SUMMARY
Animals learn to avoid harmful situations by associating a neutral stimulus with a painful one, resulting in a stable threat memory. In mammals, this form of learning requires the amygdala. Although pain is the main driver of aversive learning, the mechanism that transmits pain signals to the amygdala is not well resolved. Here we show that neurons expressing calcitonin gene-related peptide (CGRP) in the parabrachial nucleus are critical for relaying pain signals to the central nucleus of amygdala, and that this pathway may transduce the affective motivational aspects of pain. Genetic silencing of CGRP neurons blocks pain responses and memory formation, while their optogenetic stimulation produces defensive responses and a threat memory. The pain-recipient neurons in the central amygdala expressing CGRP receptors are also critical for establishing a threat memory. The identification of the neural circuit conveying affective pain signals may be pertinent for treating pain conditions with psychiatric comorbidities.
INTRODUCTION
All living organisms respond and adapt to their environment by changing their internal states. Learning to avoid physically harmful situations is critical for the survival of organisms. Aversive learning is formed when a certain neutral situation (conditioned stimulus or CS) is associated with the physically harmful situation (unconditioned stimulus or US) (Fanselow and Poulos, 2005; LeDoux, 2000). In rodents, fear (which does not mean the conscious feeling of fear, but instead, a defensive response to a threat) manifests as immobility or ‘freezing’ under environmental conditions that predict pain– the major sensory modality of the physical harm (Herry and Johansen, 2014; Pape and Pare, 2010). Study of the neural mechanisms underlying learning about threats (fear conditioning or, preferably, threat conditioning (see LeDoux 2014)) is a major endeavor of behavioral neuroscience. The amygdala, an almond-shaped structure that is a part of the limbic system, is known to be a critical brain region that integrates the sensory (CS) and pain (US) signals to create a memory that will produce a threat response when exposed to the same CS (Gross and Canteras, 2012). Although the neural circuitry engaged within the amygdala during threat learning has been studied extensively, the neural circuit that transmits pain signals from the periphery to the amygdala has not been rigorously established. The pain signal produced by a noxious stimulus, such as foot shock, is transmitted from sensory neurons to projection neurons in the most superficial layer (lamina 1) of the spinal cord and then through the two ascending pathways: the spino-thalamic pathway, and the spino-parabrachial pathway (Hunt and Mantyh, 2001; Todd, 2010). Because the sensory thalamus is anatomically connected with the lateral amygdala (LA) (LeDoux et al., 1990), the spino-thalamic pathway has been extensively studied as a potential circuit for the US during fear conditioning (Shi and Davis, 1999), but other studies suggest the existence of an alternative US circuit (Brunzell and Kim, 2001; Lanuza et al., 2004). Recent studies show that the midbrain periaqueductal gray (PAG) may transduce pain signals during fear learning through an indirect connection from the PAG to the LA (Herry and Johansen, 2014; Johansen et al., 2010; Kim et al., 2013). Because the PAG and the PBN are directly connected (Krout et al., 1998) and most of lamina 1 projection neurons project their axons to the PBN (Todd, 2010), it is possible that the PAG transmits the pain signal to the central nucleus of amygdala (CeA) via the PBN during threat learning. The spino-parabrachial pathway that relays the nociceptive signal from the spinal cord to the lateral part of CeA (CeAl) has been well characterized as a central pain-processing pathway (Hunt and Mantyh, 2001). Anterograde tracing studies show that most spinal lamina 1 projection neurons send their axonal terminals to the external lateral subdivision of the PBN (PBel) (Al-Khater and Todd, 2009), and field-potential recordings in vivo reveal that noxious stimuli (e.g., pinching, high temperature) in the periphery induce firing of PBel (Bernard and Besson, 1990; Bester et al., 2000) and CeAl (Neugebauer and Li, 2002) neurons. Neuronal tracing studies reveal that the PBel neurons directly innervate CeAl neurons (Lu et al., 2014; Sarhan et al., 2005), and electrical stimulation of axonal fibers from the PBel induces strong depolarization of neurons in the CeAl in vitro (Han et al., 2010; Watabe et al., 2013), and in vivo (Jhamandas et al., 1996). However, despite its involvement in the central pain processing, the spino-parabrachial pathway has not been studied as the circuit that transmits pain signals to the amygdala during threat conditioning.
In situ hybridization and immunohistochemistry studies revealed that the Calca gene encoding calcitonin gene-related peptide (CGRP), a 37-amino-acid neuropeptide that regulates vasodilation and pain transmission, is abundantly expressed in the PBel, and the neurons expressing CGRP project their axons directly to the CeAl (Carter et al., 2013; D'Hanis et al., 2007). Interestingly, direct infusion of CGRP into the CeA induces freezing behaviors even without foot shock (Kocorowski and Helmstetter, 2001). The generation of synaptic plasticity in the CeAl neurons by stimulating fibers coming from the PBel is enhanced by perfusing CGRP in slice preparations of CeA (Han et al., 2010; Han et al., 2005).
Based on these observations, we pursued the idea that the parabrachio-amygdaloid pathway is responsible for relaying the US pain signal to the CeAl during fear conditioning. To investigate this hypothesis, we used Cre-dependent viruses in genetically engineered mice to selectively activate or inactivate the CGRP neurons in the PBel and CGRP receptor (CGRPR) neurons in the CeAl to establish the role of this circuit in fear conditioning. Our findings reveal that the CGRP neurons in the PBel relay the US signal to the CeAl and that CGRPR neurons are the functional US-recipient neurons in the CeAl.
RESULTS
CGRP Neurons in the PBel Are Activated by Foot Shock
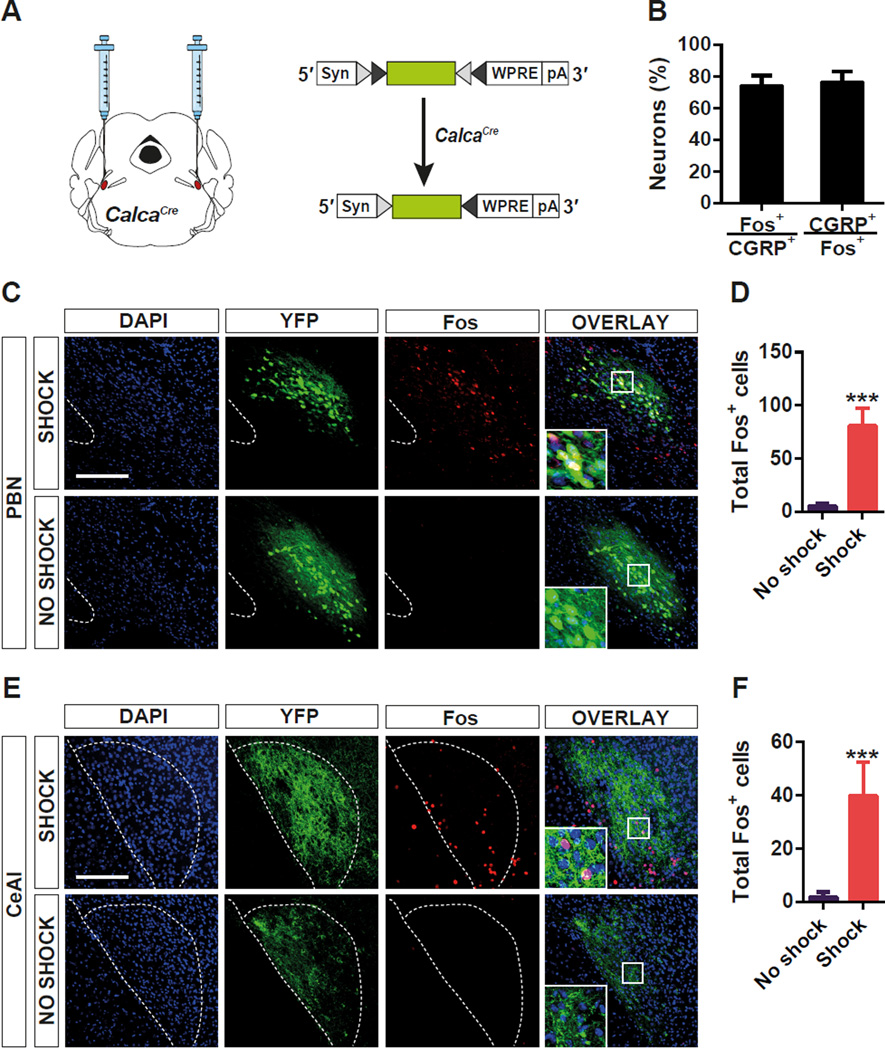
To test whether GGRP is a good marker for the neurons involved in the relay of the US information during threat conditioning, we targeted Cre recombinase to the Calca locus (CalcaCre) that encodes CGRP by differential splicing (Carter et al., 2013; Rosenfeld et al., 1983). Then, Cre-dependent adeno-associated virus (AAV) expressing Yellow Fluorescent Protein (AAV1-DIO-YFP) was injected into the PBel of CalcaCre mice (Figure 1A) (Carter et al., 2013). Two weeks after the viral delivery, foot shock was given and induction of Fos, a surrogate marker for neuronal activation, was examined by immunohistochemistry 90 min after the foot shock. Most Fos+ neurons in the PBel were YFP-expressing CGRP neurons (70%), whereas few Fos+ neurons were observed in the PBel of control mice (Figure 1B–D). Axonal terminals from CGRP neurons in the PBel densely innervated neurons in the CeAl, and the number of Fos+ neurons within the axonal terminal field was significantly increased by foot shock (Figure 1E and 1F). These data indicate that CGRP neurons in the PBel and neurons in their CeAl projection field are activated by foot shock.
Figure 1. Activation of CGRP Neurons in the PBel by Foot Shock.
(A) Stereotaxic delivery of AAV encoding a Cre-dependent YFP reporter gene into the PBN of CalcaCre mice.
(B–D) Quantification (B and D) and representative histological examples (c) of co-labeling of CGRP neurons and Fos-like immunoreactivity in the PBel after foot shock.
(E and F) Representative histological examples (E) and quantification (F) of Fos-like immunoreactivity in the CeAl where the axonal terminals of the CGRP neurons in the PBel project. All values are means ± s.e.m. from 6 brain sections of 3 animals. ***P < 0.001.
Role of PBel CGRP Neurons in Learning about Painful Threats
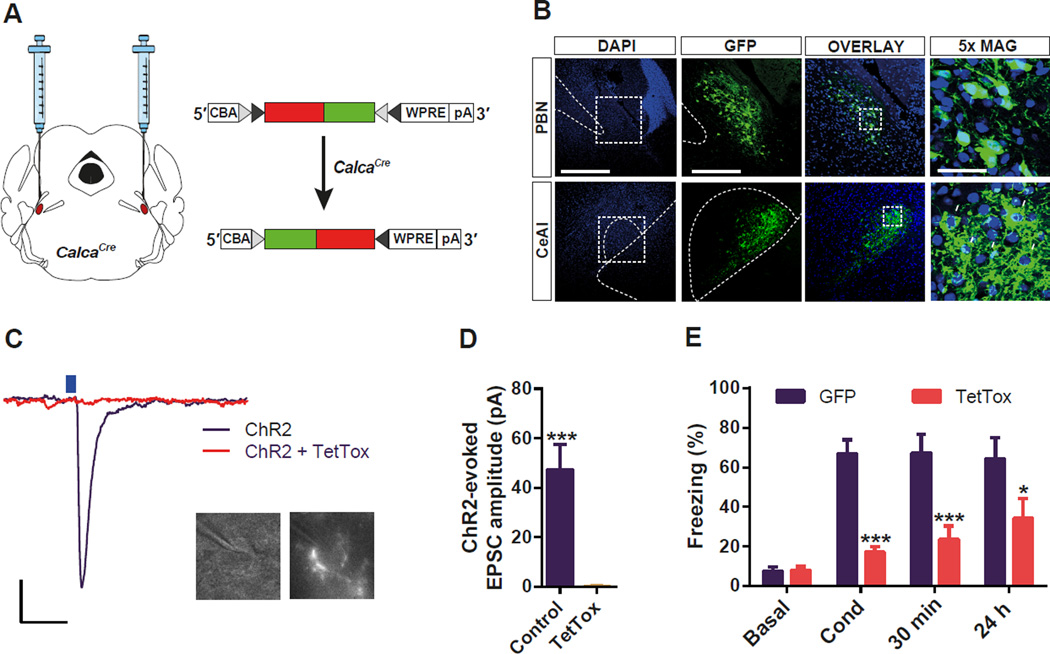
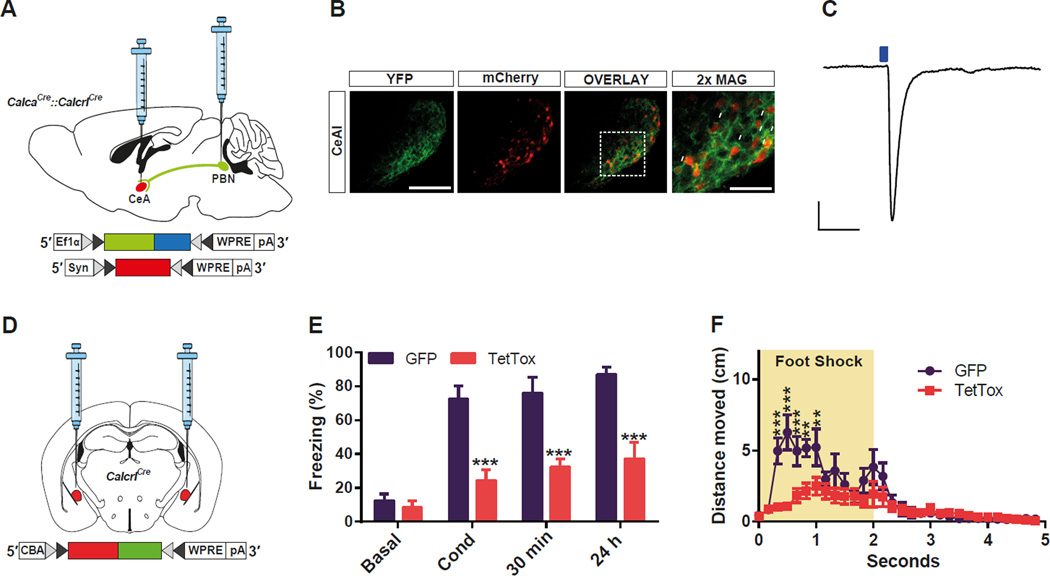
To examine whether the activation of CGRP neurons in the PBel is necessary for the formation of threat memory, we inactivated synaptic transmission specifically in PBel CGRP neurons by the Cre-dependent expression of the tetanus toxin light chain (TetTox) (Kim et al., 2009) with bilateral stereotaxic delivery of virus (AAV1-DIO-GFP:TetTox) into CalcaCre mice (Figure 2A). Expression of GFP:TetTox was visible in PBel and in the axons that project to the CeAl (Figure 2B). To ascertain how effectively TetTox inactivated synaptic transmission, mice were injected with AAV1-DIO-ChR2:YFP with or without AAV1-DIO-GFP:TetTox and CeAl neurons with soma surrounded by fluorescent boutons (Lu et al., 2014) were recorded using whole-cell patch clamp in brain slices (Figure 2C). Photoactivation of axon terminals with blue light elicited a glutamatergic excitatory post-synaptic current (EPSC) (Carter et al., 2013) in all (10/10) neurons from the ChR2 control slices, whereas no (0/10) neurons from the mice receiving TetTox showed a pronounced EPSC (Figure 2D).
Figure 2. Functional Silencing of CGRP Neurons in the PBel Attenuates Threat Learning.
(A) Bilateral delivery of AAV carrying Cre-dependent TetTox into the PBN of CalcaCre mice.
(B) Representative histological images of TetTox expression in the CGRP neurons in the PBN (upper panels), and their terminal projections to the CeAl (lower panels). White arrows indicate their characteristic perisomatic synapses in the CeAl.
(C and D) Example traces (C) and quantification (D) of photostimulation-evoked EPSCs in the CeAl neurons that receive direct inputs from the PBel CGRP neurons. Brain slices containing the CeAl were obtained from mice previously injected with Cre-dependent ChR2 plus TetTox, or ChR2 alone into the PBN. Only neurons surrounded by fluorescent boutons were recorded (c). Scale bar: 10 pA, 25 ms. Data in (D) are means ± s.e.m. from 10 neurons (3 mice) per group.
(E) Genetic silencing of CGRP neurons in the PBel by TetTox attenuated freezing responses immediately after the conditioning (Cond), and 30 min or 24 h after the conditioning when compared with the GFPexpressing control mice. All data shown are means ± s.e.m. from 8 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
A battery of behavioral tests was performed to address fear-dependent learning and memory by comparing mice injected bilaterally with AAV1-DIO-GFP:TetTox with controls injected with AAV1-DIOGFP. Context-dependent, threat conditioning was assessed by comparing the total time spent freezing (immobility monitored by video-tracking, which was verified by manual scoring; see Methods) during conditioning, and then again 30 min and 24 h later by returning the mice to the conditioning box. The TetTox-injected group displayed substantially reduced freezing during all three experimental sessions compared to the control GFP-injected group (Figure 2E, Videos S1, S2). These results reveal that the activity of PBel CGRP neurons facilitates transmission of the pain signals during threat learning.
Role of PBel CGRP Neurons in Central Pain Processing
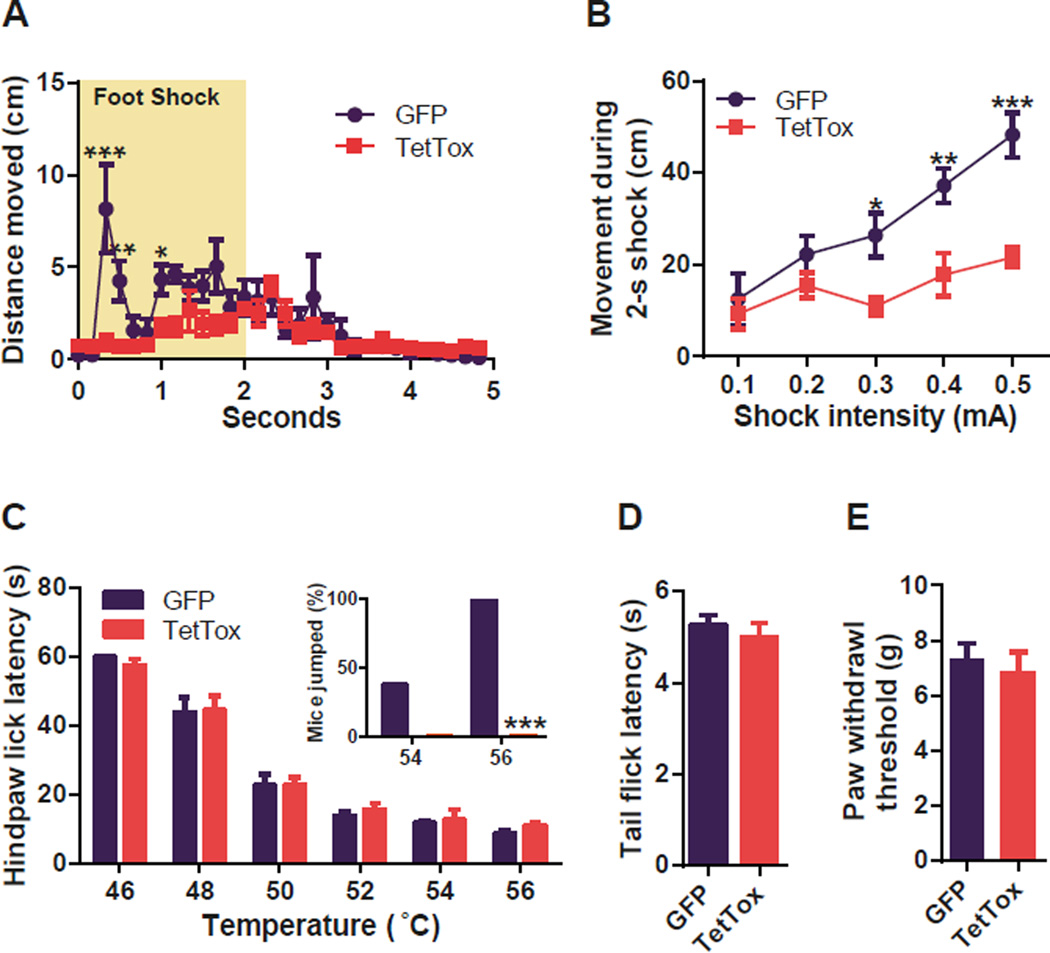
To examine whether the CGRP neurons in the PBel are necessary for the central pain processing, we performed a battery of nociceptive behavioral experiments with TetTox- and GFP-injected groups. To test the immediate defensive response to the foot shock, the total distance traveled by the mice during and immediately after a 2-s foot shock was measured. Control mice displayed a bout of activity during first 500 ms to escape from the threat. However, this defensive escape behavior was absent in the TetTox-expressing mice (Figure 3A). The escape running behavior of the control mice during the 2-s foot shock increased with shock intensity (0.1 to 0.5 mA) whereas movement by the TetTox group was barely affected by shock intensity (Figure 3B, Figure S1A). General locomotor activity of the TetTox group was, however, comparable to the control group (Figure S1B). Nociceptive responses to thermal (Figure 3C and 3D), and mechanical (Figure 3E) stimuli were preserved in TetTox-injected mice when compared with GFP-injected mice. However, the TetTox group did not display escape jumping behavior, whereas the control group jumped at 54°C and 56°C during the hot-plate test (Figure 3C inset). These results reveal that reflexive withdrawal responses are intact; however, escape behaviors depend on the activity of PBel CGRP neurons.
Figure 3. Functional Silencing of CGRP Neurons in the PBel Blocks Pain Signals during Threat Learning.
(A) Immediate escape running response of the test mice to the foot shock was attenuated by functionally silencing the PBel CGRP neurons in the CalcaCre mice.
(B) Shock intensity-dependent movement was substantially decreased in the TetTox-expressing CalcaCre mice.
(C) In the hot plate test, the nociceptive response to the thermal stimulus was intact in the TetTox-expressing CalcaCre mice. The number in each bar indicates the number of test mice that jumped to escape during the test at the indicated temperature. Inset, functional inactivation of the PBel CGRP neurons completely blocked escape jumping behavior in the CalcaCre mice.
(D) In the tail-flick test, the nociceptive response to the thermal stimulus was unaffected by functionally inactivating CGRP neurons in the PBel.
(E) In the dynamic plantar anesthesiometer test, the nociceptive response to the mechanical stimulus was unaffected by functionally inactivating CGRP neurons in the PBel. All data shown are means ± s.e.m. from 8 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
Activation of PBel CGRP Neurons Is Sufficient for Threat Learning
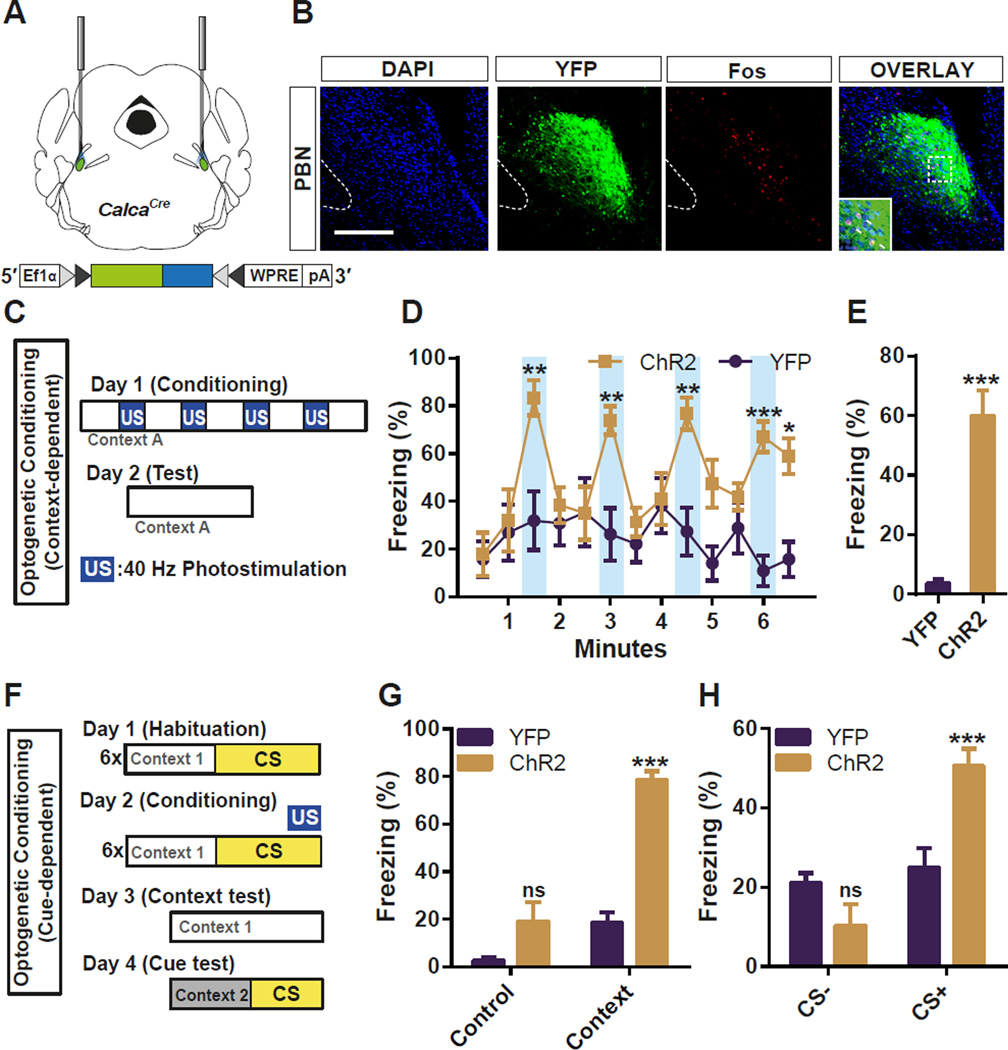
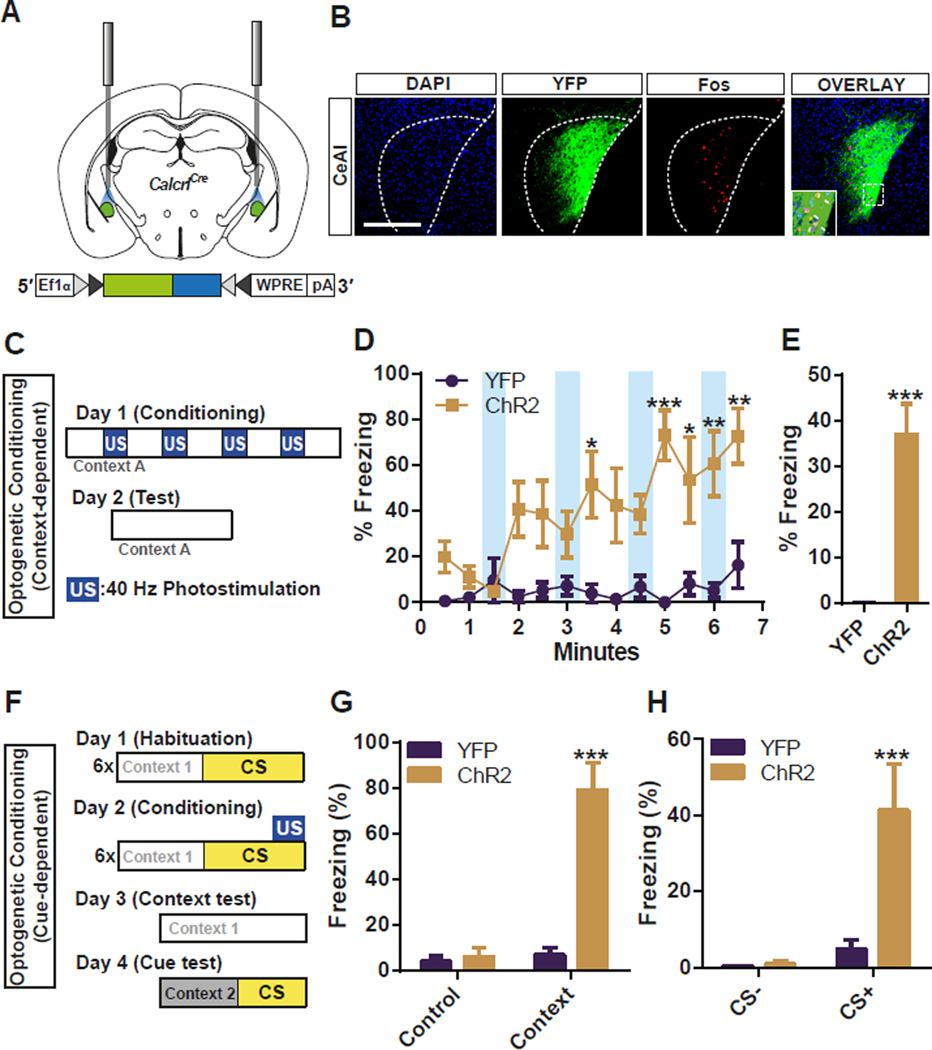
To test whether the activation of CGRP neurons in the PBel is sufficient to evoke an US pain response, PBel CGRP neurons were optogenetically stimulated instead of delivering a foot shock. ChR2:YFP was selectively expressed in the PBel CGRP neurons by bilateral stereotaxic delivery of AAV1-DIO-ChR2:YFP in the PBel of CalcaCre mice; control mice received AAV1-DIO-YFP (Figure 4A). Immunohistochemical staining after the behavioral tests revealed that the photostimulation of the PBel CGRP neurons induced Fos in ChR2-expressing CGRP neurons (Figure 4B). Mice were placed in an open field arena and photostimulated for 30 s with 60-s inter-trial intervals (Figure 4C). The 30-s photostimulation of ChR2-expressing CGRP neurons induced immobility, whereas the same stimulation had no effect on movement of the control mice (Figure 4D, Figure S2, and Video S3). Freezing by the ChR2 group was reversible at first but the baseline immobility gradually increased with repeated stimulations (Figure 4D, Figure S2). To test whether the mice learned to associate the context in which they were photostimulated, they were returned to the arena 24 h later. The ChR2 group displayed significantly more freezing than the control mice (Figure 4E). We also observed tail rattling behavior, an intense defensive response to a threat in all the ChR2 group when they were stimulated with blue light the day after optogenetic conditioning (Video S4). Mice were also trained in a classical auditory fear-conditioning paradigm in which a tone was paired with a 10-s photostimulation rather than with a foot shock. The following day, context- and cue-dependent memories were assessed by exposing the mice to the same context or to a novel context with the same tone (Figure 4F). The ChR2-expressing mice froze more than control mice when returned to the test chamber (Figure 4G) or when exposed to the tone in a novel chamber (Figure 4H). These data demonstrate that the activation of PBel CGRP neurons generates aversive teaching signals that are sufficient to induce an immediate defensive response as well as context- and cue-dependent threat memories.
Figure 4. Optogenetic Stimulation of CGRP Neurons in the PBel Induces Freezing Behaviors and Produces a Threat Memory.
(A) Diagram illustrating the placement of optic fiber bilaterally in the PBN of a CalcaCre mouse injected with AAV-DIO-ChR2:YFP.
(B) Representative histological images showing the Fos-like immunoreactivity within the PBel after 30-s photostimulation of CGRP neurons.
(C) Illustration of context-dependent optogenetic conditioning. Photostimulation (40 Hz) was used as the US signal instead of foot shock.
(D and E) Optogenetic stimulation of the PBel CGRP neurons reversibly induced freezing behaviors followed by increased basal freezing (D), and also produced fear memory 24 h after the photostimulation (E).
(F) Illustration of cue-dependent optogenetic conditioning.
(G and H) Optogenetic stimulation of the PBel CGRP neurons paired with 10-kHz pure tone produced context-dependent (G), and cue-dependent (H) fear memories. All data shown are means ± s.e.m. from 7 mice per group. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Anatomical and Molecular Characterization of CGRPR Neurons in the CeAl
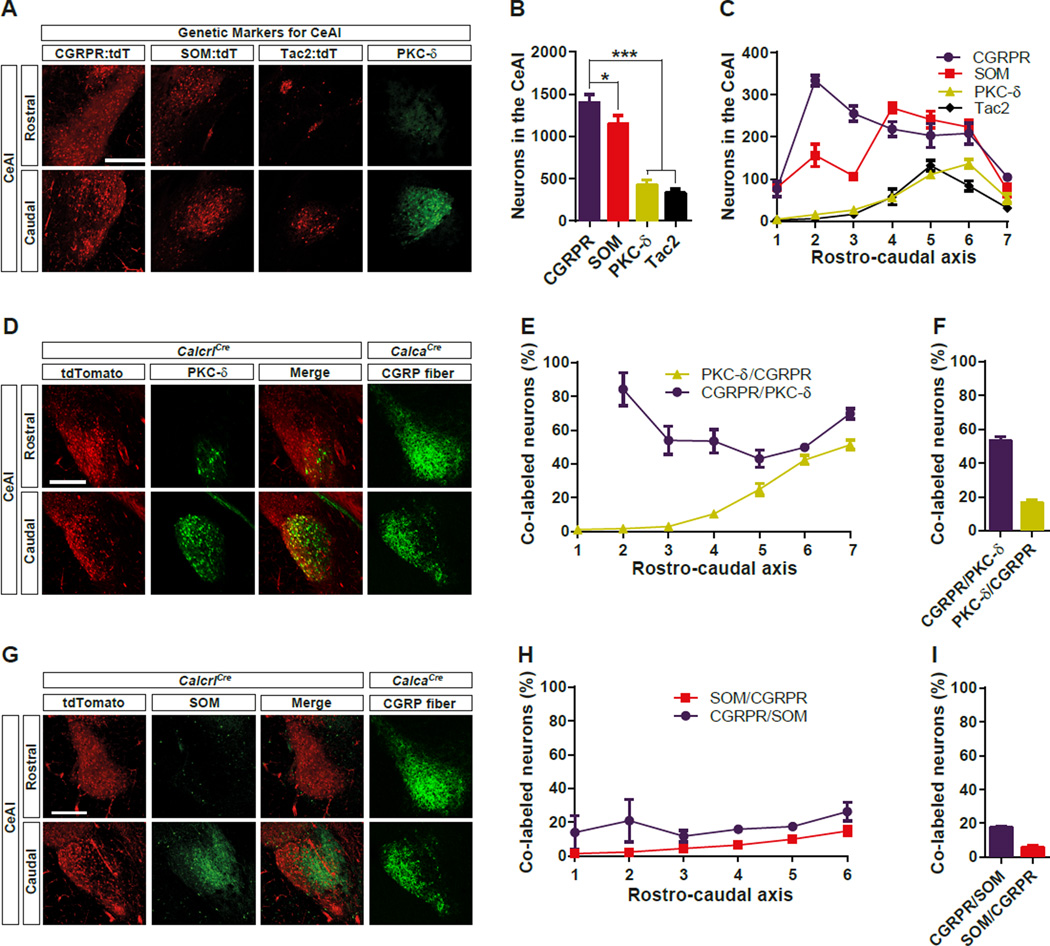
Somatostatin-positive (SOM) and PKC-δ-positive (PKC-δ) neurons in the CeAl form a reciprocal inhibitory circuit shown to be directly involved in the CS information processing during auditory threat conditioning; however, neither of these neuronal populations has been shown to be sufficient to induce threat memory (Ciocchi et al., 2010; Haubensak et al., 2010; Li et al., 2013). Therefore, we reasoned that other CeAl neurons contribute to the acquisition of threat memories. We chose CGRPR neurons, because they should receive direct synaptic input from the CGRP neurons in the PBel (Han et al., 2005; Lu et al., 2014). To genetically manipulate them, we targeted a Cre:GFP cassette with an internal ribosome entry site to the last exon of the Calcrl gene, which encodes a subunit of the CGRPR (Figure S3). To genetically label the CGRPR neurons, we crossed CalcrlCre mice with a Rosa26-flox-stop-tdTomato reporter line, Ai14 (Calcrl:tdTomato mice). The expression of CGRPR was widely distributed throughout the entire brain and it was highly expressed in the cerebral microvasculature, which is consistent with previous observations (Figure S4) (Moreno et al., 2002). We also labelled other CeAl-specific genetic markers, such as SOM, and Tackykinin 2 (Tac2) by genetic labelling, and PKC-δ with immunohistochemical staining. Immunolabelled PKC-δ virtually recapitulates what is observed with its genetic labeling (Cai et al., 2014), allowing for its comparison with knock-in tdTomato reporters. These genetic markers were all expressed in the CeAl, but the number of labelled neurons and their spatial distribution were different for each marker (Figure 5). Quantitative analysis showed that the CGRPR neurons are the most abundant population in the CeAl (Figure 5B). In fact, CGRPR was found to be expressed by 3 times as many neurons as PKC-δ, which was previously estimated to label ~50% of CeAl GABAergic neurons (Haubensak et al., 2010). The spatial distribution of marker expression throughout rostro-caudal axis differed; whereas PKC-δ, and Tac2 were expressed predominantly in the caudal part of the CeAl, CGRPR and SOM were expressed throughout the rostro-caudal axis of the CeAl (Figure 5C). Immunohistochemical staining of PKC-δ in Calcrl:tdTomato mice showed that PKC-δ and CGRPR substantially overlapped in the caudal CeAl, but much less so in rostral CeA (Figure 5D–F). CGRPR and SOM were co-expressed at a low level throughout entire rostro-caudal planes of CeA (Figure 5G–I). However, the total number of immunolabelled SOM neurons only represented ~26% of genetically labelled SOM neurons due to the difficulty in exhaustive somatic peptide labeling. Hence, the percentage of SOM neurons co-expressing CGRPR and vice versa may be underestimates and overestimates respectively. These data indicate that the CGRPR is expressed abundantly throughout the CeAl and overlaps with some SOM and PKC-δ neurons.
Figure 5. Expression of CGRPR and other Molecular Markers within the CeAl.
(A) Representative histological images showing the genetic labeling of CGRPR, SOM, and Tac2, as well as immunohistochemical labeling of PKC-δ in the rostral (−0.9 mm from Bregma) and caudal (−1.62 mm from Bregma) CeAl.
(B) The number of total neurons labeled with each genetic marker from the seven representative sections throughout the rostro-caudal axis in the CeAl.
(C) The number of labeled neurons in the rostro-caudal plane of CeAl. 1 = −0.72 mm, and 7 = −1.8 mm posterior to bregma.
(D) Representative histological images showing co-labeling of CGRPR and PKC-δ in the rostral (−0.9 mm from Bregma) and caudal (−1.62 mm from Bregma) CeAl.
(E) The percentage of co-labeled neurons in the rostro-caudal plane of CeAl. 1 = −0.72 mm, and 7 = −1.8 mm posterior to Bregma.
(F) The percentage of total neurons co-labeled with each genetic marker from the six representative sections throughout the rostro-caudal axis in the CeAl.
(G) Representative histological images showing co-labeling of CGRPR and SOM in the rostral (−0.9 mm from Bregma) and caudal (−1.62 mm from Bregma) CeAl. CGRP fiber image from the CalcaCre mouse was duplicated with the Figure 5D for the anatomical reference.
(H) The percentage of co-labeled neurons in the rostro-caudal plane of CeAl. 1 = −0.72 mm, and 6 = −1.8 mm posterior to bregma.
(I) The percentage of total neurons co-labeled with each genetic marker from the seven representative sections throughout the rostro-caudal axis in the CeAl. All data shown are means ± s.e.m. from 3 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
Although the CGRPR was specifically expressed in the CeAl among the amygdala structures, it was also expressed in the neighboring striatal structures, such as caudate putamen and striatal amygdala (Figure S4). Therefore, breeding CalcrlCre mice with other Cre-dependent mouse lines to label or manipulate the CGRPR neurons only within the CeAl was not feasible. However, we were able to specifically target the CeAl CGRPR neurons by stereotaxic delivery of Cre-dependent AAV virus into the CeAl (Figure S4).
CGRPR-expressing Neurons in the CeAl Are the US-recipient Cells
Previous studies showed that the CGRP axonal terminals, but not CGRP cell bodies, are observed in the CeAl (Dobolyi et al., 2005). We confirmed that the CGRP neurons are not present in the CeAl by injecting AAV-DIO-mCherry in the CeA of the CalcaCre mouse (data not shown). Likewise, our Calcrl:tdTomato mouse did not reveal fluorescence in the PBN (Figure S5). To test whether the CGRPR neurons in the CeAl receive direct synaptic inputs from the CGRP neurons in the PBN, we generated CalcaCre::CalcrlCre mice (Figure 6A). We injected the AAV-DIO-ChR2:YFP in the PBN to optogenetically stimulate the CGRP neurons and we also injected the AAV-DIO-mCherry in the CeA of the same mice to label the CGRPR cells in the CeAl. Dense, perisomatic green fibers were observed surrounding mCherry-positive cell bodies in the CeAl, confirming previous histological results (Lu et al., 2014) Figure 6B). Optogenetic stimulation of CGRP terminals in brain slices that included the CeA evoked postsynaptic currents in most (6 of 7) mCherry-positive CGRPR neurons (Figure 6C). These data indicate that the CGRPR neurons in the CeAl are anatomically and functionally connected to CGRP neurons located in the PBN.
Figure 6. CGRPR Neurons in the CeAl Are Functionally and Anatomically Downstream of the PBel CGRP Neurons and Relay Teaching Signals during Threat Conditioning.
(A) Dual delivery of AAV carrying Cre-dependent ChR2 into the PBN of CalcaCre mice and AAV carrying Cre-dependent mCherry into the CeA in the CalcaCre::Calcrl Cre mouse.
(B) Representative histological images of the terminal projections of the PBN CGRP neurons to the CeAl, and their direct-recipient mCherry-labelled CGRPR neurons in the CeAl. White arrows indicate their characteristic perisomatic synapses in the CeAl.
(C) Example traces of photostimulation-evoked EPSCs in the mCherry-labelled CGRPR neurons in the CeAl. The average amplitude of the EPSC from 6 neurons (2 mice) was 51.6 pA ± 19.9. Scale bar: 25 pA, 25 ms.
(D) Bilateral delivery of AAV carrying Cre-dependent TetTox into the CeAl of CalcrlCre mice.
(E) Genetic silencing of CGRPR neurons in the CeAl by TetTox attenuated freezing responses immediately after conditioning (Cond) and 30 min or 24 h after contextual fear conditioning when compared with the GFP-expressing control mice.
(F) Immediate escape running response of the test mice to the foot shock was attenuated by functionally silencing the PBel CGRP neurons in the CalcaCre mice. All data shown are means ± s.e.m. from 7 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
To test the necessity of the CeAl CGRPR neurons during threat learning, we functionally inactivated the CGRPR neurons by stereotaxically injecting AAV-DIO-GFP:TetTox virus bilaterally into the CeAl of CalcrlCre mice (Figure 6D). Expression of GFP:TetTox was visible exclusively in the CeAl (Figure S6). We examined context-dependent threat conditioning with TetTox-expressing mice and GFP-expressing control mice. Whereas GFP-expressing control mice displayed normal freezing immediately after the conditioning, TetTox-expressing mice displayed substantially reduced freezing immediately after the conditioning as well as 30 min and 24 h later when they were returned to the test chamber (Figure 6E, Video S5). To test the immediate defensive response to the foot shock, the total distance traveled by the mice during and immediately after a 2-s foot shock were measured. Control mice displayed an initial bout of activity during first 1 s to escape from the threat. However, the defensive escape running behavior was absent in the TetTox-expressing mice (Figure 6F). We also tested the activation of the CeAl CGRPR neurons during threat learning by monitoring Fos activation 90 min after foot shock; ~10% of total CGRPR neurons were Fos+, and ~40% of total Fos+ neurons were CGRPR neurons (data not shown). These data indicate that CGRPR neurons in the CeAl facilitate encoding of pain signals during threat learning.
Activation of CGRPR Neurons in the CeAl Is Sufficient to Elicit Threat Learning
To determine whether CGRPR neuronal activation is sufficient to induce defensive responses and threatassociated learning, CalcrlCre mice were injected bilaterally in the CeAl with AAV1-DIO-ChR2:YFP or AAV1-DIO-YFP as controls (Figure 7A). Immunohistochemical staining after the behavioral tests revealed that the photostimulation of the CeAl CGRPR neurons induced Fos in ChR2-expressing CGRPR neurons, but not in the LA (Figure 7B). Three weeks after viral injection, the mice were placed in an open field arena to monitor freezing behavior induced by four 30-s photostimulations with 60-s intervals between stimulations (Figure 7C). Freezing behaviors time-locked to photostimulation were not observed by either ChR2-expressing or control mice, but ChR2-expressing mice gradually developed freezing behavior during the 7-min test session, whereas controls did not (Figure 7D, Figure S7, and Video S6). To test whether the mice associated the context in which they were photostimulated as a threat, they were returned to the arena 24 h later. The ChR2 group displayed more freezing compared to the control mice (Figure 7E).
Figure 7. Optogenetic Stimulation of CGRPR Neurons in the CeAl Induces Freezing Behaviors and Produces a Threat Memory.
(A) Schematic diagram illustrating the placement of optic fiber bilaterally in the CeA in a CalcrlCre mouse injected with AAV-DIO-ChR2:YFP into the CeA.
(B) Representative histological images showing the Fos-like immunoreactivity within the CeAl after 30-s photostimulation of CGRPR neurons.
(C) Illustration of context-dependent optogenetic conditioning. Photostimulation (40 Hz) was used as the noxious teaching signal instead of foot shock.
(D and E) Optogenetic stimulation of the CeAl CGRPR neurons did not induce freezing behaviors, but increased basal freezing immediately after the photostimulation in a step-wise manner (E), and produced fear memory 24 h after the photostimulation (E).
(F) Illustration of cue-dependent optogenetic conditioning.
(G and H) Optogenetic stimulation of the CeAl CGRPR neurons paired with 10-kHz pure tone produced context-dependent (G), and cue-dependent (H) fear memories. All data shown are means ± s.e.m. from 7 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
To test whether the optical stimulation of CGRPR neurons was sufficient for mice to establish a threat memory, they were exposed to a 30-s tone in a novel context that overlapped with a 10-s photostimulation (Figure 7F). When tested the next day, the ChR2 group displayed robust freezing when returned to the test box (Figure 7G) or when placed in a novel box and exposed to the CS tone (Figure 7H). These results demonstrate that activation of CGRPR neurons in the CeAl delivers pain-like signals that are sufficient to generate both context- and cue-dependent memories.
DISCUSSION
Deciphering the neural circuitry for the US is essential to complete the current understanding of how the amygdala encodes associative threat memories. The traditional fear-conditioning model suggests that the US is transmitted from the spino-thalamic tract to the LA where the CS and US converge, thereby engaging synaptic plasticity mechanisms to establish a fear memory (Herry and Johansen, 2014; Pape and Pare, 2010). Here, using optogenetic activation and toxin-mediated silencing techniques, we demonstrate that the CGRP neurons in the PBel transmit the foot shock-driven US teaching signal to CGRPR neurons in the CeAl. Our results add to recent evidence indicating that the US and CS can converge within the CeAl, as well as the LA (Ciocchi et al., 2010; Duvarci et al., 2011; Li et al., 2013; Pare and Duvarci, 2012; Sato et al., 2015; Wilensky et al., 2006). Both the CS and US may promote synaptic plasticity at multiple nodes along their paths to the CeAl. The CeAl directs its output to the medial CeA and from there to distal brain regions that regulate appropriate physiological and behavioral responses.
Previous reports showed that the local inhibitory microcircuits in the CeAl are formed with two functionally and genetically distinct neuronal subpopulations (Ciocchi et al., 2010); PKC-δ neurons decrease their firing rate in response to the CS (CeAloff) (Haubensak et al., 2010), whereas SOM neurons increase their firing rate in response to the CS (CeAlon) (Li et al., 2013). A previous study showed that stimulation of the PBN increased the firing rate of CeAlon neurons in vivo (Ciocchi, 2009). Therefore, our results suggest that the CGRPR neurons that we manipulated include the CeAlon neurons. However, our double-labeling study showed that only 6% of CGRPR neurons co-express SOM (Figure 5G–I). Repeated photostimulation of SOM neurons reversibly induced freezing during stimulation, but the mice failed to develop a fear (threat) memory (Li et al., 2013). In contrast, photostimulation of CGRPR neurons failed to induce an immediate freezing during the stimulation (unlike photostimulation of CGRP neurons in PBel), but the mice gradually developed freezing behavior during repeated trials (Figure 7F and G). Perhaps stimulation of the PBel CGRP neurons recruits both the CGRPR and SOM neurons during threat conditioning; immediate freezing is achieved by the activation of SOM neurons while the US-CS association is acquired by activation of CGRPR neurons. Alternatively, a single injection of virus may not be enough to transduce the entire population of CGRPR neurons in the CeAl. Our results also reveal that the CGRPR neuronal population partially overlaps with PKC-δ (CeAloff) neurons in caudal CeAl, but much less so in rostral CeAl (Figure 5D–F). PKC-δ neurons also partially overlap with tachykinin 2 (Tac2; ~50%) (Cai et al., 2014) and oxytocin receptor (Oxtr; ~65%) (Haubensak et al., 2010) neuronal populations, both of which are known to suppress fear expression (Andero et al., 2014; Knobloch et al., 2012). These results suggest that PKC-δ is expressed in the multiple populations of neurons in the CeAl; the CeAloff neurons may represent the subpopulation of PKC-δ neurons that is Tac2+, and/or Oxtr+, but CGRPR13 negative. The relationships and connectivity of CGRPR neurons in the CeAl to the other neuronal populations implicated in threat conditioning remains to be established.
We observed that expression of TetTox in CGRP neurons prevents the immediate locomotor activity during the 2-s foot shock (Figure 3A and 3B), whereas photoactivation of CGRP neurons in the PBel generates immobility without stimulating the initial burst of activity (Figure 4C and 4D). There are two potential explanations for this dichotomy. The level of activation of CGRP neurons that occurs during the foot shock may exceed that which occurs with photostimulation and high activity may be necessary to initiate the burst in locomotor activity. Alternatively, the burst of activity may require simultaneous activation of two pathways; hence, blocking one pathway may be sufficient to prevent the response, but activating just one pathway may be insufficient to produce the response. We also observed the incomplete block of freezing behaviors during threat conditioning by TetTox in both CalcaCre and CalcrlCre mice. This may be due to incomplete silencing of the target neurons by single bilateral injections of AAV-DIO-GFP:TetTox. Alternatively, the spino-thalamic pathway and the spinoparabrachial pathway may send pain signals in parallel during threat learning. Previous studies support this idea. Although foot shock-induced Fos activation in the sensory thalamus has not been described, electrophysiological measurements showed that foot shock and pinch do activates the sensory thalamic neurons (e.g. posterior intralaminar thalamic nuclei, PIN) (Asede et al., 2015; Bienvenu et al., 2015), as well as the PBel neurons (Bernard and Besson, 1990; Bester et al., 2000), and the PIN sends excitatory projections to both intercalated neurons and principal neurons in the LA (Asede et al., 2015; Bienvenu et al., 2015). Thus, the spino-thalamic and spino-parabrachial circuits may coordinately activate the CeA and LA to establish robust learning about threats.
We showed that the modulation of CGRP neurons in the PBN or CGRPR neurons in the CeAl affects cue-dependent and context-dependent threat memory acquisition and retrieval by attenuating the aversive sensory inputs during associative threat learning. Based on these observations, we speculate that both cue- and context-dependent threat learning utilize the same spino-parabrachio-amygdaloid pathway as an aversive US circuit during associative learning. In a recent study, we showed that PBel CGRP neurons are also critical for conditioned taste aversion (CTA). Genetic or optogenetic inactivation of PBel CGRP neurons substantially attenuated the aversion to a novel taste paired with LiCl injection. And, pairing a novel taste with optogenetic stimulation of PBel CGRP neurons, instead of LiCl injection, induced strong CTA response (Carter et al., 2015). These results indicate that the CGRP neurons in the PBel transmit aversive signals from the vagus nerve as well as spinal lamina 1 neurons.
The CGRP neurons in the PBel express Fos in response to anorectic peptides (cholecystokinin and amylin), inflammation, and visceral malaise (Carter et al., 2013). These signals are relayed by vagal stimulation of the nucleus tractus solitarius (NTS) to the PBel. The PBel can also mediate pain-induced loss of appetite (Malick et al., 2001; Petrovich et al., 2009). Importantly, appetite suppression without freezing was observed with low frequency stimulation of CeAl PKC-δ neurons (Cai et al., 2014), which overlap with some CGRPR neurons (Figure 5D). These differential behavioral effects may reflect two different CGRP neuronal populations in the PBel—one activated by spinal inputs (mediating pain) and the other activated by inputs from the vagus via the NTS (mediating visceral malaise)—that have different stimulation thresholds. Alternatively, a single population of CGRP neurons may activate different populations of downstream neurons by secreting different neurotransmitters in a frequency-dependent manner. The latter idea is more congruent with the previous reports because recording in vivo showed that the same neurons in the PBel could be activated by both visceral stimuli (colorectal distension) and cutaneous noxious heat (Bernard et al., 1994). In addition, PBel neurons fire at lower frequency range when stimulated by visceral stimuli, whereas they fire at higher frequency range when stimulated by cutaneous noxious stimuli (Bernard et al., 1994).
We also provide behavioral evidence that the two main ascending pain pathways may have different roles in central pain processing. Inhibiting the activity of CGRP neurons in the PBel not only blocked the immediate escape behavior during the foot shock, but also blocked the escape jumping response at high temperatures during the hot-plate test (Figure 3C). However, inactivation of CGRP neurons did not affect latency of paw withdrawal to thermal or mechanical stimuli (Figure 3D and 3E). These results imply that the spino-parabrachio-amygdaloid pathway may transduce the affective motivational aspects of pain, whereas the spino-thalamic pathway may transduce the sensory and discriminative aspects of pain (Auvray et al., 2010; Bernard et al., 1996; Strobel et al., 2014; Veinante et al., 2013). Our data suggest that CGRP neurons in the PBel transmit the affective component of pain. Alternatively, the transduction of these different pain signals may be cell-type specific, not brain structure-specific. CGRP-positive sensory neurons may transduce affective pain signals, and non-CGRP neurons may transduce sensory pain signals, regardless of which brain structures they innervate. Further study should address the cell-type specificity in transducing different aspects of pain.
Identification of a neural circuit that transmits only affective pain signals has clinical relevance. Blockade of affective pain without changing sensory pain would be an ideal target for treatment of chronic neuropathic pain and related-psychiatric comorbidities. CGRPR antagonists are already considered as good candidates for the treatment for the chronic affective pain disorders, such as osteoarthritis, and migraine headaches (Hirsch and Birklein, 2014; Hirsch et al., 2013; Ho et al., 2014).
In summary, by employing recently available technologies, such as optogenetic circuit mapping and genetic silencing techniques, our results emphasize the importance of a previously ignored contribution of the spino-parabrachial-amygdaloid pain circuit as an important aversive signaling pathway during associative threat learning by providing compelling evidence of followings: First, the CGRP neurons in the PBel selectively transmit affective pain signals. Second, the same neurons send aversive teaching signal (US) to the CeA during aversive threat learning. Third, the US-CS association occurs within the CGRPR neurons in the CeA.
METHODS SUMMARY
Mice
CalcaCre mice were made as described (Carter et al., 2013). CalcrlCre mice were made by inserting a 6 kb 5’ arm and a 4.1 kb 3’ arm into a targeting vector with ires-Cre:GFP, frt-flanked SV-Neo (for positive selection), HSV-TK and Pgk-DTa (for negative selection). The SV-Neo gene was removed by a cross with Gt(ROSA)26Sor-FLP recombinase mice and then CalcrlCre mice were continuously backcrossed to C57Bl/6J mice. SstCre and Tac2Cre mice were obtained from Jackson Laboratory.
Virus Production and Stereotaxic Surgery
AAV vectors were co-transfected with AAV serotype 1 helper plasmid into human embryonic kidney (HEK293T) cells, and purified by multi-step, sucrose- and CsCl-gradient ultracentrifugation. Stereotaxic surgery was performed as described (Carter et al., 2013). Cre-dependent virus (0.5 µl) was bilaterally injected in the PBN (antero-posterior (AP), −5.1 mm; medio-lateral (ML), ±1.3 mm; dorso-ventral (DV), 3.25 mm) or in the CeA (AP, −1.2 mm; ML, ±2.9 mm; DV, 4.9 mm) for 5 min (0.1 µl/min).
Immunohistochemistry
Fos, PKC-δ and SOM immunolabeling and quantification were performed as described (Carter et al., 2013). We used CalcrlCre::tdTomato, SstCre::tdTomato, Tac2Cre::tdTomato, or wild-type mice for genetic labelling. Detailed experimental procedures are described in the supplemental information.
Slice Electrophysiology
Coronal brain slices (250 µm) were prepared as described (Carter et al., 2013). For light-evoked EPSCs, neurons were held in voltage clamp at −70 mV and EPSCs were sWmulated by 10-ms pulses of blue laser light at 0.1 Hz using a fiber optic placed in the bath above the slice. Detailed experimental procedures are described in the supplemental information.
Behavioral Tests for Sensory Pain Signals
Hot/Cold Plate Analgesia Meter (Coulbourn Instruments) was used for the hot-plate test. Plantar Test apparatus (Ugo Basile model 37370) was used for the tail-flick test. Dynamic Plantar Aesthesiometer (Ugo Basile, model 37450) was used to test the mechanical sensation of the test mouse. Detailed experimental procedures are described in the supplemental information.
Behavioral Tests for Affective Pain Signals
The open field test was performed to measure general locomotor behaviors. Context-dependent, and auditory cue-dependent threat-conditioning tests were performed to measure the response to the painful threats, as well as the threat-dependent memory. The test was performed as described with minor modification (Han et al., 2012). Detailed experimental procedures are described in the supplemental information.
Optogenetic Threat Conditioning
The optic fibers were bilaterally connected to the optic ferrules on the head of the test mouse. Ten minutes after the habituation to the optic fibers, the test mouse was introduced to a behavioral arena for the optogenetic conditioning. During the conditioning, the test mouse received photostimulation (40 Hz frequency and 14 mW/mm2 intensity) instead of foot shock. Detailed experimental procedures are described in the supplemental information.
Supplementary Material
ACKNOWLEDGMENTS
Authors thank Dr. S. Dymecki for a GFP:TetTox construct, and Dr. K. Deisseroth for AAV-ChR2:YFP and YFP constructs. M. Chiang assisted with animal husbandry; A. Bowen helped with histology; K. Kafer and S. Tsang helped generate CalcrlCre mice. We appreciate feedback on the manuscript from laboratory members and Drs. M. Carter, J. Kim, A. Dhaka, and D. Anderson. L.S.Z is supported by a grant from National Institute of Health (R01MH094536).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions S.H. and R.D.P. conceived and designed the study. S.H. performed and analyzed histological and behavioral experiments, M.T.S performed some histological experiments, M.E.S. performed electrophysiology experiments and R.D.P. produced the CalcaCre and CalcrlCre mouse lines. L.S.Z. and R.D.P. provided equipment, reagents and expertise. S.H. and R.D.P wrote the manuscript with help from the other authors.
REFERENCES
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. The Journal of comparative neurology. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83:444–454. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asede D, Bosch D, Luthi A, Ferraguti F, Ehrlich I. Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron. 2015;86:541–554. doi: 10.1016/j.neuron.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Auvray M, Myin E, Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neuroscience and biobehavioral reviews. 2010;34:214–223. doi: 10.1016/j.neubiorev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. Journal of neurophysiology. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Progress in brain research. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. Journal of neurophysiology. 1994;71:15. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. Journal of neurophysiology. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Micklem BR, Mansouri M, Magill PJ, Ferraguti F, Capogna M. Large intercalated neurons of amygdala relay noxious sensory information. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:2044–2057. doi: 10.1523/JNEUROSCI.1323-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Kim JJ. Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behavioral neuroscience. 2001;115:365–375. [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nature neuroscience. 2014;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related Peptide neurons mediate conditioned taste aversion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S. PhD Thesis. University of Basel Ph.D; 2009. Fear conditioning- and extinction-induced neuronal plasticity in the mouse amygdala; p. 58. [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- D'Hanis W, Linke R, Yilmazer-Hanke DM. Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala. The Journal of comparative neurology. 2007;505:268–291. doi: 10.1002/cne.21495. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. The Journal of comparative neurology. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual review of psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nature reviews Neuroscience. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Molecular pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:10717–10728. doi: 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nature neuroscience. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Birklein F. [Pain-relieving effect of CGRP antagonism on inflammatory pain] Schmerz (Berlin, Germany) 2014;28:532–535. doi: 10.1007/s00482-014-1460-0. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain. 2013;154:700–707. doi: 10.1016/j.pain.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, Lines C, Edvinsson L, Goadsby PJ, Michelson D. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–966. doi: 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nature reviews Neuroscience. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Petrov T, Harris KH, Vu T, Krukoff TL. Parabrachial nucleus projection to the amygdala in the rat: electrophysiological and anatomical observations. Brain research bulletin. 1996;39:115–126. doi: 10.1016/0361-9230(95)02084-5. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature neuroscience. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Horovitz O, Pellman BA, Tan LM, Li Q, Richter-Levin G, Kim JJ. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14795–14800. doi: 10.1073/pnas.1310845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kocorowski LH, Helmstetter FJ. Calcitonin gene-related peptide released within the amygdala is involved in Pavlovian auditory fear conditioning. Neurobiology of learning and memory. 2001;75:149–163. doi: 10.1006/nlme.2000.3963. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nature neuroscience. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Chen YZ, Wei YY, He XT, Li X, Hu W, Yanagawa Y, Wang W, Wu SX, Dong YL. Neurochemical properties of the synapses between the parabrachial nucleus-derived CGRPpositive axonal terminals and the GABAergic neurons in the lateral capsular division of central nucleus of amygdala. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8713-x. [DOI] [PubMed] [Google Scholar]
- Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MJ, Terron JA, Stanimirovic DB, Doods H, Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. Journal of neurophysiology. 2002;87:103–112. doi: 10.1152/jn.00264.2001. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Current opinion in neurobiology. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:15205–15212. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissuespecific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Sarhan M, Freund-Mercier MJ, Veinante P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. The Journal of comparative neurology. 2005;491:418–442. doi: 10.1002/cne.20697. [DOI] [PubMed] [Google Scholar]
- Sato M, Ito M, Nagase M, Sugimura YK, Takahashi Y, Watabe AM, Kato F. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Molecular brain. 2015;8:22. doi: 10.1186/s13041-015-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fearpotentiated startle: lesion studies. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel C, Hunt S, Sullivan R, Sun J, Sah P. Emotional regulation of pain: the role of noradrenaline in the amygdala. Science China Life sciences. 2014;57:384–390. doi: 10.1007/s11427-014-4638-x. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nature reviews Neuroscience. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. Journal of Molecular Psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Molecular brain. 2013;6:11. doi: 10.1186/1756-6606-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.