Abstract
The current study describes everyday executive function (EF) profiles in young children with Down syndrome. Caregivers of children with Down syndrome (n = 26; chronological ages = 4-10 years; mental ages = 2-4 years) completed the Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P; G. A. Gioia, K. A. Espy, & P. K. Isquith, 2003), a caregiver report measure of everyday/functional EF skills in multiple domains. On the BRIEF-P, elevations were noted on a global EF composite as well as the Working Memory and Plan/Organize scales in particular (relative to norms developed for typically developing children of a similar mental age). These results suggest a specific pattern ofEF weaknesses in young children with Down syndrome, consistent with the extant literature that has focused primarily on older individuals who have been tested using laboratory EF tasks.
Down syndrome, the most common genetic syndrome associated with intellectual disability, occurs in 1 in 732 live births (Canfield et al., 2006). The neuropsychological phenotype of Down syndrome is characterized by a pattern of relative weaknesses and strengths, with weaknesses in expressive language, problem solving, and both verbal short-term and working memory and strengths in receptive language and visuospatial processing (for a review, see Fidler & Nadel, 2007). Research on executive function (EF) profiles, particularly in children with Down syndrome is scant, despite studies of the Down syndrome neuroanatomical phenotype suggesting specific reductions in the size of the frontal lobes (for a review, see Nadel, 2003), a region of the brain most often associated with EF (Roberts, Robbins, & Weiskrantz, 1998). Thus, in the current study, we add to what is known about EF in Down syndrome by describing patterns of everyday EF in various domains (e.g., working memory, inhibition) in a population-based sample of children with Down syndrome (chronological ages = 4-10 years; mental ages = 2-4 years) by using a caregiver-report measure, the Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P; Gioia, Espy, & Isquith, 2003).
EF refers to a collection of skills, including working memory, planning, inhibition, and cognitive flexibility, which are necessary to solve novel problems and cope with changing task demands (Lezak, Howieson, & Loring, 2004; Miyake et al., 2000). These functions are thought to be related but distinct, as evidenced by low correlations among various EF tasks (Miyake et al., 2000). In the last 10-15 years, researchers have begun to distinguish between more affect-related executive processes, called hot EF (associated with the ventromedial prefrontal cortex), and primarily cognitively mediated executive processes, called cool EF (associated with the dorsolateral prefrontal cortex; Metcalfe & Mischel, 1999; Zelazo & Muller, 2002). These distinctions have been supported by both patient and animal studies in which lesions in the ventromedial or dorsolateral prefrontal cortices have been associated with deficits on hot or cool EF tasks, respectively (Bechara, Damasio, Tranel, & Anderson, 1998; Dias, Robbins, Roberts, 1996). Last, some researchers are beginning to apply these distinctions to developmental disorders as well (e.g., Zelazo & Muller, 2002). Thus, the question arises: Could this model be used to examine the nature of EF deficits in Down syndrome?
Different laboratory measures have been implemented to assess hot and cool EFs (for a review, see Zelazo & Müller, 2002). Examples of hot EF tasks include gambling tasks, such as the Iowa Gambling Task, and delay discounting tasks (tasks in which children must choose between a small reward now or a large reward later). Examples of cool EF tasks include working memory tasks, such as backward digit span, and tests of cognitive flexibility, such as the Wisconsin Card Sorting Task (Heaton, Chelune, Talley, Kay, & Curtiss, 1993). Although the BRIEF-P was not developed to assess hot and cool EF per se, its five clinical scales, which include Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organize, assess behaviors that on face value map onto hot and cool EF domains. For example, the Inhibition and Emotional Control Scales assess skills related to regulating emotion and behavior (which we conceptualize as relating more to hot EF), whereas the Working Memory and Plan/Organize Scales tap skills related to attention and completion of cognitive tasks (which we conceptualize as relating more to cool EF). See Table 1 for a description of the five EF scales as well as examples of behaviors assessed by each.
Table 1.
Descriptions of BRIEF-P Clinical Scales
| Clinical scale | Clinical scale description and behavioral examples | Corresponding index(es) |
|---|---|---|
| Inhibit (I) | Measures behavioral regulation or the ability to inhibit responses and avoid engaging in impulsive or inappropriate behavior. Behavioral examples: impulsive; behavior is “too wild.” | Inhibitory Self-Control (ISO) |
| Shift (S) | Measures ability to flexibly move between tasks, parts of a problem, or situations and to alternate attentional focus when completing tasks. Behavioral examples: resistant to change; difficulty with new situations. | Flexibility (Fl) |
| Emotional Control (EC) | Measures emotion modulation and corresponding behavioral responses. Behavioral examples: overreacts to minor difficulties; easily upset. | ISO; Fl |
| Working Memory (WM) | Measures the ability to maintain information in the focus of one's attention to complete a task or generate the appropriate response. Behavioral examples: problems completing multistep tasks or activities; difficulties finishing tasks. | Emergent Metacognition (EMI) |
| Plan-Organize (PO) | Measures the ability to anticipate and prepare for future activities or tasks, handle current demands, and to impose order on information to accomplish a goal. Behavioral examples: has difficulty locating possessions; leaves messes. | EMI |
Compared with other developmental disorders, such as autism, research on EF skills in individuals with Down syndrome is limited. A summary of existing studies of EF in children and young adults with Down syndrome using direct neuropsychological assessment is provided in Table 2. Because Down syndrome is associated with premature onset of Alzheimer's disease (Zigman & Lott, 2007), we did not include research focused on middle-aged to older adults in our summary (i.e., we wanted to exclude studies with participants who may have been experiencing cognitive decline associated with dementia). As can be seen, the table is organized by EF domain. We included the following domains: inhibition, planning–problem solving, cognitive flexibility–shifting, and working memory.
Table 2.
Studies Examining Executive Function in Children and Young Adults With Down Syndrome
| Executive function domain and studies | Control group(s) | Chronological age of group with Down syndrome: M (range) | Task | Findings |
|---|---|---|---|---|
| Inhibition | ||||
| Kopp, Krakow, & Johnson, 1983 | MA-match TD | ~4 y (2-5 y) | Delayed reward | DS < TD DS: decreased latency to touch prohibited (but desired) object |
| Pennington, Moon, Edgin, Stedron, & Nadel, 2003 | MA-match TD | ~15 y (11-19 y) | Stop | DS-TD DS: similar number of correct inhibitions |
| Rowe, Lavender, & Turk, 2006 Lanfranchi, Jerman, Dal Pont, Alberti, & Vianello, 2010 | CA-match ID | ~33 y (23-40 y) | Finger tapping | DS < ID DS: less able to inhibit following examiner's (prepotent) tapping response (this finding did not survive correction for multiple comparisons) |
| MA-match TD | ~15 y (11-18 y) | Day-night (modified Stroop) | DS < TD on experimental but not control condition | |
| Planninq-problem solving | ||||
| Kasari & Freeman, 2001 | CA-match ID MA-match TD |
~8y (5-12 y) | Solvable & unsolvable puzzles | DS < ID, TD DS: increased latency to initiate task & complete puzzles |
| Pennington et al., 2003 | MA-match TD | ~15 y (11-19 y) | Stockings of Cambridge (Tower) | DS ~ TD DS: similarly efficient solutions |
| Fidler, Hepburn, Mankin, & Rogers, 2005 | CA-match ID MA-match TD |
~3 y (2-4 y) | Object retrieval | DS < ID, TD DS: less efficient strategy to obtain desired object |
| Rowe et al., 2006 | CA-match ID | ~33 y (23-40 y) | Tower of London | DS < ID DS: less efficient & slower solutions (this finding did not survive correction for multiple comparisons) |
| Lanfranchi et al., 2010 | MA-match TD | ~15 y (11-18 y) | Tower of London | DS < TD DS: fewer problems solved overall and on first attempt |
| Cognitive flexibility-shifting | ||||
| Zelazo, Burack, Benedetto, & Frye, 1996 | MA-match TD | ~23 y (16-30 y) | Card sort | DS < TD DS: less able to sort using more than one rule |
| Edgin, 2003 | CA-match ID (Williams syndrome) | ~18 y (13-23 y) | Dimensional card sort | DS < ID DS: worse performance on post-shift trials |
| Rowe et al., 2006 | CA-match ID | ~33 y (23-40 y) | Weigl color form | DS < ID DS: less able to sort by second category |
| Lanfranchi et al., 2010 | MA-match TD | ~15 y (11-18 y) | Rule shift task Modified card sort |
DS < TD DS: greater shift difficulties on shift task; fewer categories and more errors on card sort task |
| Working memory: Part 1a | ||||
| Vicari, Carlesimo, & Caltagirone, 1995 | CA-match ID MA-match TD |
~17 y (range not noted) | Verbal & spatial backward span | DS < ID, TD DS: reduced backward span for both verbal & spatial conditions |
| Pennington et al., 2003 | MA-match TD | ~15 y (11-19 y) | Spatial working memory & counting span | DS - TD DS: similar number of errors on spatial working memory & similar counting span length |
| Lanfranchi, Cornoldi, & Vianello, 2004 | MA-match TD | 2 DS groups Ms = ~12, 15 y Ranges = 7-18; 11-18y |
Various verbal/spatial span tasks; other working memory tasks | DS < TD on all tasks (verbal & visuospatial) with greater working memory demands |
| Kogan et al., 2009 | MA-match TD CA-match TD MA-match ID (fragile X) |
~17 (11-36 y; all males) | Delayed nonmatching-to-position & sample | DS < CA-match TD only on position task (DS ~ other groups on sample task) |
| Lanfranchi, Jerman, & Vianello, 2009 | MA-match TDs (2 groups) | ~13 y (8-19 y) | Similar to Lanfranchi et al., 2004 | DS < TDs (both groups) on all tasks (verbal & visuospatial) with greater working memory demands |
| Lanfranchi et al., 2010 | MA-match TD | ~15 y (11-18 y) | Verbal- visuospatial dual tasks | DS < TD on verbal & visuospatial dual tasks |
| Edgin, Pennington, & Mervis, 2010 | CA-match ID (Williams syndrome) | ~18 y (13-23 y) | Verbal & spatial backward span | DS ~ ID DS: similar performance on backward span for both verbal and spatial conditions |
| Workinq Memory: Part 2 | ||||
| Numerous studies; see Jarrold & Baddeley, 2001 for a review | Studies have included: CA-match ID & MA-match TD | Ages vary | Digit span, spatial span, nonword repetition | In general, DS < ID & TD on verbal short-term memory, but not visuospatial short-term memory tasks |
Note. MA = mental age; TD = typically developing; CA = chronological age; ID = intellectual disability; DS = Down syndrome.
The working memory section is divided into studies that have used more traditional working memory tests in which manipulation of the to-be-recalled items is required or delay is involved (Part 1, e.g., backward digit or spatial span, spatial working memory, delayed nonmatch to sample-position) and a section on working memory studies that have used tasks in which short-term memory, such as the phonological memory component of working memory, is probed requiring verbatim recall of items (Part 2, e.g., digit span, nonword repetition).
The large majority of studies examining EF in Down syndrome have documented weaknesses relative to typically developing children matched on mental age (MA) or children with another form of intellectual disability (in most cases, idiopathic intellectual disability) matched on chronological age (CA) in all four EF domains studied to date (but see Pennington, Moon, Edgin, Stedron, & Nadel, 2003, in which EF deficits were not reported, but the authors speculated about whether deficits may have been found using more developmentally appropriate tasks). The greatest evidence for EF weaknesses comes from the working memory domain; however, most of these studies have focused on short-term memory, which requires only verbatim item recall and no item manipulation (for a review of short-term memory research in Down syndrome, see Baddeley & Jarrold, 2007; Jarrold & Baddeley, 2001).
Despite the consistency across many of the studies completed to date, there remain several unanswered questions about EF skills in Down syndrome. First, very little is known about EF skills in young children (< 10 years old) with Down syndrome. Although there are three studies that included children under the age of 10 (Fidler, Hepburn, Mankin, & Rogers, 2005; Kasari & Freeman, 2001; Kopp, Krakow, & Johnson, 1983), these studies were limited in that they used only one task thought to tap EF skills. Given that intellectual functioning in Down syndrome has been reported to decline from infancy to adult-hood (Carr, 2005; Hodapp & Zigler, 1990), it is important to describe different aspects of cognition, including EF, at different ages to augment our understanding of the developmental course of this disorder. Furthermore, from a treatment standpoint, identifying cognitive deficits in young children with Down syndrome may be informative for early intervention programs aimed at lessening cognitive impairments experienced by individuals with Down syndrome.
Second, the lack of comprehensive studies ofEF (across multiple domains, including both hot and cool EF measures) makes it difficult to draw conclusions about profiles of EF strengths and weak-nesses in Down syndrome. This deficiency in the literature seems particularly important to address, because research on other developmental disorders suggests that different disorders may be associated with unique patterns of EF strengths and weaknesses rather than global depressions in EF across multiple domains of functioning (for a review, see Pennington & Ozonoff, 1996; Zelazo & Müller, 2002). Again, a clarification of which EF domains are impacted may inform treatment studies and may also help researchers tie neuropsychological deficits to their genetic and neuroanatomical underpinnings.
Third, none of the studies completed to date have described everyday or functional EF skills in Down syndrome but instead have reported on laboratory EF testing. Given the importance of everyday EF skills (e.g., planning; inhibiting inappropriate or prepotent responses) for people with Down syndrome to live more independently in the community, it is essential that we gain a better understanding of everyday EF profiles. Because it has been suggested that the BRIEF-P (Gioia, Espy, & Isquith, 2003) and the school-age BRIEF may measure different aspects of EF than laboratory measures (Anderson, Anderson, Northam, Jacobs, & Mikiewicz, 2002; Mahone & Hoffman, 2007; Mahone, Zabel, Levey, Verda, & Kinsman, 2002; Vriezen & Pigott, 2002), the current investigation complements existing studies. Furthermore, research with other developmental disorders, such as autism (Gilotty, Kenworthy, Sirian, Black, & Wagner, 2002), has identified correlations between parent reports of EF using the BRIEF and adaptive functioning skills, suggesting that parent reports of EF may be important in predicting daily living skills for children with Down syndrome as well.
In the current study, our goal was to provide a description of functional EF skills in a population-based sample of young children (ages 4–10 years) with Down syndrome using the BRIEF-P, a measure that is appropriate for the MA of the participants included in this study. In particular, we sought to answer the following questions:
Do young children with Down syndrome present with clinically elevated levels of everyday EF weaknesses using norms appropriate for their MA?
Are EF skills uniformly elevated in children with Down syndrome or do they vary as a function of EF domain? In particular, do these children present with EF deficits in more hot or cool EF domains?
Method
Participants
Twenty-six children with Down syndrome participated in the current study. These participants were a subsample of children with Down syndrome (who were free of a comorbid autism spectrum disorder) enrolled in a cross-sectional surveillance study of autism spectrum disorders in children with Down syndrome (see DiGuiseppi et al., 2010, for more details). Participants were included in the current study if they met the following criteria: (a) They had a complete BRIEF-P protocol filled out by their primary caregiver; (b) their MA, as estimated by a direct developmental assessment (see below for details), fell within the normative sample age range of the BRIEF-P, which is 2 years, 0 months to 5 years, 11 months; and (c) they were free of a clinical diagnosis of autism spectrum disorder as determined by expert clinical opinion, which integrated information obtained through the Autism Diagnostic Observation Schedule (Lord, Rutter, DiLavore, & Risi, 1994) and the Autism Diagnostic Interview–Revised (Lord, Rutter, & Le Couteur, 1994).
Demographic information about the participants, including CA, MA, developmental quotient (described below), gender, race/ethnicity, and parental education is summarized in Table 3.
Table 3.
Demographic Characteristics of the Sample
| M | SD | Range | |
|---|---|---|---|
| Chronological age (months) | 75.15 | 23.05 | 48-129 |
| Mental age (months) | 36.57 | 8.98 | 24-57 |
| Developmental quotient | 50.23 | 9.95 | 33-68 |
| N | % | |
|---|---|---|
| Total sample | 26 | — |
| Female | 11 | 42.3 |
| White, Non-Hispanica | 20 | 80.0 |
| Mother ed.: collegeb,c | 14 | 58.3 |
| Father ed.: collegeb,d | 15 | 62.5 |
n = 25.
n = 24.
Mother's education: number/% who completed college.
Father's education: number/% who completed college.
Procedures
Written consent was obtained from the parents of participants prior to completing any measures. All evaluations were completed either at JFK Partners (the University Center for Excellence in Developmental Disabilities for the state of Colorado) at the University of Colorado School of Medicine in Denver or at Colorado State University in Fort Collins. Caregivers completed one BRIEF-P form per participant. Children were evaluated by experienced clinicians using a developmentally appropriate evaluation.
Measures
Developmental assessment
Participants with an estimated developmental level at or below 68 months were administered the Mullen Scales of Early Learning (MSEL; Mullen, 1995). This developmental assessment tool provides an estimate of verbal and nonverbal abilities for children between the ages of 1 and 68 months. Participants with an estimated developmental level above 68 months were evaluated using the Differential Ability Scales (DAS; Elliott, 1990), an assessment of overall cognitive ability normed for children aged 2.5 through 17 years, which also provides an estimate of verbal and nonverbal abilities. Both tasks yield age-based standard scores and age-equivalent scores for each of the scales or subtests. Age-equivalent scores from the scales (MSEL) or subtests (DAS) were averaged for each participant to generate an estimate of overall MA. This score was used to determine if a participant would be included in the current study (i.e., if they had a MA within the BRIEF-P normative age range). To avoid floor effects for participants with significant intellectual impairment and to use a consistent measure of developmental level for all participants, developmental quotients (DQs) were calculated by dividing mean age-equivalent scores (i.e., MA) by CA, consistent with previous studies (Munson et al., 2008). Although this is not the preferred method to describe cognitive abilities, we reasoned it was most appropriate for the current study for two reasons. First, some of the children included in this study had very significant levels of intellectual impairment that prohibited the administration of the age-appropriate cognitive assessment and the ability to rely on more robust age-normed standard scores. Second, because we were not interested in the relations between EF and general cognitive ability, as measured by an IQ test, but needed an approximation of developmental level to probe EF abilities that were developmentally relevant to our participants, we determined that using DQs (and age-equivalent scores) was adequate.
Everyday executive function skills assessment
EF skills were assessed for all26 participants utilizing the BRIEF-P (Gioia, Espy, & Isquith, 2003), which was completed by one caregiver. Although most of the children (n = 19) in the current study were within the CA range for the school-age BRIEF, the BRIEF-P was used exclusively in this study, because it was deemed to be more developmentally appropriate for our participants. This decision was supported by parents’ comments about the inappropriateness of many of the questions on the school-age BRIEF for this young sample of children with Down syndrome (i.e., some questions on the school-age BRIEF probed homework completion and other tasks that were beyond the developmental level of our participants).
The BRIEF-P is a 63-item questionnaire that assesses EF in various domains. Caregivers describe their child's behavior using a 3-point Likert scale, indicating how frequently their child engages in a given behavior (never, sometimes, often) The scale was normed on 460 children (214 girls) ages 2 years, 0 months to 5 years, 11 months who were deemed representative of the U.S. population. The scale has adequate internal consistency (Cronbach's αs = .80–.97 for the scales) and test–retest reliability (test–retest rs = .78–.90 for the scales and .87–.90 for the indexes). Convergent and discriminant validities were established through an examination of correlations between the BRIEF-P clinical scales–indexes and other ratings scales thought to assess similar or dissimilar domains. However, it is important to note that when the BRIEF-P was created, it was not validated with laboratory EF measures. Thus, the BRIEF-P may measure different aspects of EF than traditional laboratory tasks.
The BRIEF-P includes five clinical scales-Inhibit (I), Shift (S), Emotional Control (EC), Working Memory (WM), and Plan/Organize (PO)—that are both theoretically and empirically derived. These clinical scales are combined to form three index scales: the Inhibitory Self-Control Index (ISCI; I + EC Scales), Flexibility Index (FI; S + EC Scales), and Emergent Metacognition Index (EMI; WM + PO Scales). Last, a global measure of overall EF is generated—the Global Executive Composite (GEC). Details about the clinical scales and their corresponding indexes are provided in Table 1.
Raw scores from each of the scales and indexes were used to generate age- and sex-referenced normative T-scores. In this study, MA was used rather than CA to generate age-referenced T-scores. Higher T-scores denote greater levels of difficulty. The manual indicates that T-scores at or above 65 may be suggestive of clinical significance.
Data Analyses
First, the T-score means on the GEC and the three BRIEF-P indexes (ISCI, FI, and EMI) were compared with the normative mean of 50 using one-sample t tests. If any of the these t tests reached statistical significance, that index's corresponding clinical scales were compared with the normative mean of 50 using one-sample t tests. Next, percentages of children with Down syndrome within the sample with T-scores above 65 (the suggested cutoff to denote possible clinical significance) were examined for the GEC, three indexes, and five clinical scales. These percentages were compared with expected rates of clinical elevation (i.e., ~7% of the population will have T-scores ≥ 65) using chi-square tests. Following these analyses, within-group patterns of performance on the three indexes were examined using within-group analysis of variance (ANOV A). Last, to take a more detailed look at patterns of performance, differences among the five clinical scales (I, S, EC, WM, PO) were examined using within-group ANOVA. Bonferroni adjustment was applied to control for multiple comparisons.
Results
Comparisons of GEC, Index, and Clinical Scale T-Scores to BRIEF-P Normative Mean of 50
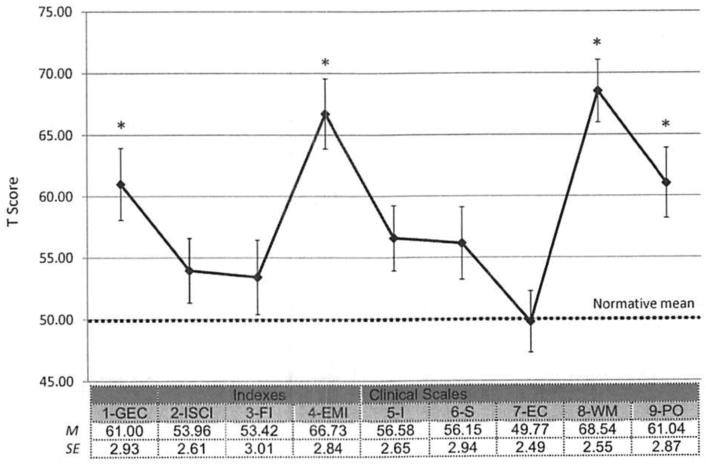
Means (and standard errors) for the GEC, index scores, and clinical scales are summarized in Figure 1. When the GEC and three index T-scores were each compared with the normative mean of 50 using one-sample t tests, only the GEC and the EMI significantly exceeded the normative mean (after Bonferroni adjustment, .05/ 4 = .01); ts for GEC, EMI, ISCI, and FI were as follows: t(25) = 3.76, p < .01; t(25) = 5.89, p < .001; t(25) = 1.52, p > .14; t(25) = 1.39, p > .26, respectively. Because only the EMI T-score significantly exceeded the normative mean, its corresponding clinical scales (WM, PO) were compared with the normative mean of 50 for additional analysis. Both significantly exceeded the mean (after Bonferroni adjustment, .05/2 = .025); WM, t(25) = 7.26, p < .001; PO: t(25) = 3.85, p < .01. These results indicate that children with Down syndrome presented with greater impairments in EF than typically developing children of a similar MA. However, this finding may have been driven, in part, by significantly higher (more impaired) scores on the two clinical scales that constitute the EMI, namely WM and PO.
Figure 1.
Means (with SE bars) for GEC, Index, and Clinical Scale T- Scores for DS group. 1 = Global Executive Composite, 2 = Inhibitory Self-Control Index, 3 = Flexibility Index, 4 = Emergent Metacognition Index, 5 = Inhibition Scale, 6 = Shift Scale, 7 = Emotional Control Scale, 8 = Working Memory Scale, 9 = Plan/ Organize Scale; * = scores for which the group mean significantly exceeded the normative mean T score of 50 (higher scores are associated with greater impairment); Bonferroni-adjusted p < .05.
Percentage of Participants With Clinically Elevated Scores on GEC, Indexes, and Clinical Scales
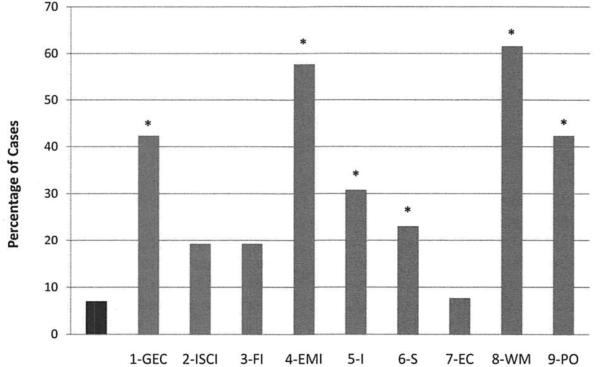
The percentage of participants who received clinically elevated T-scores (≥65) for the GEC, indexes, and clinical scales is summarized in Figure 2. Also presented in this figure is the expected rate of such elevations in the general population (~7%) to be used as a comparison. As can be seen, the percentage of participants with Down syndrome who had clinically elevated scores was significantly higher on GEC and EMI. Furthermore, elevations were noted on all scales, with the exception of the EC Scale (all χ2s[1] > 10; all ps < .006; Bonferroni-adjusted p, .05/9 = .006).
Figure 2.
Expected percentage of clinically elevated scores (T ≥ 65) on the BRIEF-P Scales (black bar) and the percentage of participants with Down syndrome with clinically elevated scores on the GEC, index, and clinical scales (gray bars); 1 = Global Executive Composite, 2 = Inhibitory Self-Control Index, 3 = Flexibility Index, 4 = Emergent Metacognition Index, 5 = Inhibition Scale, 6 = Shift Scale, 7 = Emotional Control Scale, 8 = Working Memory Scale, 9 = Plan/Organize Scale; * = scales for which the percentage of participants with Down syndrome with elevated scores significantly exceeded the expected value of 7%. Bonferroni-adjusted p < .05.
Within-Group Patterns of T-Scores on Indexes and Clinical Scales
To examine patterns of performance on the three indexes and five scales, within-group ANOVAs were completed. For the index T-scores, there was a main effect of index, F(2, 50) = 24.60, p < .001. Paired t tests revealed that the EMI T-score significantly exceeded the T-scores for ISCI and FI (after Bonferroni adjustment, .05/3 = .017; both ps <. 001), which did not differ significantly from one anotl1er (p > .71). For the clinical scale T-scores, there was a main effect of clinical scale, F(4, 100) = l 9.78, p < .001. When these T-scores were compared with one another, it was revealed that the EC Scale was significantly lower (indicating relatively better functioning) than the other four scales and the WM Scale was significantly higher (indicating relatively worse functioning) than the other four scales. (All of these comparisons survived Bonferroni adjustment, .05/ 10 = .005; all ps < .005.)
These results suggested a specific pattern of performance on the BRIEF-P for individuals with Down syndrome. In particular, a significant relative weakness was noted on the EMI, with the highest T-score (denoting greater difficulty) on the WM Scale. In addition, the EC score was significantly lower than the other index scores, suggesting that this was the domain that was least impaired in this sample.
Discussion
In this study, we describe EF profiles of a population-based sample of young children (CAs = 4-10 years; MAs= 2-4 years) with Down syndrome (who were free of a comorbid autism spectrum disorder diagnosis) on a caregiver report measure created for preschoolers, the BRIEF-P. We compared performance of this group to published norms for typically developing children of a similar MA to document the nature of everyday EF skills in young children with Down syndrome relative to their general cognitive functioning.
Consistent with prior studies using laboratory tests (see Baddeley & Jarrold, 2007, for a review), significant working memory deficits were noted, with the group receiving the highest T-score (indicating worse performance) on the WM Scale relative to published norms for typically developing children of a similar MA. Furthermore, an elevated T-score that significantly exceeded the population mean was observed for the PO Scale, consistent with the limited evidence for planning weaknesses documented in laboratory studies in the literature (Fidler et al., 2005; Kasari & Freeman, 2001). Both of these clinical scales are a part of the EMI, which was the only index that was elevated relative to norms. In contrast, mean T -scores for the ISCI and FI were not significantly higher than the normative means reported for the BRIEF-P, suggesting that, at this young age (M = ~6 years), difficulties in these EF domains are not in excess of the overall cognitive impairment present in this group. Furthermore, scores were the lowest for the EC Scale, indicating that this domain was least impaired in the current sample.
It is important to emphasize that the norm-referenced T-scores reported here were developed for typically developing children whose chronological ages were similar to the MAs of the participants with Down syndrome. Thus, if we used a questionnaire normed for children with similar CAs to those of our participants, such as the school-age BRIEF, we would anticipate even greater elevations in scores across all domains tested. However, we chose this instrument for two reasons. First, the items on the BRIEF-P were more developmentally appropriate for our participants. Second, we sought to identify areas of EF weakness that were in excess of overall cognitive deficits, similar to laboratory studies of EF that match participants on MA (e.g., Kopp et al., 1983). Thus, it is particularly noteworthy that we found clinically elevated rates of EF difficulties in this sample of children with Down syndrome who have far greater life experience than the typically developing children on which the BRIEF-P was normed.
Considering these results within the context of cool versus hot EFs (Metcalfe & Mischel, 1999; Zelazo & Müller, 2002), these results indicate that at this young age, EF deficits in Down syndrome are more pronounced in the cool than hot EF domain, suggesting possibly greater involvement of the dorsolateral prefrontal cortical system than the ventromedial prefrontal cortical system in Down syndrome. Unfortunately, neuroimaging studies of children with Down syndrome have not used fine-grained analyses to measure gray matter volumes or cortical thickness in these specific frontal subregions. Thus, it is difficult to determine how these behavioral findings fit with the studies of frontal lobe morphometry in Down syndrome (which have generally described lobar-level reductions in frontal volume). However, it is noteworthy that one pediatric neuroimaging study (Pinter et al., 2001) found that the amygdala, a brain structure that has been implicated in emotion regulation and hot EF processes (Zelazo & Muller, 2002), did not differ in size between participants with Down syndrome and typical children of a similar CA (after reductions in overall brain volume were controlled). This finding fits with the current study's results of lesser difficulties in the hot EF domain and studies of psychiatric comorbidities in Down syndrome that have reported lower rates of significant psychiatric and behavior disturbances in childhood relative to other groups with intellectual disability (see Dykens, 2007, for a review).
To the best of our knowledge, this is the first study to describe EF profiles across multiple domains in a sample of young children with Down syndrome (ages 10 years and under), albeit with a parent-report measure rather than with laboratory tasks. This study documents (everyday) working memory deficits in Down syndrome at a younger age than most studies using laboratory tests (see Baddeley & Jarrold, 2007), suggesting that this deficit may be present very early. This finding is particularly significant, because often traditional measures of working memory (e.g., forward and backward digit span) cannot be administered to children with MAs as young as those of the children included in this sample. Thus, caregiver report may be an appropriate method with which to assess certain cognitive abilities in young (or more cognitively impaired) children with Down syndrome, who are often difficult to test using traditional neuropsychological measures. Furthermore, the current study provides additional evidence for (everyday) planning–organization difficulties in young children with Down syndrome. Last, this study probed behaviors that map onto cool and hot EF domains, an area that has been relatively understudied in Down syndrome research. The results indicate greater impairments in cool rather than hot EF in this young sample.
Longitudinal research is needed to examine the developmental unfolding of EF differences from early childhood to adolescence and adulthood in Down syndrome. From a neoconstructivist standpoint (Karmiloff-Smith, 2009), it is possible that these early difficulties with working memory and planning can result in later learning and behavioral difficulties, including rigid behaviors and difficulties with behavior control sometimes reported for children and adults with Down syndrome (Capone, Goyal, Ares, & Lannigan, 2006). Thus, it may be that when children with Down syndrome are assessed later in development, they will present with behavioral and learning difficulties that are suggestive of both cool and hot EF deficits. However, such hot EF deficits may be a secondary consequence of earlier cool EF difficulties.
Furthermore, identifying the nature of early EF weaknesses in Down syndrome may help educators identify which skills should be targeted for early intervention. Recent research (Diamond, Barnett, Thomas, & Munro, 2007) has suggested that early behavioral approaches, such as the Tools of the Mind Program (Bodrova & Leong, 2007), have been effective in improving EF skills in young, typically developing children. This program draws on Vygotsky's theory of child development and encourages children to use “mental tools” (e.g., external aides to increase attention/memory and self-talk to promote self-regulation) to aid problem solving and classroom learning. Programs such as Tools of the Mind and others may be adapted for use with children with Down syndrome to encourage the development of early executive skills that appear to be critical for academic success.
There were some weaknesses in the current study that should be noted. Then, future directions for behavioral and neuroimaging studies are discussed. An obvious weakness of the current study is the lack of a comparison group, either a group of typically developing children (matched on MA or CA) or a group of children with another form of intellectual disability (either idiopathic or a specific form of intellectual disability). The latter comparison group would have been particularly informative, as it would have allowed us to speak to the specificity of this pattern of EF weaknesses to Down syndrome. Because we did not have such a comparison group, we cannot conclude that the pattern of EF deficits reported here is specific to Down syndrome and not generally descriptive of young children with intellectual disability. Thus, future studies should compare Down syndrome profiles on the BRIEF-P (or BRIEF) with children with another form of intellectual disability to identify the specificity of this profile to Down syndrome.
Another weakness of the study is that the BRIEF-P was not validated as a measure of EF in children with Down syndrome, nor was it developed to assess hot–cool EF domains. In addition, this study did not use laboratory measures of EF, thus reducing the comparability of these findings with those of the studies completed to date using EF tasks in the laboratory. This latter point is important to acknowledge, because some studies have indicated that the BRIEF-P may be measuring different aspects of EF than laboratory tasks (Mahone & Hoffman, 2007) and, thus, could be considered a complementary measure of EF. However, it was encouraging to find convergence between reports of impairments using laboratory assessments of working memory and the current findings, given that the greatest amount of data on the Down syndrome neuropsychological phenotype exists for the working memory or verbal short-term memory deficit. Moreover, research by Alloway and colleagues (2009) has suggested that the BRIEF (school-age) may be effective in measuring everyday difficulties associated with working memory impairments and in describing differing EF profiles among clinical groups, such as children with attention-deficit–hyperactivity disorder (ADHD) and children with working memory impairments (without ADHD). Thus, although the BRIEF and potentially the BRIEF-P have promise for describing the nature ofEF difficulties in children with different developmental disorders, more research is needed, including collecting data on both laboratory EF measures and caregiver report of EF in Down syndrome and other clinical groups.
Last, there is a great need for additional research on the Down syndrome neuroanatomical phenotype in childhood. In particular, finer grained descriptions of lobar subregions, such as possible reductions in (or sparing of) gray matter volumes and cortical thickness of the dorsolateral and ventromedial prefrontal cortex, are needed. Furthermore, studies directly examining correlations between brain morphometry and physiology (using structural and functional MRI, respectively) and various EF tasks would be particularly informative in the search for answers about biological endophenotypes associated with EF difficulties in Down syndrome. These studies could inform research seeking to develop novel biomedical therapies aimed at improving cognitive functioning and quality of life for those with Down syndrome and their families.
Acknowledgments
This investigation was supported by the National Center on Birth Defects and Developmental Disabilities, U.S. Centers for Disease Control and Prevention, through Cooperative Agreement RTOI2005–1/2–416 with the Association of University Centers on Disabilities; University Center of Excellence in Developmental Disabilities Education, Research, and Service Grant 90DD0632 from the Administration on Developmental Disabilities, Administration for Children and Families; and Leadership Education in Neurodevelopmental Disabilities Grant 5-T73-MC11044–02-00 from the Maternal and Child Health Bureau, Health Resources and Services Administration. We are grateful to the children and families who contributed to this research and to our colleagues, Erin Flanigan, Amy Philofsky, and Kay Ridge, for their contributions to participant recruitment, testing, and data management.
Contributor Information
Nancy Raitano Lee, University of Colorado School of Medicine.
Deborah J. Fidler, Colorado State University
Audrey Blakeley-Smith, University of Colorado School of Medicine.
Lisa Daunhauer, Colorado State University.
Cordelia Robinson, University of Colorado School of Medicine.
Susan L. Hepburn, University of Colorado Health Science Center
References
- Alloway TP, Gathercole SE, Holmes J, Place M, Elliott JG, Hilton K. The diagnostic utility of behavioral checklists in identifying children with ADHD and children with working memory deficits. Child Psychiatry and Human Development. 2009;40:353–366. doi: 10.1007/s10578-009-0131-3. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8:231–240. doi: 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C. Working memory and Down syndrome. Journal of Intellectual Disability Research. 2007;51:925–931. doi: 10.1111/j.1365-2788.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrova E, Leong DJ. Tools of the mind: The Vygotskian approach to ear[y childhood education. Merrill/Prentice-Hall; New York: 2007. [Google Scholar]
- Canfield MA, Henein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76:747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- Capone G, Goyal P, Ares W, Lannigan E. Neurobehavioral disorders in children, adolescents, and young adults with Down syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2006;142C:158–172. doi: 10.1002/ajmg.c.30097. [DOI] [PubMed] [Google Scholar]
- Carr J. Stability and change in cognitive ability over the life span: A comparison of populations with and without Down's syndrome. Journal of Intellectual Disability Research. 2005;49:915–928. doi: 10.1111/j.1365-2788.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi C, Hepburn S, Davis JM, Fidler DJ, Hartway S, Lee NR, Miller L, Ruttenber M, Robinson C. Screening for autism spectrum disorders in children with Down syndrome: Population prevalence and screening test characteristics. Journal of Developmental and Behavioral Pediatrics. 2010;31:181–191. doi: 10.1097/DBP.0b013e3181d5aa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Mental Retardation and Developmental Disability Research Reviews. 2007;13:272–278. doi: 10.1002/mrdd.20159. [DOI] [PubMed] [Google Scholar]
- Edgin J. A neuropsychological model for the development of the cognitive profiles in mental retardation syndromes: Evidence from Down syndrome and Williams syndrome. University of Denver; Denver, CO.: 2003. Unpublished doctoral dissertation. [Google Scholar]
- Edgin JO, Pennington BF, Mervis CB. Neuropsychological components of intellectual disability: The contributions of immediate, working, and associative memory. Journal of Intellectual Disability Research. 2010;54:406–417. doi: 10.1111/j.1365-2788.2010.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. The Differential Ability Scales. The Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Fidler DJ, Hepburn SL, Mankin G, Rogers SJ. Praxis skills in young children with Down syndrome, other developmental disabilities, and typically developing children. American Journal of Occupational Therapy. 2005;59:129–138. doi: 10.5014/ajot.59.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler DJ, Nadel L. Education and children with Down syndrome: Neuroscience, development, and intervention. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:262–271. doi: 10.1002/mrdd.20166. [DOI] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology. 2002;8:241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, Isquith PK. Behavior rating inventory of executive function-Preschool version. Psychological Assessment Resources; Lutz, FL: 2003. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: Revised and expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Hodapp RM, Zigler E. Applying the developmental perspective to individuals with Down syndrome. In: Cicchetti D, Beeghly M, editors. Children with Down syndrome. Cambridge University Press; New York: 1990. pp. 1–28. [Google Scholar]
- Jarrold C, Baddeley AD. Short-term memory in Down syndrome: Applying the working memory model. Down Syndrome Research and Practice. 2001;7:17–23. doi: 10.3104/reviews.110. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Preaching to the converted? From constructivism to neuroconstructivism. Child Development Perspectives. 2009;3:99–102. [Google Scholar]
- Kasari C, Freeman SF. Task-related social behavior in children with Down syndrome. American Journal on Mental Retardation. 2001;106:253–264. doi: 10.1352/0895-8017(2001)106<0253:TRSBIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Graham GE, Berry-Kravis E, Drouin A, Milgram NW. A comparative neuropsycho-logical test battery differentiates cognitive signatures of Fragile X and Down syndrome. Journal of Intellectual Disabi1ity Research. 2009;53:125–142. doi: 10.1111/j.1365-2788.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Kopp CB, Krakow JB, Johnson KL. Strategy production by young Down syndrome children. American Journal of Mental Deficiency. 1983;88:164–169. [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Vianello R. Verbal and visuospatial working memory deficits in children with Down syndrome. American Journal on Mental Retardation. 2004;109:456–466. doi: 10.1352/0895-8017(2004)109<456:VAVWMD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Dal Pont E, Alberti A, Vianello R. Executive function in adolescents with Down syndrome. Journal of Intellectual Disability Research. 2010;54:308–319. doi: 10.1111/j.1365-2788.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Vianello R. Working memory and cognitive skills in individuals with Down syndrome. Child Neuropsychology. 2009;15:1–20. doi: 10.1080/09297040902740652. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DN, Loring DW. Neuropsychological assessment. 4th ed. Oxford University Press; New York: 2004. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule-WPS edition. Western Psychological Services; Los Angeles, CA: 1994. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Hoffman J. Behavior ratings of executive function among pre-schoolers with ADHD. Clinical Neuropsychologist. 2007;21:569–586. doi: 10.1080/13854040600762724. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Zabel TA, Levey E, Verda M, Kinsman S. Parent and self-report ratings of executive function in adolescents with myelomeningocele and hydro-cephalus. Child Neuropsychology. 2002;8:258–270. doi: 10.1076/chin.8.4.258.13510. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Services; Circle Pines, MN: 1995. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. American Journal on Mental Retardation. 2008;113:439–-452. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L. Down's syndrome: A genetic disorder in biobehavioral perspective. Genes Brain and Behavior. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: Evidence for hippocampal dysfunction. Child Development. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psycho-pathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: A high-resolution MRI study. Neurology. 2001;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Weiskrantz L. The prefrontal cortex: Executive and cognitive functions. Oxford University Press; Oxford, United Kingdom: 1998. [Google Scholar]
- Rowe J, Lavender A, Turk V. Cognitive executive function in Down's syndrome. British Journal of Clinical Psychology. 2006;45:5–17. doi: 10.1348/014466505X29594. [DOI] [PubMed] [Google Scholar]
- Vicari S, Carlesimo A, Caltagirone C. Short-term memory in persons with intellectual disabilities and Down's syndrome. Journal of Intellectual Disability Research. 1995;39:532–537. doi: 10.1111/j.1365-2788.1995.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Vriezen ER, Pigott SE. The relationship between parental report on the BRIEF and performance-based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuro-Psychology. 2002;8:296–303. doi: 10.1076/chin.8.4.296.13505. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Burack JA, Benedetto E, Frye D. Theory of mind and rule use in individuals with Down's syndrome: A test of the uniqueness and specificity claims. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:479–484. doi: 10.1111/j.1469-7610.1996.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Blackwell; Oxford, United Kingdom: 2002. pp. 445–469. [Google Scholar]
- Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: Neurobiology and risk. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]