Abstract
The accumulation of toxic metals in the human body is influenced by exposure and mechanisms involved in metabolism, some of which may be under genetic control. This is the first genome-wide association study to investigate variants associated with whole blood levels of a range of toxic metals. Eleven toxic metals and trace elements (aluminium, cadmium, cobalt, copper, chromium, mercury, manganese, molybdenum, nickel, lead and zinc) were assayed in a cohort of 949 individuals using mass spectrometry. DNA samples were genotyped on the Infinium Omni Express bead microarray and imputed up to reference panels from the 1000 Genomes Project. Analyses revealed two regions associated with manganese level at genome-wide significance, mapping to 4q24 and 1q41. The lead single nucleotide polymorphism (SNP) in the 4q24 locus was rs13107325 (P-value = 5.1 × 10−11, β = −0.77), located in an exon of SLC39A8, which encodes a protein involved in manganese and zinc transport. The lead SNP in the 1q41 locus is rs1776029 (P-value = 2.2 × 10−14, β = −0.46). The SNP lies within the intronic region of SLC30A10, another transporter protein. Among other metals, the loci 6q14.1 and 3q26.32 were associated with cadmium and mercury levels (P = 1.4 × 10−10, β = −1.2 and P = 1.8 × 10−9, β = −1.8, respectively). Whole blood measurements of toxic metals are associated with genetic variants in metal transporter genes and others. This is relevant in inferring metabolic pathways of metals and identifying subsets of individuals who may be more susceptible to metal toxicity.
Introduction
Toxic metals are ubiquitous in the environment, with sources ranging from shipyard and construction business to agricultural and domestic industries. These metals are bio-accumulative and have a variety of toxic effects in the human body (1). For example, exposure to cadmium could result in liver and renal damage, as well as bone demineralization, while lead poisoning could affect the gastrointestinal and neurological systems (2).
The accumulation of these metals in the body is not only governed by environmental exposure but also by mechanisms involved in the absorption, distribution, metabolism and elimination (ADME) of the compounds. Knowledge about the pathways involved in the ADME of different metals has generally been generated in experimental studies.
For example, several gene transporter families have been implicated in manganese transport in plants (3). These transporter families include NRAMP (natural resistance-associated macrophage protein), YSL (yellow stripe-like), ZIP [zinc-regulated transporter/iron-regulated transporter (ZRT/IRT1)-related protein], CAX (cation exchanger), CCX (calcium cation exchangers), CDF/MTP (cation diffusion facilitator/metal tolerance protein) and VIT (vacuolar iron transporter).
Another example is that the P(1B)-type heavy metal ATPases (HMAs) are diverse in terms of tissue distribution, subcellular localization and metal specificity. Functional studies of HMAs have shown that these transporters can be divided into two subgroups based on their metal–substrate specificity: a copper (Cu)/silver (Ag) group and a zinc (Zn)/cobalt (Co)/cadmium (Cd)/lead (Pb) group (4).
However, it is not known if all of these mechanisms involved in the ADME of metals are operating and valid in humans. One way to examine such mechanisms in humans is to evaluate if genetic variation in the genes encoding the critical proteins involved in these ADME steps are related to circulating levels of relevant metals. Mendelian disorders such as Wilson's disease and Menke's disease are examples of direct genetic influence on metal ADME steps. The former is an autosomal recessive disorder involving a mutation in ATP7B gene, causing abnormal hepatic copper deposition, and the latter is an X-linked recessive disorder involving a mutation in the ATP7A gene, causing copper deficiency. Additionally, hereditary factors and environmental factors together have been suggested to influence blood levels of cadmium and lead in healthy individuals (5). A twin study using genetic linkage analysis showed significant linkage for lead and suggestive linkage for cadmium, mercury, selenium and zinc (6).
Today, we have the ability to investigate genetic variation at multiple sites across all chromosomes through genome-wide association studies (GWAS). This approach has the advantage that associations with genetic variation mapping to any gene, not just some pre-specified subset, can be investigated. So far, there has been one GWAS that examined the levels of copper, zinc and selenium, revealing several loci with biologically relevant genes (7). For example, the locus showing association with zinc contains genes for the Zn-containing enzyme carbonic anhydrase, and the locus associated with selenium includes genes involved in the metabolism of sulphur-containing amino acids. Identification of these loci may help to identify population subgroups that may be more susceptible to deficiency or toxicity.
Using a GWAS approach, our study aimed to investigate genetic variants associated with serum levels of a range of metals in a cohort of 949 individuals. The metals were aluminium, cadmium, cobalt, copper, chromium, mercury, manganese, molybdenum, nickel, lead and zinc. To our knowledge, this is the first GWAS studying serum levels of toxic metals, in a comprehensive manner and not restricted to trace elements.
Results
A total of 949 individuals from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study were included in the sample. Following imputation, a total of 8 736 858 high-quality single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) > 1% were included in the analysis. Median values for the studied metals are presented in Table 1, together with the genomic control inflation factor, all of which were close to 1. No additional correction for residual population structure after adjustment for principal components was required. Further details are available in Materials and Methods.
Table 1.
Median values (with 25th and 75th percentiles) and genomic control lambda are given for the different metals included in the study
| Metal | Median serum level (SD) (mmol/l) | Genomic inflation factor |
|---|---|---|
| Al | 0.637 (0.504–0.795) | 1.02 |
| Cd | 2.41 (1.72–3.74) | 1.00 |
| Co | 1.43 (1.09–1.90) | 1.00 |
| Cr | 11.8 (9.58–16.00) | 1.01 |
| Cu | 12.8 (11.6–14.1) | 1.00 |
| Hg | 8.92 (5.86–13.65) | 1.00 |
| Mn | 138 (115–165) | 1.01 |
| Mo | 9.74 (8.09–11.80) | 0.991 |
| Ni | 90.6 (68.3–109.0) | 0.982 |
| Pb | 0.083 (0.059–0.110) | 1.01 |
| Zn | 96.0 (87.7–104.0) | 1.02 |
A total of three metals demonstrated signals at a genome-wide significance level—manganese, cadmium and mercury—defined here as P < 4.5 × 10−9 with a Bonferroni correction for 11 tested metals.
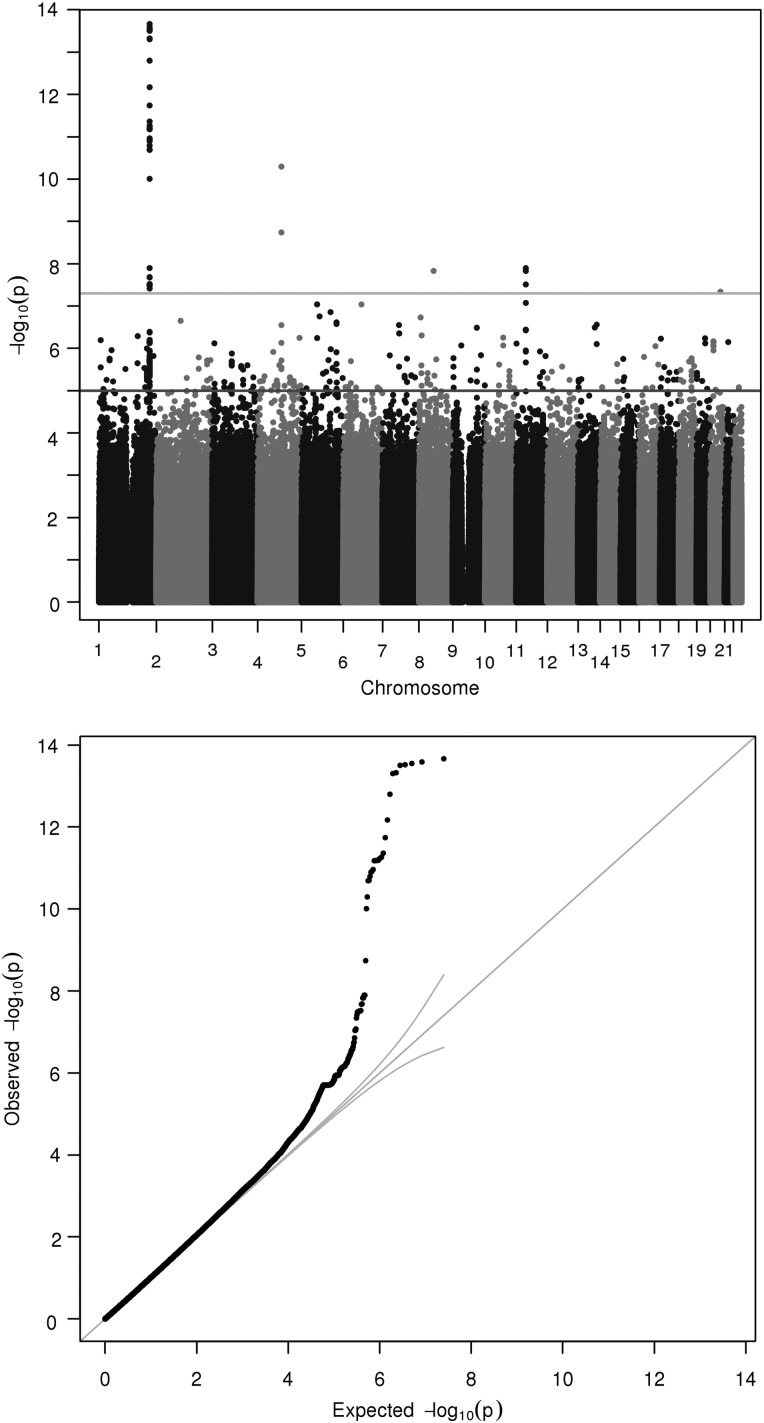
The GWAS analyses revealed two regions that were associated with manganese level. These mapped to 4q24 and 1q41 (Fig. 1 shows the Manhattan and quantile–quantile plots). The lead SNP in the 4q24 locus was a coding variant, rs13107325 (P-value of 5.08 × 10−11, β value of −0.767, MAF of 0.038), located in SLC39A8, a gene that encodes a well-known protein involved in manganese and zinc transport (8). The amino acid change for this SNP was alanine to threonine. ‘Sorting Tolerant From Intolerant’ (SIFT) algorithm (9) prediction revealed that the variation may have damaging effects on protein formation. The lead SNP in the 1q41 locus was rs1776029 (P-value of 2.17 × 10−14, β value of −0.456, MAF of 0.182). The SNP lies just downstream of SLC30A10, another well-established ion transporter protein (10). There are no non-synonymous SNPs in the region that pass the genome-wide significance threshold, and conditional analysis revealed no secondary signals. Supplementary Material, Figs S1 and S2 show the local association plots for these two loci. The lead SNP rs1776029 lies within a region known to be marked by histone-3-lysine-27 trimethylation (H3K27Me3), which is thought to be inhibitory to transcription (11).
Figure 1.
Manhattan and quantile–quantile plot of GWAS of serum manganese. Each point corresponds to a SNP passing quality control (QC), plotted according to genomic position on the x-axis and the strength of association (−log10 P-value) on the y-axis. The horizontal lines indicate genome-wide significance threshold (5 × 10−8) and also a nominal threshold of 10−05.
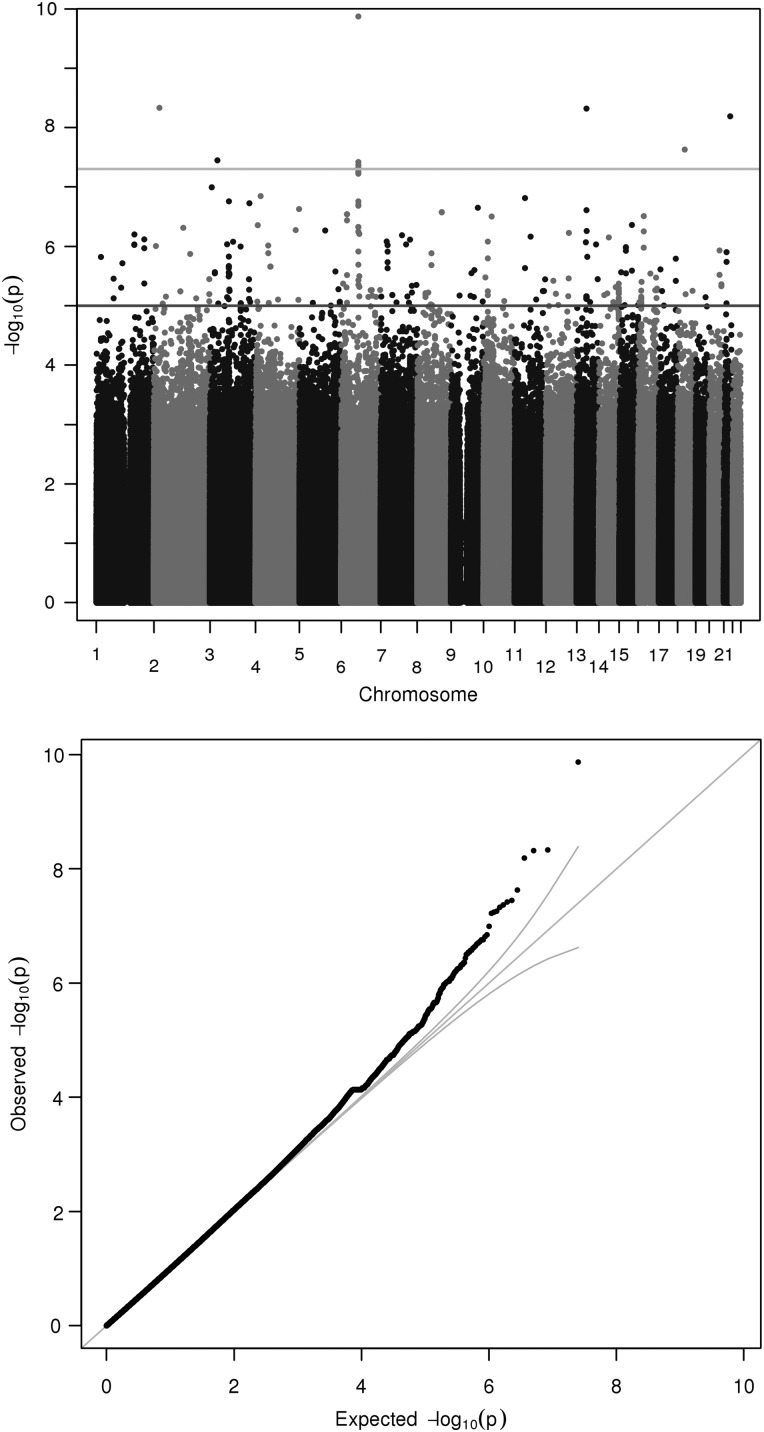
The locus 6q14.1 was associated with cadmium levels. The lead SNP was rs9350504 (P-value of 1.35 × 10−10, β value of −1.16, MAF of 0.0175), which is an intronic variant within CD109, a glycosyl phosphatidylinositol-linked glycoprotein (Fig. 2). Since smoking was correlated with cadmium levels, we also tested the model with smoking included as a covariate. In this model, the P-value of rs9350504 increased marginally to 1.90 × 10−11. This SNP lies within a region known to be marked by H3K27Me3 as well as histone-3-lysine-4 monomethylation (H3K4Me1), the latter of which is thought to be an enhancer of transcription (12).
Figure 2.
Manhattan and quantile–quantile plot of GWAS of serum cadmium. Each point corresponds to a SNP passing QC, plotted according to genomic position on the x-axis and the strength of association (−log10 P-value) on the y-axis. The horizontal lines indicate genome-wide significance threshold (5 × 10−8) and also a nominal threshold of 10−05.
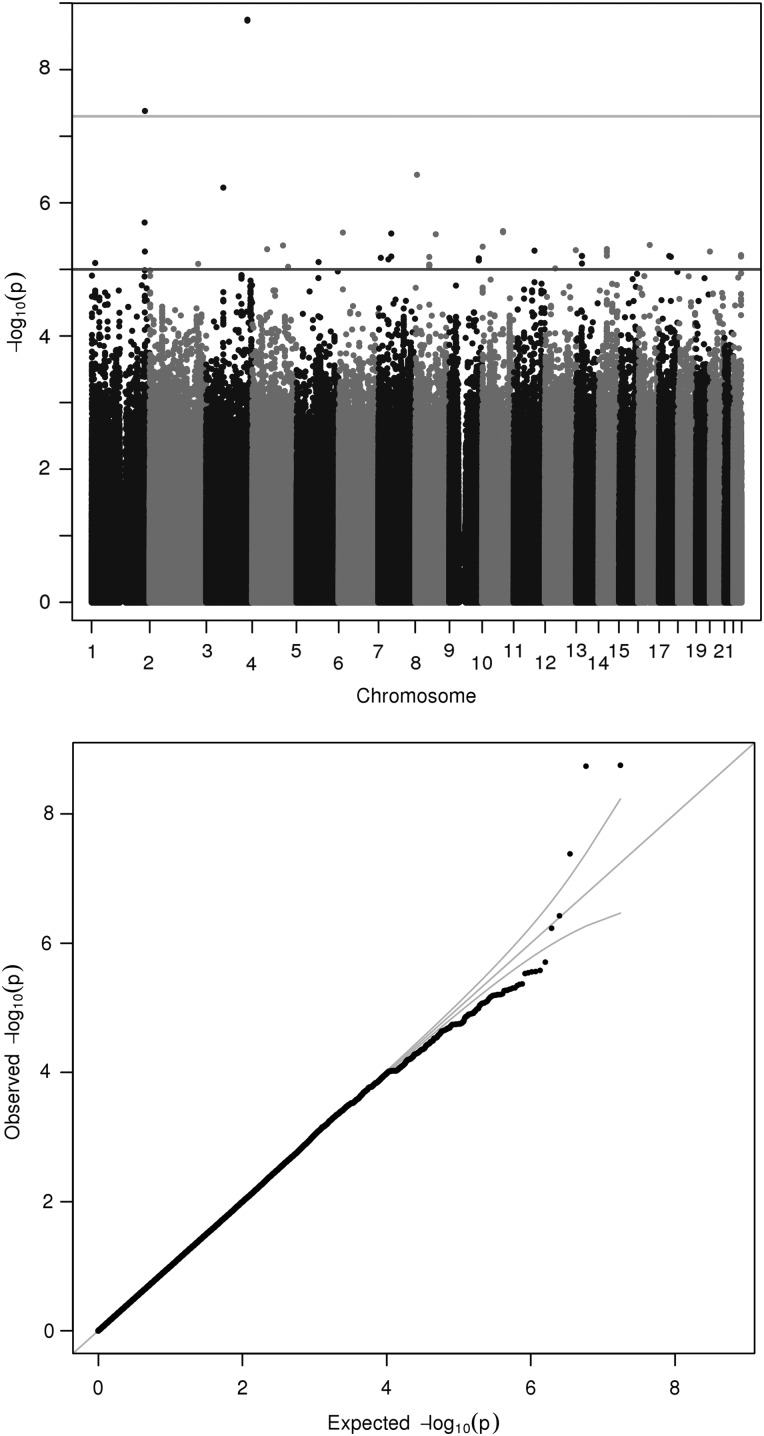
The 3q26.32 locus was associated with mercury levels (P-value of 1.76 × 10−9, β value of −1.80, MAF of 0.0140). The lead SNP is rs148534631, an intron variant within LINC00578, an intergenic non-protein-coding RNA (Fig. 3). Local association plots for these two metals can be found in Supplementary Material, Figs S2 and S3. The lead SNP rs148534631 also lies within a region known to be marked by H3K27Me3.
Figure 3.
Manhattan and quantile–quantile plot of GWAS of serum mercury. Each point corresponds to a SNP passing QC, plotted according to genomic position on the x-axis and the strength of association (−log10 P-value) on the y-axis. The horizontal lines indicate genome-wide significance threshold (5 × 10−8) and also a nominal threshold of 10−05.
Supplementary Material, Table S2 provides a list of lead SNPs associated with metals at a P-value threshold of 1 × 10−5. A SNP associated with chromium levels (rs12915189) was associated with information processing speed in a GWAS on three cohorts of individuals (13). This SNP is an intron variant in CRTC3, which regulates CREB-dependent gene transcription in a phosphorylation-independent manner. A SNP associated with manganese (rs11006464) was associated with oleic acid levels in a GWAS on five population-based cohorts (14). It is an intron variant in the FAM13C1 gene.
When comparing our results with that of Evans et al. (7), the SNPs that showed genome-wide significance in their study for Zn levels (rs1532423 and rs2120019) had P-values of 0.000142 and 1.03 × 10−5 in our study and same direction of effects, demonstrating consistency with other cohorts.
Discussion
The present study showed that the GWAS approach is useful to investigate mechanisms in humans that are of interest for circulating levels of toxic metals and trace elements. By this approach, we identified novel associations between genetic variation mapping to genes encoding for proteins known to be involved in ion transport in experimental studies. This approach could validate that these previously described ADME pathways are also valid in humans. Besides the associations with ion transporters, we also found an association between cadmium and CD109 not previously described. As evidenced by the negative β values, for all four significant SNPs, the presence of minor alleles is associated with lower metal serum levels compared with the median values, possibly suggesting increased clearance or decreased absorption.
Manganese is a trace metal that is essential to the body, but can also be neurotoxic at higher chronic exposure levels, with symptoms persisting long after cessation of exposure. Occupational studies have demonstrated that exposure to manganese can cause cognitive and motor defects, in addition to changes in mood and short-term memory, altered reaction time and reduced hand–eye coordination (15,16). Affected workers often show accumulation of manganese in the globus pallidus (17). Serum manganese levels can be influenced by a range of factors, including dietary characteristics for example carbohydrate source, presence of phytate and presence of animal protein and dietary iron (18). In addition, serum ferritin concentration has also been associated with serum manganese levels (19,20). Henn et al. studied a cohort of 332 women and reported that women with human haemachromatosis protein (HFE) variant alleles at rs1800562 or rs1799945 had 12% lower blood manganese concentrations than women with no variant alleles. The reason for this is postulated to be a common cellular transport mechanism; hence, excess of one element inhibits transport of the other (21). In our study, rs1800562 was weakly associated with manganese levels (P-value of 0.000484) but not rs1799945 (P-value of 0.817).
In our study, rs13107325 was associated with manganese levels. Several studies have uncovered an association between this SNP and other phenotypes, including metabolic traits such as blood pressure, body mass index and lipid levels (22–25). Rs13107325 is a non-synonymous SNP within SLC39A8. This gene encodes a zinc transporter that functions in the cellular importation of zinc at the onset of inflammation. Waterworth's reports postulate that since the gene is induced by tumour necrosis factor alpha (TNF-α), it is possible that the SLC39A8 molecule might be associated with lipids such as high-density lipoprotein (HDL) in an inflammatory context. This SNP has also been associated with neurological phenotypes including schizophrenia (26) and stress (27).
This SNP gives rise to substitution of alanine (hydrophobic) to alanine (hydrophilic). It is in low linkage disequilibrium (LD) with nearby SNPs in HapMap sample (26). SLC39A8 is one of the members of the family of 39 solute carrier transporters. It transports manganese and zinc, which are crucial for the functioning of various organs, but may be neurotoxic at levels beyond a certain concentration. Excessive exposure to manganese may lead to Parkinsonian features, as well as other neuropsychiatric symptoms such as agitation and hallucination. SLC39A8 is responsible for the uptake of manganese and zinc from blood into various tissues. It is expressed at high levels in various tissues (including placenta), but low levels in the brain. The lead SNP in the 1q41 locus is rs1776029. The SNP lies within the intronic region of SLC30A10, which is a member of the solute carrier family 30 member 10. SLC30A10 is a member of the SLC30 solute carrier subfamily of the cation diffusion facilitator (28). It is a manganese efflux transporter localized to the cell surface, which decreases intracellular manganese and protects against manganese toxicity (29). Recessive mutations in SLC30A10 have been linked to a syndrome characterized by severe manganese accumulation, polycythaemia, neurological disturbances and liver complications (10,30). Of note, these patients were not known to be exposed to excessive manganese, yet they developed symptoms of toxicity. SLC30A10 is the only mutated protein known to cause manganese toxicity, suggesting that it may play a key role in manganese clearance (31). Of note, it was originally suggested to be a zinc transporter; however, in our study, serum zinc levels were not significantly associated with the presence of mutation (28).
Cadmium is a common environmental pollutant, with exposure arising from tobacco smoke (32), foods such as shellfish, liver and kidney, as well as inhalation of cadmium-contaminated air from industry sectors. Adverse health effects have been reported in association with low-level environmental cadmium exposure, including liver and kidney damage (33). In addition, there is increased risk of cancer, including breast, kidney, pancreas and urinary bladder (34).
The genetic basis of cadmium ADME is poorly understood. A SNP in the core promoter region of metallothionein 2A (MT2A), rs28366003, has previously been shown to be associated with cadmium levels (P-value = 0.004 in the present sample) (35). Metallothioneins (MTs) are low-molecular weight, cysteine-rich proteins that bind to the biologically essential metals and perform these metals' homeostatic regulations; absorb the heavy metals and assist with their transportation and extraction. In another study on 172 Andean women, a polymorphism in the transferrin receptor gene TFRC, rs3804141, was associated with urinary cadmium levels, with mean urinary cadmium concentrations that were 22% higher in women with GA and AA genotypes (P-value = 0.78 in the present sample when related to circulating Cd concentrations) (36). Our study demonstrated an association between cadmium blood levels and an SNP within the CD109 gene. CD109 encodes a glycosyl phosphatidylinositol-linked glycoprotein that localizes to the surfaces of endothelial cells, T cells and platelets. It is associated with various cancers. For example, upregulation of CD109 is associated with oral cancer and lung cancer (37,38). High expression of CD109 antigen is associated with poor prognosis of soft tissue sarcoma (39), and CD109+ endothelial cells is a prognostic indicator for glioblastomas (40). It is of interest to note that cadmium exposure also has been discussed in the aetiology of several cancers (41,42). Nevertheless, CD109 now emerges as a new player in cadmium ADME, but this has to be replicated in an independent sample.
A few genetic variants have previously been associated with mercury toxicokinetics, mainly in the glutathione-related genes (33,43,44), as well as multi-specific transporter family genes (45). However, there have been no genome-wide studies that address this question. Our study demonstrates an association between mercury levels and a SNP in LINC00578, but the function of this intergenic non-protein-coding RNA is not clear.
The overlap between these significant SNPs and regions of known histone modification is notable since the latter may enhance or repress nearby gene transcription. The fact that the top SNP for cadmium (rs9350504) lies within the region known to be marked by both H3K27Me3 and H3K4Me1 is particularly interesting because the former is a repressor of transcription while the latter is thought to be an enhancer (12). In the genome, there are regions hypothesized to exist in a ‘poised’ state. These bivalent domains tend to coincide with transcription factor genes expressed at low levels and have been postulated to silence developmental genes in embryonic stem cells while keeping them in a state ready for activation (46).
This study includes a large number of metals and trace elements measured in sample for which extensive genotyping and imputation have been performed. However, statistical power is limited due to correction for multiple testing; hence, only large gene versus phenotype effects could be discovered. Also, the GWAS-significant findings in the present study have to be replicated in order to be fully validated. The SNPs found to be associated with metal levels in this study may not be the biological cause of association but rather in LD with causative variants; hence, it may be interesting to further investigate this possibility through deep sequencing in associated LD blocks.
A GWAS approach to study association between genetic variation and circulating levels of toxic metals and some trace elements disclosed novel associations between some ion transporters and whole blood manganese concentrations. In addition, other less well-known genes were related to cadmium and mercury concentrations, showing GWAS to be a valuable tool to explore ADME pathways for metals in humans.
Materials and Methods
The PIVUS study was initiated in 2001 to investigate the predictive power of different measurements of vascular characteristics for future cardiovascular events. Secondary aims included measurements of cardiac and metabolic functions, as well as serum biomarkers and levels of environmental pollutants including polychlorinated biphenyls. Sample details and methods are described more fully by Lind et al. (47). Briefly, subjects aged 70 years living in the community of Uppsala were randomly selected from the community register and invited to participate in the study. Of the 2025 subjects invited, 1016 subjects agreed to participate. After excluding individuals for which phenotypical data were missing, 949 subjects remained.
All 11 toxic metals and trace elements (Al, Cd, Co, Cu, Cr, Hg, Mn, Mo, Ni, Pb and Zn) in this study were determined in whole blood. The analysis was performed using inductively coupled plasma sector field mass spectrometry, after microwave-assisted digestion with nitric acid (48). Details on the analysis have been described by Lind et al. (49).
DNA samples were genotyped according to the manufacturer's instructions on Illumina Infinium Omni Express bead microarrays. Heterozygosity was calculated as the proportion of autosomal SNPs at which the individual carries a heterozygous genotype. Samples were excluded if the call rate was <95%, if they had extreme heterozygosity (>3 SD from the mean), if they were ethnic outliers, or if they were gender discordant. SNP quality control measures included exact P-value for deviation from Hardy–Weinberg equilibrium <10−10 and missing genotype rate >0.01 (MAF < 5%) or missing genotype rate >0.05 (MAF ≥ 5%). Multidimensional scaling was performed to obtain principal components to adjust for population structure. Prior to imputation, variants with MAF < 1% were removed from the GWAS scaffold. Samples were pre-phased with SHAPEIT2. Genotype data were then imputed up to the ‘all ancestries’ reference panel from the 1000 Genomes Project Consortium Phase 1 interim release (June 2010) (50) using IMPUTEv2 (51,52).
Metal measurements were then ranked inverse normalized to generate a Gaussian distribution for downstream association analyses and minimize the impact of outliers. Association testing for each transformed metal was performed in a linear regression framework under an additive model in the minor allele for each variant in turn, after adjusting for triglycerides, cholesterol, gender and two principle components from multidimensional scaling to account for population structure as covariates. Age was not included as a covariate because all individuals were of the same age. Association testing was performed in SNPTEST, allowing for uncertainty in the imputation in a missing data likelihood. The association analysis was restricted to SNPs with MAF > 1% and imputation quality information score (info) of >0.4. The genomic control inflation factor (52,53) for each metal was used to assess evidence of residual population structure. Conditional analyses to search for secondary signals of association were performed by including genotype dosage at the lead SNP as an additional covariate in the linear regression model. To assign function to these genomic variants, we annotated all SNPs that met a significance criterion of 10−5 using ANNOVAR (53) and the Encyclopaedia of DNA variants (ENCODE et al.) (54).
Supplementary Material
Funding
Financial support was received from the Wellcome Trust (grant numbers WT098017, WT090532, 097306/Z/11/Z and WT064890). C.M.L. is a Wellcome Trust Research Career Development Fellow (086596/Z/08/Z). Funding to pay the Open Access publication charges for this article was provided by Wellcome Trust.
Supplementary Material
Acknowledgments
Conflict of Interest statement. None declared.
References
- 1.Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., Groneberg D.A. (2006) The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol., 1, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flora G., Gupta D., Tiwari A. (2012) Toxicity of lead: a review with recent updates. Interdiscip. Toxicol., 5, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socha A.L., Guerinot M.L. (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci., 5, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi R., Bashir K., Ishimaru Y., Nishizawa N.K., Nakanishi H. (2012) The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal Behav., 7, 1605–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björkman L., Vahter M., Pedersen N.L. (2000) Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ. Health Perspect., 108, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitfield J.B., Dy V., McQuilty R., Zhu G., Heath A.C., Montgomery G.W., Martin N.G. (2010) Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium, and zinc in erythrocytes. Environ. Health Perspect., 118, 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans D.M., Zhu G., Dy V., Heath A.C., Madden P.A., Kemp J.P., McMahon G., St Pourcain B., Timpson N.J., Golding J., et al. (2013) Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet., 22, 3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambe T. (2011) An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci. Biotechnol. Biochem., 75, 1036–1043. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P., Henikoff S., Ng P.C. (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc., 4, 1073–1081. [DOI] [PubMed] [Google Scholar]
- 10.Quadri M., Federico A., Zhao T., Breedveld G.J., Battisti C., Delnooz C., Severijnen L.A., Di Toro Mammarella L., Mignarri A., Monti L., et al. (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet., 90, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young M.D., Willson T.A., Wakefield M.J., Trounson E., Hilton D.J., Blewitt M.E., Oshlack A., Majewski I.J. (2011) ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res., 39, 7415–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hon G.C., Hawkins R.D., Ren B. (2009) Predictive chromatin signatures in the mammalian genome. Hum. Mol. Genet., 18, R195–R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luciano M., Hansell N.K., Lahti J., Davies G., Medland S.E., Räikkönen K., Tenesa A., Widen E., McGhee K.A., Palotie A., et al. (2011) Whole genome association scan for genetic polymorphisms influencing information processing speed. Biol. Psychol., 86, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J.H., Lemaitre R.N., Manichaikul A., Guan W., Tanaka T., Foy M., Kabagambe E.K., Djousse L., Siscovick D., Fretts A.M., et al. (2013) Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ. Cardiovasc. Genet., 6, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowler R.M., Gocheva V., Harris M., Ngo L., Abdelouahab N., Wilkinson J., Doty R.L., Park R., Roels H.A. (2011) Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology, 32, 596–605. [DOI] [PubMed] [Google Scholar]
- 16.Antonini J.M., Santamaria A.B., Jenkins N.T., Albini E., Lucchini R. (2006) Fate of manganese associated with the inhalation of welding fumes: potential neurological effects. Neurotoxicology, 27, 304–310. [DOI] [PubMed] [Google Scholar]
- 17.Criswell S.R., Perlmutter J.S., Huang J.L., Golchin N., Flores H.P., Hobson A., Aschner M., Erikson K.M., Checkoway H., Racette B.A. (2012) Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med., 69, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postawa A., Hayes C. (2013) Best Practice Guide on the Control of Iron and Manganese in Water Supply. IWA publishing, New York. [Google Scholar]
- 19.Davis C.D., Malecki E.A., Greger J.L. (1992) Interactions among dietary manganese, heme iron, and nonheme iron in women. Am. J. Clin. Nutr., 56, 926–932. [DOI] [PubMed] [Google Scholar]
- 20.Finley J.W. (1999) Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr., 70, 37–43. [DOI] [PubMed] [Google Scholar]
- 21.Gunshin H., Mackenzie B., Berger U.V., Gunshin Y., Romero M.F., Boron W.F., Nussberger S., Gollan J.L., Hediger M.A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature, 388, 482–488. [DOI] [PubMed] [Google Scholar]
- 22.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M., Pirruccello J.P., Ripatti S., Chasman D.I., Willer C.J., et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature, 466, 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Lango Allen H., Lindgren C.M., Luan J., Mägi R., et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet., 42, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehret G.B., Munroe P.B., Rice K.M., Bochud M., Johnson A.D., Chasman D.I., Smith A.V., Tobin M.D., Verwoert G.C., Hwang S.J., et al. (2011) Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature, 478, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterworth D.M., Ricketts S.L., Song K., Chen L., Zhao J.H., Ripatti S., Aulchenko Y.S., Zhang W., Yuan X., Lim N., et al. (2010) Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol., 30, 2264–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrera N., Arrojo M., Sanjuán J., Ramos-Ríos R., Paz E., Suárez-Rama J.J., Páramo M., Agra S., Brenlla J., Martínez S., et al. (2012) Association study of nonsynonymous single nucleotide polymorphisms in schizophrenia. Biol. Psychiat., 71, 169–177. [DOI] [PubMed] [Google Scholar]
- 27.Bruenig D., White M.J., Young R.M., Voisey J. (2014) Subclinical psychotic experiences in healthy young adults: associations with stress and genetic predisposition. Genet. Test. Mol. Biomarkers, 18, 683–689. [DOI] [PubMed] [Google Scholar]
- 28.Seve M., Chimienti F., Devergnas S., Favier A. (2004) In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genomics, 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leyva-Illades D., Chen P., Zogzas C.E., Hutchens S., Mercado J.M., Swaim C.D., Morrisett R.A., Bowman A.B., Aschner M., Mukhopadhyay S. (2014) SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci., 34, 14079–14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuschl K., Clayton P.T., Gospe S.M., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R.T., Barsottini O.G., Zaki M.S., et al. (2012) Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet., 90, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen P., Parmalee N., Aschner M. (2014) Genetic factors and manganese-induced neurotoxicity. Front. Genet., 5, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson I.M., Bensryd I., Lundh T., Ottosson H., Skerfving S., Oskarsson A. (2002) Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ. Health. Perspect., 110, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engström A., Michaëlsson K., Vahter M., Julin B., Wolk A., Åkesson A. (2012) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone, 50, 1372–1378. [DOI] [PubMed] [Google Scholar]
- 34.Huff J., Lunn R.M., Waalkes M.P., Tomatis L., Infante P.F. (2007) Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health, 13, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayaaltı Z., Aliyev V., Söylemezoğlu T. (2011) The potential effect of metallothionein 2A-5A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels. Toxicol. Appl. Pharmacol., 256, 1–7. [DOI] [PubMed] [Google Scholar]
- 36.Rentschler G., Kippler M., Axmon A., Raqib R., Ekström E.C., Skerfving S., Vahter M., Broberg K. (2013) Polymorphisms in iron homeostasis genes and urinary cadmium concentrations among nonsmoking women in Argentina and Bangladesh. Environ. Health Perspect., 121, 467–472, 472e461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagiwara S., Murakumo Y., Sato T., Shigetomi T., Mitsudo K., Tohnai I., Ueda M., Takahashi M. (2008) Up-regulation of CD109 expression is associated with carcinogenesis of the squamous epithelium of the oral cavity. Cancer Sci., 99, 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato T., Murakumo Y., Hagiwara S., Jijiwa M., Suzuki C., Yatabe Y., Takahashi M. (2007) High-level expression of CD109 is frequently detected in lung squamous cell carcinomas. Pathol. Int., 57, 719–724. [DOI] [PubMed] [Google Scholar]
- 39.Emori M., Tsukahara T., Murase M., Kano M., Murata K., Takahashi A., Kubo T., Asanuma H., Yasuda K., Kochin V., et al. (2013) High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancer-initiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS One, 8, e84187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuppini L., Calleri A., Bruzzone M.G., Prodi E., Anghileri E., Pellegatta S., Mancuso P., Porrati P., Di Stefano A.L., Ceroni M., et al. (2013) Prognostic value of CD109+ circulating endothelial cells in recurrent glioblastomas treated with bevacizumab and irinotecan. PLoS One, 8, e74345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahim F., Jalali A., Tangestani R. (2013) Breast cancer frequency and exposure to cadmium: a meta-analysis and systematic review. Asian Pac. J. Cancer. Prev., 14, 4283–4287. [DOI] [PubMed] [Google Scholar]
- 42.Feki-Tounsi M., Hamza-Chaffai A. (2014) Cadmium as a possible cause of bladder cancer: a review of accumulated evidence. Environ. Sci. Pollut. Res. Int., 21, 10561–10573. [DOI] [PubMed] [Google Scholar]
- 43.Harari R., Harari F., Gerhardsson L., Lundh T., Skerfving S., Strömberg U., Broberg K. (2012) Exposure and toxic effects of elemental mercury in gold-mining activities in Ecuador. Toxicol. Lett., 213, 75–82. [DOI] [PubMed] [Google Scholar]
- 44.Custodio H.M., Harari R., Gerhardsson L., Skerfving S., Broberg K. (2005) Genetic influences on the retention of inorganic mercury. Arch. Environ. Occup. Health, 60, 17–23. [DOI] [PubMed] [Google Scholar]
- 45.Engström K., Ameer S., Bernaudat L., Drasch G., Baeuml J., Skerfving S., Bose-O'Reilly S., Broberg K. (2013) Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ. Health Perspect., 121, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell, 125, 315–326. [DOI] [PubMed] [Google Scholar]
- 47.Lind L., Fors N., Hall J., Marttala K., Stenborg A. (2005) A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler. Thromb. Vasc. Biol., 25, 2368–2375. [DOI] [PubMed] [Google Scholar]
- 48.Rodushkin I., Odman F., Olofsson R., Axelsson M. (2000) Determination of 60 elements in whole blood by sector field inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom., 15, 937–944. [Google Scholar]
- 49.Lind P.M., Olsén L., Lind L. (2012) Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci. Total Environ., 416, 80–88. [DOI] [PubMed] [Google Scholar]
- 50.Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A., Consortium G.P. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature, 491, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howie B., Fuchsberger C., Stephens M., Marchini J., Abecasis G.R. (2012) Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet., 44, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devlin B., Roeder K. (1999) Genomic control for association studies. Biometrics, 55, 997–1004. [DOI] [PubMed] [Google Scholar]
- 53.Wang K., Li M., Hakonarson H. (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res., 38, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ENCODE project consortium. (2011) A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol., 9, e1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.