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J. Cell Sci., 120, 1178–1188. [DOI] [PubMed] [Google Scholar]
- 10.Cereghetti G.M., Stangherlin A., Martins de Brito O., Chang C.R., Blackstone C., Bernardi P., Scorrano L. (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl Acad. Sci. USA, 105, 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C.R., Blackstone C. (2007) Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem., 282, 21583–21587. [DOI] [PubMed] [Google Scholar]
- 12.Cribbs J.T., Strack S. (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep., 8, 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X.J., Lu Y.F., Li S.A., Kaitsuka T., Sato Y., Tomizawa K., Nairn A.C., Takei K., Matsui H., Matsushita M. (2008) CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol., 182, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem., 282, 11521–11529. [DOI] [PubMed] [Google Scholar]
- 15.Cho B., Cho H.M., Kim H.J., Jeong J., Park S.K., Hwang E.M., Park J.Y., Kim W.R., Kim H., Sun W. (2014) CDK5-dependent inhibitory phosphorylation of Drp1 during neuronal maturation. Exp. Mol. Med., 46, e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strack S., Wilson T.J., Cribbs J.T. (2013) Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. J. Cell Biol., 201, 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z., Okamoto K., Hayashi Y., Sheng M. (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell, 119, 873–887. [DOI] [PubMed] [Google Scholar]
- 18.Barsoum M.J., Yuan H., Gerencser A.A., Liot G., Kushnareva Y., Graber S., Kovacs I., Lee W.D., Waggoner J., Cui J., et al. (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J., 25, 3900–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang E., Qu D., Zhang Y., Venderova K., Haque M.E., Rousseaux M.W., Slack R.S., Woulfe J.M., Park D.S. (2010) The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat. Cell Biol., 12, 563–571. [DOI] [PubMed] [Google Scholar]
- 20.Park D.S., Levine B., Ferrari G., Greene L.A. (1997) Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J. Neurosci., 17, 8975–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu D., Rashidian J., Mount M.P., Aleyasin H., Parsanejad M., Lira A., Haque E., Zhang Y., Callaghan S., Daigle M., et al. (2007) Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron, 55, 37–52. [DOI] [PubMed] [Google Scholar]
- 22.Rashidian J., Iyirhiaro G., Aleyasin H., Rios M., Vincent I., Callaghan S., Bland R.J., Slack R.S., During M.J., Park D.S. (2005) Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc. Natl Acad. Sci. USA, 102, 14080–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Cicero S.A., Wang L., Romito-Digiacomo R.R., Yang Y., Herrup K. (2008) Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc. Natl Acad. Sci. USA, 105, 8772–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhavan R., Tsai L.H. (2001) A decade of CDK5. Nat. Rev. Mol. Cell Biol., 2, 749–759. [DOI] [PubMed] [Google Scholar]
- 25.Meuer K., Suppanz I.E., Lingor P., Planchamp V., Goricke B., Fichtner L., Braus G.H., Dietz G.P., Jakobs S., Bahr M., et al. (2007) Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ., 14, 651–661. [DOI] [PubMed] [Google Scholar]
- 26.Sung J.Y., Engmann O., Teylan M.A., Nairn A.C., Greengard P., Kim Y. (2008) WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc. Natl Acad. Sci. USA, 105, 3112–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahani-Asl A., Cheung E.C., Neuspiel M., MacLaurin J.G., Fortin A., Park D.S., McBride H.M., Slack R.S. (2007) Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death . J. Biol. Chem., 282, 23788–23798. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare M.J., Kushwaha N., Zhang Y., Aleyasin H., Callaghan S.M., Slack R.S., Albert P.R., Vincent I., Park D.S. (2005) Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J. Neurosci., 25, 8954–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Herrup K. (2008) Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle, 7, 3487–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang E., Qu D., Park D.S. (2010) Cdk5: Links to DNA damage. Cell Cycle, 9, 3142–3143. [DOI] [PubMed] [Google Scholar]
- 31.Maestre C., Delgado-Esteban M., Gomez-Sanchez J.C., Bolanos J.P., Almeida A. (2008) Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J., 27, 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith P.D., Mount M.P., Shree R., Callaghan S., Slack R.S., Anisman H., Vincent I., Wang X., Mao Z., Park D.S. (2006) Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci., 26, 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawasli A.H., Benavides D.R., Nguyen C., Kansy J.W., Hayashi K., Chambon P., Greengard P., Powell C.M., Cooper D.C., Bibb J.A. (2007) Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat. Neurosci., 10, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagace D.C., Benavides D.R., Kansy J.W., Mapelli M., Greengard P., Bibb J.A., Eisch A.J. (2008) Cdk5 is essential for adult hippocampal neurogenesis. Proc. Natl Acad. Sci. USA, 105, 18567–18571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortin A., Cregan S.P., MacLaurin J.G., Kushwaha N., Hickman E.S., Thompson C.S., Hakim A., Albert P.R., Cecconi F., Helin K., et al. (2001) APAF1 is a key transcriptional target for p53 in the regulation of neuronal cell death. J. Cell Biol., 155, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA, 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zolotukhin S., Potter M., Zolotukhin I., Sakai Y., Loiler S., Fraites T.J., Jr, Chiodo V.A., Phillipsberg T., Muzyczka N., Hauswirth W.W., et al. (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods, 28, 158–167. [DOI] [PubMed] [Google Scholar]
- 38.Irrcher I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M., Lutz A.K., Rousseaux M.W., Bevilacqua L., Jahani-Asl A., et al. (2010) Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet., 19, 3734–3746. [DOI] [PubMed] [Google Scholar]
- 39.Cheung E.C., Joza N., Steenaart N.A., McClellan K.A., Neuspiel M., McNamara S., MacLaurin J.G., Rippstein P., Park D.S., Shore G.C., et al. (2006) Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J., 25, 4061–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishijima H., Nishitani H., Seki T., Nishimoto T. (1997) A dual-specificity phosphatase Cdc25B is an unstable protein and triggers p34(cdc2)/cyclin B activation in hamster BHK21 cells arrested with hydroxyurea. J. Cell Biol., 138, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]