Abstract
Previous lesion studies have shown compromised complex object discrimination in rats, monkeys and human patients with damage to the perirhinal cortical region (PRC) of the medial temporal lobe. These findings support the notion that the PRC is involved in object discrimination when pairs of objects have a high degree of overlapping features but not when object discrimination can be resolved on the basis of a single feature (e.g., size or color). Recent studies have demonstrated age-related functional changes to the PRC in animals (rats and monkeys) resulting in impaired complex object discrimination and object recognition. To date, no studies have compared younger and older humans using paradigms previously shown to engage the PRC. To investigate the influence of age on complex object discrimination in humans, the present study used an object matching paradigm for blob-like objects that have previously been shown to recruit the PRC. Difficulty was manipulated by varying the number of overlapping features between objects. Functional MRI data was acquired to determine the involvement of the PRC in the two groups during complex object discrimination. Results indicated that while young and older adults performed similarly on the easy version of the task, most older adults were impaired relative to young participants when the number of overlapping features increased. fMRI results suggest that older adults do not engage bilateral anterior PRC to the same extent as young adults. Specifically, complex object matching performance in older adults was predicted by the degree to which they engage left anterior PRC. These results provide evidence for human age-related changes in PRC function that impact complex object discrimination.
Introduction
There is considerable evidence suggesting that the perirhinal cortex (PRC) is necessary for disambiguating complex objects that share conjunctions of features. A common finding across studies in rats (Bartko et al. 2007), monkeys (Buckley and Gaffan, 1997; Bussey et al. 2002, 2003), and humans with perirhinal lesions (Barense et al. 2005, Barense et al. 2007) is that damage to the PRC, Brodmann areas 35 and 36, results in impaired ability to discriminate complex objects with many overlapping features (e.g., faces or complex novel objects) but not simple visual stimuli that differ on a single feature (e.g., shapes that differ in size or color). For example, Barense et al. (2007) tested human patients with lesions that were either confined to the hippocampus proper or extended beyond the hippocampus to include the PRC. Participants were asked to indicate which object was different from all other objects in an array. When the objects in the array differed on only one feature (size or color), hippocampal and PRC lesioned patients performed similarly. However, when the objects were created from combinations of four features which were then varied across objects, patients with PRC lesions were impaired, while patients with lesions confined to the hippocampus performed equivalently to appropriately matched controls (see also Lee and Rudebeck, 2010; Barense et al., 2005, 2011b; Taylor et al., 2007; Lee et al., 2005; Barense et al., 2010b).
Functional neuroimaging studies with healthy young participants provide additional support for these findings (O’Neil et al., 2009; Lee et al., 2008; Devlin and Price, 2007; Barense et al., 2010a). For example, Devlin and Price (2007) used a perceptual odd-one-out discrimination task with two levels of difficulty that was originally developed for monkeys (Buckley et al., 2001). In the easy condition, participants chose the nonmatching item out of an array of three other visually identical objects. In the difficult condition, the three matching objects were shown from different viewpoints. This latter task can only be solved by integrating multiple visual features into a single object representation that can be used to identify the object from various angles. They also administered easy and difficult matching conditions, in which participants selected the nonmatching shape or color. Perirhinal activation was observed when the objects were shown from different viewpoints, but not for the simple object matching condition, or for either the easy or hard feature matching conditions. Importantly, Barense, Henson, and Graham (2011a) showed that increased PRC activation during object discrimination occurs even when the objects are not well remembered later on, strengthening the view that PRC activation reflects increased perceptual processing rather than incidental encoding into long-term memory. This is consistent with work showing that lesions to the PRC result in object discrimination impairments, even when the task does not demand extensive declarative memory (i.e., a zero delay between presentation and test; Bartko et al., 2007 or when the perceptual task has no working memory requirement (Barense et al., 2011b; Lee and Rudebeck, 2010). Taken together, these studies establish a similar perceptual specialization for the PRC across species that suggests that this region is critical for the integration of multiple visual features into an abstract, view-invariant, representation (Murray and Bussey, 1999; Bussey et al., 2002; Bussey et al., 2005).
Recently, Burke et al. (2010) demonstrated that aged rats have difficulty identifying novel objects compared to younger rats in a spontaneous object recognition task. While the age groups did not differ behaviorally when the objects were distinctive, old rats were impaired on recognition performance relative to young animals when the objects to be distinguished shared multiple common features, even at very short delays (see also Pitsikas et al., 2005; Pieta Dias et al., 2007; Pitsikas and Sakellaridis, 2007: Hopkins et al., 2011). Burke et al. (2010) further demonstrate that the age-related object discrimination impairment is not due to forgetting over the delay period, but rather a failure to represent the objects at a sufficient level of complexity. This age-related deficit in object discrimination, although not as severe, is reminiscent of the behavior of young animals who have sustained damage to the perirhinal cortex (McTighe et al., 2010). In a second study, young and aged monkeys learned which object from a pair of objects provided a food reward (Burke et al., 2011). Objects were created from Lego pieces so that the specific amount of feature overlap between objects could be quantified. When the objects had few overlapping features, young and old monkeys performed similarly on the reward learning task. However, older monkeys took significantly more trials to learn the task when feature overlap was increased. Again, this object discrimination deficit is similar to those observed after lesions to the PRC in young animals (Bussey et al., 2002; Bartko et al., 2007). Taken together, these studies suggest that aging may result in a loss of functional integrity of the PRC, resulting in a decreased ability to disambiguate between objects with multiple similar features.
Human aging and object perception
It has long been recognized that age-related changes in sensory and perceptual functions negatively impact age-related cognitive abilities including memory, decision making, and processing speed (Baltes and Lindenberger, 1997; Ghisletta and Lindenberger, 2005; Lindenberger and Ghisletta, 2009). As sensory and perceptual mechanisms become less efficient and more prone to error, perceptual task demands may require additional executive control mechanisms such as sustained attention and inhibitory control, mechanisms which are themselves negatively impacted by advancing age (Craik, 1983; Craik and Rose, 2011; Park and Reuter-Lorenz, 2009).
Although alterations in both visual and auditory perceptual processing have been shown to account for a significant amount of age-related variance on cognitive tests (Baltes and Lindenberger, 1997; Lindenberger and Baltes, 1997), the neuroanatomical bases for these perceptual changes remain relatively unknown. One theory is that age-related changes in dopaminergic neuromodulation result in less distinct neuronal signaling (Li and Lindenberger, 1999; see also Braver et al., 2001; Nieuwenhuis et al., 2002), resulting in less differentiated cortical representations (Andrews-Hanna et al., 2007; Park et al., 2004; Park and Reuter-Lorenz, 2009). The reduced uniqueness of stimulus representations could play an important role in age-related memory impairment for visually presented stimuli.
Additionally, as suggested by the recent work of Burke et al. (2010) described above, some of the difficulty that older adults have in producing distinctive visual representations may arise because of age-related functional changes to the PRC. To date, no studies have compared younger and older adults on complex object discrimination tasks using similar paradigms to those previously shown to engage the PRC in both animals and humans (Buckley et al., 2001; Bussey et al., 2002; Devlin and Price, 2007; Barense et al., 2010b; 2011a; 2012). The present study explored this hypothesis by comparing the performance of young and older adults on an object matching task with two levels of difficulty – one that required the consideration of multiple overlapping features, and another than could be judged based on a single feature – while undergoing functional magnetic resonance imaging. This task has previously been shown with fMRI in young adults to recruit the PRC and to be impaired by lesions that include the PRC (Barense et al., 2012; see also Newsome et al., this issue). Based on the previous literature, we expected that older adults would be impaired in object discrimination when the objects share multiple common features, but not when they are distinct from one another. Further, we expected that older adults would show decreased activation in the PRC while engaging in complex object discrimination compared to young adults (see Burke et al., this issue), and that the degree to which an individual older adult engages the PRC would be directly related to their ability to discriminate between highly ambiguous objects.
Methods
Participants
Young adults (n=11, ages 18–25; mean age 19.7, sd 2.14) were recruited from undergraduate psychology courses at the University of Arizona and were given course credit for participation in the study. Older adults (n=25, ages 65–79; mean age 70.4, SD 4.06) were recruited from an existing database of participants in our laboratory. The older adults in the study had significantly more years of education than young participants (16.3 years, SD 2.4 versus 13.5 years, SD 2.2, respectively, t(34)=3.36, p<.01), reflecting the fact that more than half of the older participants had completed a college degree. Participants were screened to rule out current depression, a history of psychiatric disorder, head injury, other illnesses that may affect cognitive function, and contraindications to MRI. Older adults with a diagnosis of hypertension or who were taking anti-hypertensive medications were excluded from the study due to the difficulty in interpreting the hemodynamic response in these individuals. Older participants had extensive neuropsychological data available including assessment of intellectual, memory, executive, and processing speed functions. All neuropsychological scores fell within the normal range for their age (i.e., not lower than −1.5 SD from the mean). Vision problems were assessed using a questionnaire that screened for near sightedness, far sightedness, cataracts, color blindness, glaucoma, prior eye surgeries, and other significant eye conditions that might interfere with vision. Participants with significant visual impairment were excluded from the study and those who wore glasses were asked to bring a copy of their prescription to the imaging session. One older adult was excluded because of partial blindness to one eye. Appropriate corrective lenses were placed on the fMRI presentation goggles to correct for vision. All participants viewed examples of the stimuli through the goggles to ensure that their vision was adequate.
Materials and task
Participants decided if two simultaneously presented stimuli were identical (a match) or different (a nonmatch). Novel objects for the study were developed by Barense and colleagues (2012; see also Newsome et al., this issue). The objects were created with two curved blob-like shapes, one inside the other, separated by a pattern (see Figure 1). Thus, objects could differ from one another on the basis of their three features: the outer shape, the inner shape, and the pattern. Objects were paired to create two levels of discrimination difficulty. In the Easy Objects (nonmatch) condition, pairs of objects differed in all three features, so that any single feature could be used to discriminate the objects. For Difficult Objects (nonmatch), pairs overlapped in two of the three features, so that only one feature could be used to discriminate objects pairs (which of the three features differed was fully counterbalanced across trials). For example, in Figure 1 the two objects in the Difficult Object discrimination task have an identical outer shape and fill pattern, but they have a different inner shape. Matched Objects pairs were identical to one another on all three features. To make the task more demanding and to discourage a simple feature-matching strategy, objects were rotated with respect to each other (see Figure 1).
Figure 1.
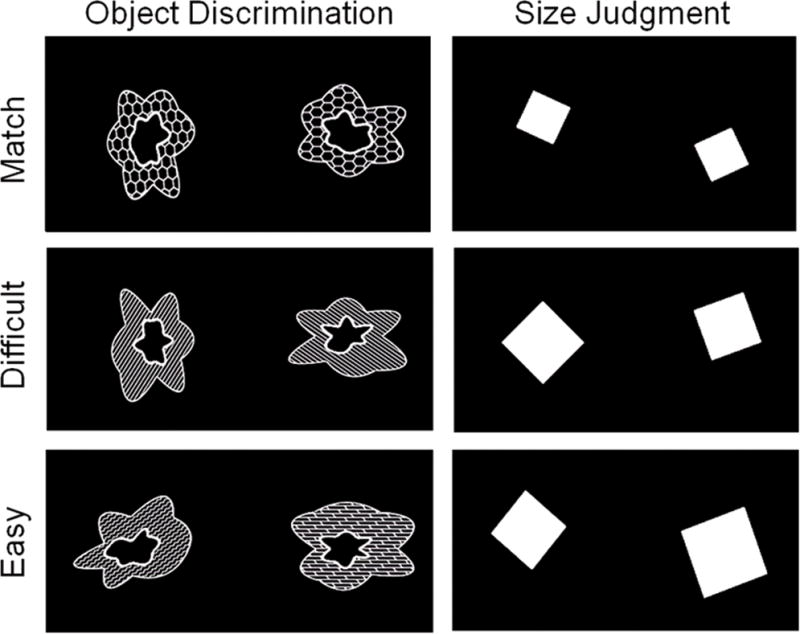
Stimuli for the object discrimination and size judgment tasks. Participants viewed pairs of squares or blob-like objects (comprised of three features – outer shape, inner shape, and fill pattern) and indicated whether or not the simultaneously presented stimuli were a match or a nonmatch. In the match conditions, both objects and squares were identical to one another. There were two nonmatch conditions for each stimulus type: Difficult and Easy. The Difficult Object pairs differed by one of the three features (in this figure, the inner shape differs). Easy Object pairs differed on all three features. For the Difficult and Easy Size judgments, squares differed from one another by 2–4mm and 5–8mm, respectively.
As a perceptual control task, pairs of squares of varying sizes were created with two levels of discrimination difficulty. Difficult Size (nonmatch) pairs differed by a minimum of 2mm to a maximum of 4 mm. Easy Size (nonmatch) pairs differed by a minimum of 5mm to a maximum of 8mm. Matched Size pairs were identical to one another. The squares were rotated with respective to each other to ensure sufficient difficulty on the task, and to match the rotation component included in the object discrimination task. Thus, a total of six conditions were included in the experiment: Difficult Objects (nonmatched), Easy Objects (nonmatched), Matched Objects, Difficult Size (nonmatched), Easy Size (nonmatched), and Matched Size. The difficult condition for both objects and squares included 60 nonmatched with an additional 30 matched pairs. The easy condition for both objects and squares included 40 nonmatched with 20 matched pairs. Thus, the total ratio of nonmatched to matched pairs in the experiment was 2:1.
Pairs of objects were presented in the scanner, one at a time, through fMRI compatible goggles (Resonance Technology, Inc., California) using E-Prime presentation software (Psychology Software Tools, Inc. Pennsylvania). Participants were instructed to judge whether or not the pair of objects (blobs or squares) were identical to one another. Participants were informed that the objects would be rotated relative to one another, and that the degree of rotation in each trial was random. They were instructed to base their decision on the differences between object features. Each pair was presented for seven seconds allowing sufficient time for older participants to respond. Trials were presented in mini-blocks of three pairs of blobs or squares – 2 nonmatching (either both difficult or both easy) and 1 matching. The mini-blocks were then presented in pseudo-random order in five separate scans, each lasting eight minutes. Each scan included a relatively equal number of the six conditions described earlier.
Participant responses were gathered using the E-Prime response box. The response box was placed in the dominant hand of the participants, and they were instructed to press the left button to indicate “Matching pair” and the right button to indicate “Nonmatching pair.”
Prior to entering the scanner, participants were given practice on the task to familiarize them with the stimuli and ensure that they understood the task. First, participants were given 21 self-paced practice trials that included examples of all six conditions in random order, and were provided with accuracy feedback that was displayed at the top of the computer screen. Once the experimenter was confident that the participant understood the task, they were given a second set of practice trials without feedback lasting 8 minutes with each stimulus presented for 7 seconds, identical to the task as it would be presented subsequently in the scanner.
fMRI protocol and analyses
Images were collected in a one hour session on a GE 3.0T Signa Excite system with an 8 channel phased array coil. Functional MRI scans were acquired using a single-shot spiral pulse sequence to obtain inward and outward spiral data sets (Glover and Law, 2001) that were combined during post-processing using a weighted average. Sections were aligned axially along the AC-PC plane, covering whole brain, TR=2400, TE=30msecs, flip angle=90, matrix 64×64, 3.4mm sections, no skip.
Functional images were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The first three trials of each scan were excluded in order to allow for the MR signal to reach equilibrium. Images were corrected for differences in slice acquisition timing using the middle section in each volume as a reference image, then motion corrected by aligning all images to the first image of the scanning session. Each participant’s functional scans were normalized to the MNI standard EPI template, interpolated to 2mm isotropic voxels, and smoothed using a Gaussian 6mm FWHM kernel. Resulting images were visually inspected for artifacts and adequacy of realignment.
Statistical analyses were first conducted at the single subject level. For each participant and each condition, trial-related activity was modeled by convolving a vector of trial onsets with a regressor created by convolving the canonical hemodynamic response function with a boxcar function equal in duration to the stimulus presentation. Only correct trials for each condition were included in the analyses. Incorrect trials and trials for which the participant did not respond within the time limit were modeled separately as conditions of no interest. Thus, although the trials were presented in mini-blocks of three, each trial was modeled separately so that incorrect trials and trials of no interest could be excluded from the analyses. The resulting functions were entered into a General Linear Model (GLM) and high pass filtered (cut-off 1/128 sec) to remove low frequency noise. Parameter estimates for each trial type were calculated at each voxel to create a single contrast image for each participant and each condition.
For second-level analyses, t-contrasts were performed voxel-by-voxel on the parameter estimates treating participants as a random-effects factor. The resulting t-statistics were thresholded by applying a familywise error (FWE) correction for multiple comparisons, p < 0.05, using Random Field Theory. Statistical parametric maps (SPMs) were created for young and older adults separately.
Regions of increased activation within the PRC associated with the Difficult Objects nonmatched condition were identified in the young group, since our hypothesis was that older adults would not engage this region to the same degree as young adults. The PRC region of interest (ROI) was defined by the probability map created by Devlin and Price (2007; available at http://joedevlin.psychol.ucl.ac.uk/perirhinal.php) that included areas of medial temporal lobe with a 70% probability of being the PRC. Because medial temporal lobe regions generally show smaller percent signal changes than other cortical regions (Ryan et al., 2008a; 2008b; Addis et al., 2007), we applied a more liberal statistical criterion of p<.01 with a cluster extent of 10 voxels or greater, providing a cluster threshold of p<.001 (Forman et al., 1995).
Based on the peak activation within significant clusters in the PRC, spherical masks were created with a diameter of 6mm using MarsBar (http://marsbar.sourceforge.net/). The masks were then applied to the parameter estimate maps for each participant to obtain mean contrast values for all voxels within the mask for each condition of interest. The mean contrast values were then output to SPSS for further statistical analyses.
Results
Behavioral results
Accuracy means and standard deviations along with false alarm rates for the size judgment and object matching tasks are listed in Table 1. False alarm refers to the probability of incorrectly judging a matching pair to be nonmatching.
Table 1.
Accuracy (probability correct), false alarm rates (FA), and d prime scores for young and older adults on the easy and difficult versions of the object matching task and the size judgment task. False alarms refer to the probability of judging a matching pair to be “nonmatched”.
| Accuracy | D prime | |||
|---|---|---|---|---|
|
|
||||
| Young | Older | Young | Older | |
|
|
||||
| Easy Objects | .98 (.01) | .97 (.04) | 2.79 (.47) | 2.53 (.62) |
| Difficult Objects | .82 (.06) | .76 (.12) | 1.57 (.34) | 1.26 (.44) |
| Objects FA | .27 (.11) | .33 (.22) | ||
| Easy Size | .97 (.03) | .98 (.04) | 4.03 (.54) | 3.67 (.76) |
| Difficult Size | .84 (.10) | .82 (.13) | 2.79 (.53) | 2.7 (.55) |
| Size FA | .06 (.07) | .09 (.09) | ||
Size judgments
In order to compare the performance of older adults and young adults, we considered both false alarm rates and accuracy. For the size judgment task, false alarm rates were low (below 10%) for both groups and did not differ between groups (t<1). Size judgment accuracy was therefore compared directly with a mixed factor ANOVA, comparing the between-subjects factor group (young, older) and the level of difficulty (Easy Size, Difficult Size). The results indicated that the two age groups did not differ in performance on the size judgment task. While overall accuracy dropped significantly in the Difficult Size condition, F(1,34)=55.42, p<.0001, neither the main effect of group or the interaction between group and level of difficulty approached significance (F’s <1). Note that a similar ANOVA carried out on d prime scores calculated for the size judgment conditions provided an identical pattern of results.
Object matching
Although the mean difference in false alarms between the groups did not differ significantly, t(34)=1.06, ns, false alarm rates for the older adults were significantly more variable than for young adults, Levene’s F=6.16, p<.01. False alarm rates for young adults ranged from .11 to .42, while false alarm rates for older adults ranged from .02 to as high as .68. To account for the wide range of false alarm rates across individuals, analyses were carried out on discrimination scores (d prime) calculated separately for Easy Objects and Difficult Objects. The means in each condition were compared using independent t-tests assuming unequal variances. D prime scores were similar for young and older adults for the Easy Objects condition, t(24.8)=1.42, ns, but were significantly lower for older adults compared to young adults in the Difficult Objects condition, t(24.8)=2.29, p<.05, suggesting that older adults had significantly more difficulty than young adults in discriminating between matching pairs of objects and unmatched pairs with two overlapping features. Importantly, in both the Easy Objects and Difficult Objects conditions, d prime scores for older adults were significantly more variable than for young adults, Levene’s F’s > 4.83, p’s <.05. This is particularly striking in the Difficult Objects condition, as depicted in the scatter plots for young and older adults in Figure 2. Some older adults performed just as well as young adults on the Difficult Objects task, while others performed well below the lowest score obtained by young adults on this task.
Figure 2.
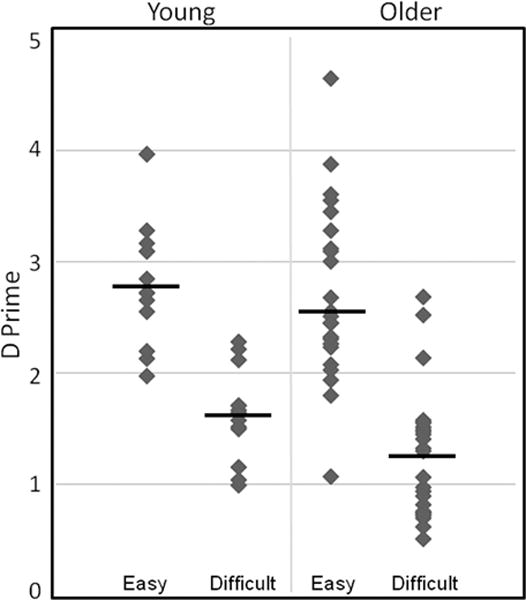
Scatter plots of d prime scores for Easy Objects and Difficult Objects. Older adults were significantly impaired relative to young participants on the difficult object discrimination condition, but performed similarly to young on the easy version of the discrimination task.
fMRI results
Whole brain patterns of activation for young and older adults
In order to assess the brain regions that were specifically engaged during the Difficult Objects task, we report the results of the contrast Difficult Objects > Difficult Size. The Difficult Size condition provided a good comparison baseline because it controlled for overall task difficulty and produced similar numbers of correct trials for both groups. Note, however, that comparing the Difficult Objects condition to other baselines, including Easy Objects, Easy Size, or a combination of all conditions (Easy Objects, Easy Size, and Difficult Size) produced whole-brain activation maps that were similar to one another. Figure 3 depicts the results for young and older adults separately, FWE corrected p<.05. Both groups engaged extensive regions of the ventral visual stream, including primary visual cortex, posterior temporal-occipital gyrus, and fusiform gyrus, as well as bilateral superior parietal lobule, posterior hippocampus, and the basal ganglia. Interestingly, consistent with other fMRI studies of aging (e.g., Park et al. 2010, Grady et al. 1999, 2006), only older adults showed extensive activations bilaterally in lateral prefrontal regions and medial prefrontal regions compared to young adults, as well as bilateral thalamus, brainstem, and insular cortex (see Figure 3 and Table 2 for region coordinates).
Figure 3.
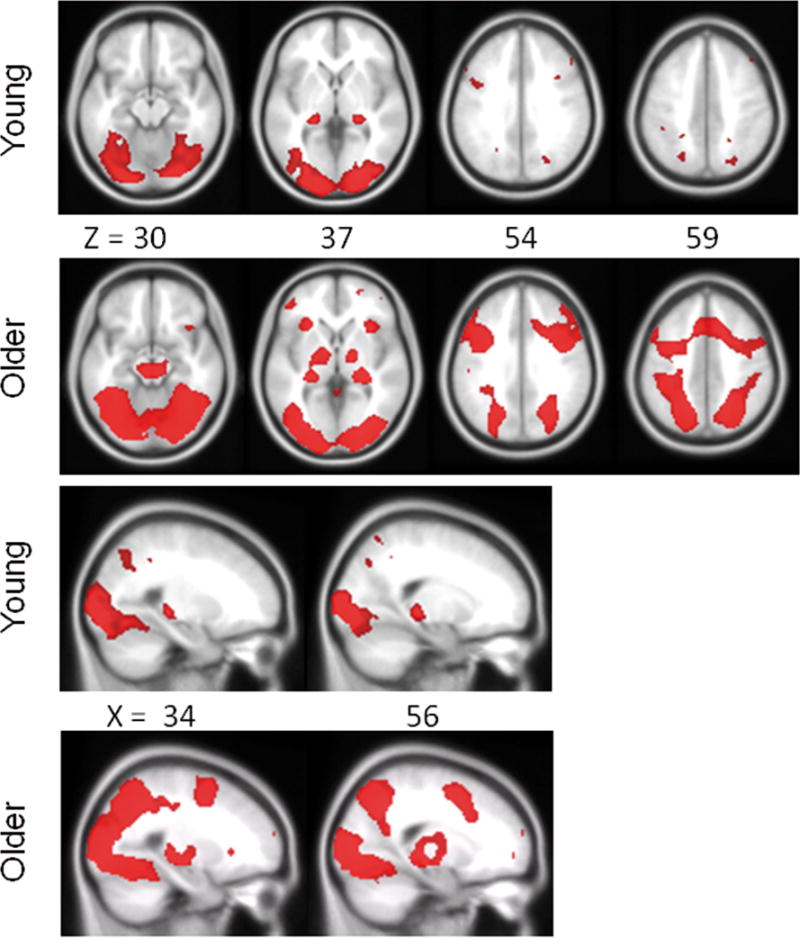
Regions of increased activation shown separately for older and young adults from the comparison Difficult Objects > Difficult Size. SPMs are thresholded at p<.05, FWE corrected for multiple comparisons. The upper two panels highlight the similar distribution of posterior ventral-visual stream regions for the two groups, as well as more extensive bilateral and medial frontal activation for older adults compared to young adults. The lower two panels show similar bilateral posterior hippocampal activation for both groups. X and Z refer to MNI coordinates for axial and saggital sections, respectively.
Table 2.
Regions of significantly increased activation for older and young adults from the comparison Difficult Objects > Difficult Size (FWE, p < 0.05) MNI coordinates (X, Y, Z) refer to the peak amplitude voxel within the cluster and the t-statistic for that voxel. K refers to the number of significant voxels within the cluster.
| OLDER ADULTS | X | Y | Z | t | k | |
|---|---|---|---|---|---|---|
| Inferior Occipital Gyrus | Right | 28 | −88 | −8 | 16.32 | 606 |
| Left | −32 | −90 | −2 | 15.92 | 739 | |
| Superior Parietal Lobule | Right | 27 | −66 | 48 | 10.07 | 1101 |
| Left | −19 | −67 | 48 | 11.28 | 1219 | |
| Inferior Temporal Gyrus | Right | 43 | −58 | −8 | 9.05 | 522 |
| Left | −44 | −58 | −8 | 100.76 | 403 | |
| Fusiform Gyrus | Right | 31 | −66 | −12 | 11.14 | 1258 |
| Left | −38 | −69 | −12 | 12.43 | 1181 | |
| Posterior Hippocampus | Right | 24 | −26 | −4 | 10.59 | 2891 |
| Left | −22 | −24 | −8 | 7.06 | 2536 | |
| Middle Occipital Gyrus | Right | 34 | −79 | 18 | 9.89 | 1297 |
| Left | −34 | −79 | 18 | 10.68 | 2845 | |
| Mid Brain | 6 | −25 | 12 | 9.33 | 981 | |
| Insula | Right | 38 | 19 | −4 | 6.82 | 237 |
| Left | −29 | 20 | 0 | 6.66 | 204 | |
| Frontal Precentral Gyrus | Right | 42 | 6 | 28 | 10.43 | 11362 |
| Left | −42 | 6 | 34 | 8.75 | 1175 | |
| Medial Prefrontal | Right | 3 | 11 | 52 | 8.03 | 505 |
| Left | −4 | 11 | 52 | 8.37 | 477 | |
| Superior Frontal Gyrus | Right | 22 | 6 | 52 | 8.21 | 1679 |
| Left | −22 | 6 | 54 | 7.74 | 1001 | |
| Inferior Frontal Gyrus | Right | 36 | 24 | 22 | 8.37 | 745 |
| Left | −38 | 24 | 24 | 8.96 | 922 | |
| Lateral Prefrontal | Right | 35 | 54 | 12 | 5.85 | 184 |
| Left | −36 | 54 | 16 | 5.76 | 197 |
| YOUNG ADULTS | X | Y | Z | t | k | |
|---|---|---|---|---|---|---|
| Inferior Occipital Gyrus | Right | 20 | −86 | −6 | 14.11 | 667 |
| Left | −25 | −88 | −10 | 13.14 | 783 | |
| Superior Parietal Lobule | Right | 22 | −72 | 40 | 6.66 | 125 |
| Left | −22 | −70 | 48 | 6.56 | 176 | |
| Middle Occipital Gyrus | Right | 26 | −90 | 6 | 7.92 | 686 |
| Left | −26 | −90 | 6 | 8.05 | 1374 | |
| Fusiform Gyrus | Right | 26 | −61 | −14 | 7.55 | 997 |
| Left | −26 | −61 | −14 | 7.70 | 898 | |
| Posterior Hippocampus | Right | 24 | −30 | −4 | 9.95 | 268 |
| Left | −20 | −30 | −2 | 10.23 | 196 | |
| Middle Frontal Gyrus | Right | 35 | 12 | 36 | 6.06 | 15 |
| Left | −48 | 10 | 36 | 6.81 | 70 |
Identifying PRC activation in young adults
Activation within the anatomically defined boundary of the PRC (Price and Devlin, 2007) was first identified within the young group only with the contrast Difficult Objects > Difficult Size. Using a less stringent statistical criterion (cluster threshold p<.001), four regions were identified within the PRC mask, bilateral posterior regions (left −32, −4, −36; right 34, −8, −38) and bilateral anterior PRC regions (left −26, 4, −18; right 26, 4, −18), the latter region situated at the boundary of the anterior PRC and the amygdala. To ensure that the activations within the PRC were robust and specific to the Difficult Objects condition, we also compared this condition with several alternative baselines including Easy Objects, all combined conditions (Difficult Size, Easy Size, and Easy Objects) and the interaction between the four non-match conditions (Difficult Objects – Easy Objects) > (Difficult Size – Easy Size). The latter interaction analysis was used in previous studies to identify regions of PRC activation specific to complex object discrimination (Barense et al., 2012). All these additional comparisons produced significant activation that overlapped with the coordinates reported above.
Based on the four peak PRC activations identified in the young group, spherical masks were created with a diameter of 6mm. Because masks were created from coordinates from the young data only, the masks provide unbiased regions of interest to assess activation within the older adults. The resulting ROI masks (see Figure 4) were used to extract the mean contrast values for the four non-match conditions from each younger and older adult.
Figure 4.
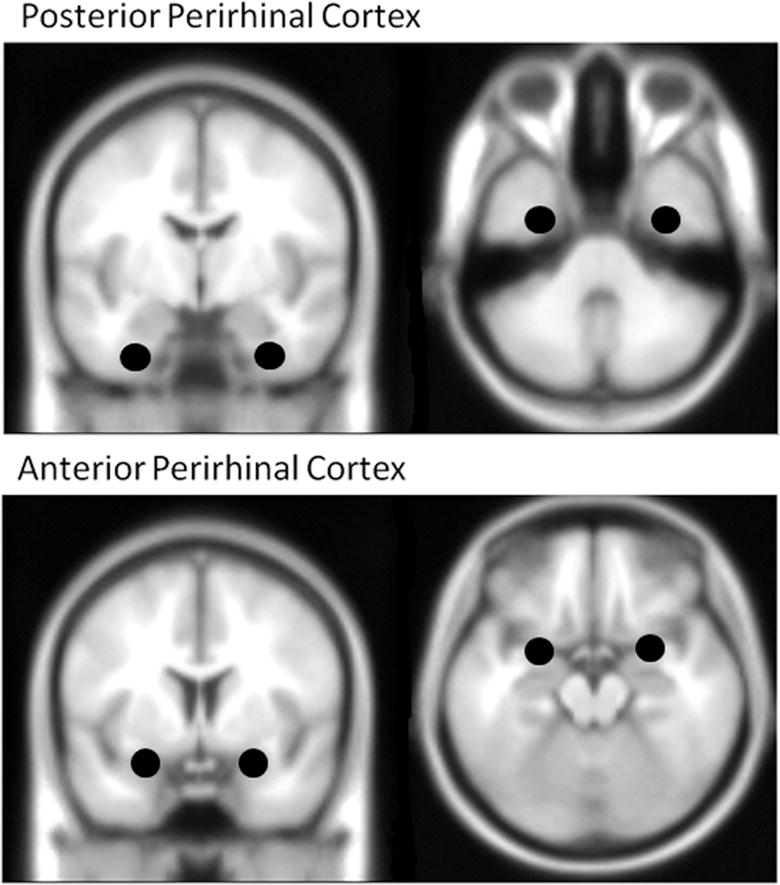
Masks for posterior and anterior PRC regions, derived from activation for the young group within the anatomical boundary of the PRC (Price and Devlin, 2007), using the contrast Difficult Objects > Difficult Size. MNI coordinates for the four regions: Posterior PRC (left −32, −4, −36; right 34, −8, −38), Anterior PRC (left −26, 4, −18; right 26, 4, −18).
Comparing PRC activation in young and older adults
This analysis examined the a priori hypothesis that young adults would engage the PRC to a greater degree than older adults. The mean contrast values obtained from the four PRC ROIs (described above) for young and older adults are depicted in Figure 5. To test the hypothesis that older adults would show decreased activation in the PRC relative to young adults, contrast values were analyzed using a mixed factor ANOVA, comparing age group (young, older) as the between-subjects factor, and region (right anterior PRC, left anterior PRC, right posterior PRC, left posterior PRC) as a repeated measure. Follow up t-tests were one-tailed due to the directional hypothesis. The ANOVA indicated a significant interaction between age group and region, F(1,34)=5.64, p<.02. Older and younger adults activated right and left posterior PRC to similar degrees (t’s<1). However, older adults showed significantly less activation in the right anterior PRC region compared to young adults, t(34)=2.68, p<.01 and a marginally lower level of activation compared to young adults in the left anterior PRC, t(34)=1.53, p<.06. In fact, the contrast values for Difficult Objects and Difficult Size within the right anterior PRC did not differ for older adults, paired t<1, suggesting that older adults simply did not recruit right anterior PRC during complex object discrimination. They did, however, engage left anterior PRC to a significant degree over baseline, paired t(24)=3.98, p<.05.
Figure 5.
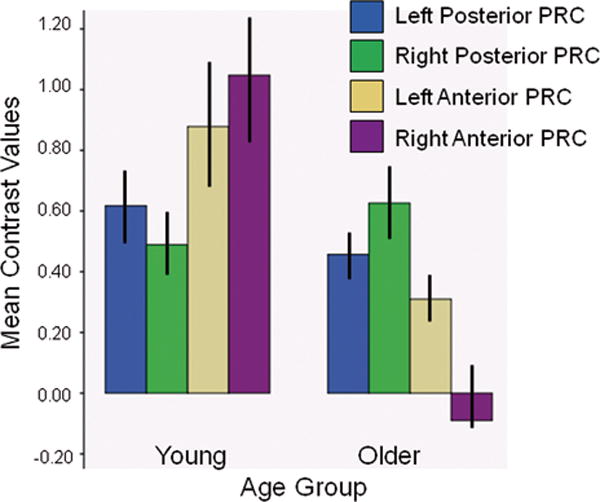
Mean (SEM) contrast values obtained from the four PRC regions of interest for young and older adults. Young and older adults activated posterior PRC regions to the same degree. Activation in anterior PRC was significantly lower for older adults compared to young adults in the right hemisphere (p<.01), and marginally lower in the left hemisphere (p=.06). The contrast values for Difficult Objects and Difficult Size within the right anterior PRC did not differ for older adults, suggesting that older adults did not recruit the right anterior PRC during complex object discrimination compared to baseline.
Predicting behavioral performance
Because activation within the two anterior PRC regions differed between age groups, we investigated whether activation within these regions would predict performance in the Difficult Objects condition. Difference scores were calculated from mean contrast values (Difficult Objects – Difficult Size) that reflected the degree to which each participant engaged the PRC region during the Difficult Objects condition above the Difficult Size baseline. The difference scores were then correlated with behavioral d prime scores from the Difficult Objects condition. Neither posterior nor anterior PRC regions predicted performance for young adults. For older adults, activation in the right anterior PRC did not correlate with d prime, (r=.09, ns), a finding not particularly surprising given that older adults showed very little response in this region relative to the baseline condition. However, the left anterior PRC showed a significant correlation with d prime scores for older adults (r=.56, p<.01; Figure 6A), suggesting that as activation in this region increased, the ability to discriminate between matched and nonmatched object pairs increased. To better understand the influence of the PRC on performance, activation scores from left anterior PRC were correlated with both accuracy and false alarm rates separately. While older adults’ accuracy did not correlate significantly (Pearson r=.10, ns; Figure 6C), false alarm rates were negatively correlated with activation in this region (Pearson r=−.54, p<.01; Figure 6B), such that increasing activation in left anterior PRC was related to decreases in false alarm rates, thereby resulting in better discrimination performance and higher d prime scores (see Figure 6).
Figure 6.
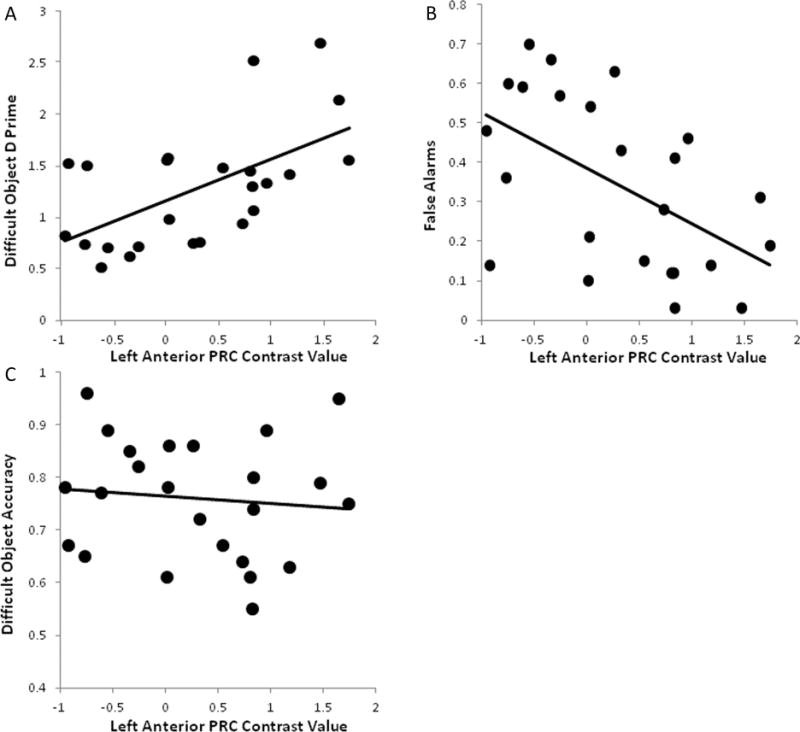
Scatter plots depicting the Pearson r correlations between mean contrast values for the left anterior PRC and d prime (A), false alarm (B), and accuracy scores (C) for older adults in the Difficult Objects condition. Individual differences in left anterior PRC activation accounted for 29% and 31% of the variance in false alarm and d prime scores, respectively. In contrast, this region did not predict individual differences in accuracy for Difficult Objects, accounting for less than 1% of the variance in accuracy.
Discussion
The present study is the first to show human age-related changes in complex object discrimination using a paradigm that has been shown previously to be sensitive to PRC damage (Barense et al., 2012). While young and older adults did equally well on the version of the object matching task when objects were distinctive, their performance dropped significantly when the matching task was made more difficult by presenting object pairs that shared overlapping features. This finding cannot be attributed to a more general perceptual impairment because young and older adults did equally well on the difficult version of the size judgment task in which pairs of objects differed on a single perceptual feature only. It was only when the objects to be discriminated shared many overlapping features that impairments in the older adults emerged. Importantly, d prime scores best captured the differences in performance between groups in the object matching task. Older adults were very good at identifying object pairs that differed on all three features (Easy Objects). However, older adults had significant difficulty discriminating between objects that shared two features (Difficult Objects) and those sharing all three features (Matching Objects), reflected in both a drop in accuracy and increasing false alarms. This finding is particularly striking given that the older adults in the study were healthy (no hypertension, diabetes, or cardiovascular disorders) and high functioning (over half of the sample had at least one college degree).
It should be noted that both older and younger groups had a tendency to judge the matched object pairs as “nonmatching”. This bias likely occurred for several reasons. First, the instructions to participants emphasized the importance of finding the nonmatching pairs. Second, in order to obtain sufficient numbers of trials in each condition while keeping the fMRI task at a reasonable length, the paradigm included twice as many nonmatched pairs (including easy and difficult conditions) as matched pairs. The ratio of 2:1 likely contributed to a bias towards judging pairs as “nonmatching”. However, the same ratio applied to the easy and difficult size judgment conditions, yet both younger and older adults showed high accuracy and low false alarms rates for these pairs.
Importantly, not all older adults were impaired on the difficult object discrimination task. The d prime scatter plots clearly demonstrate that some older adults continue to perform very well on this task, while others performed well below the range of scores obtained for young adults. The intriguing question is why some older adults are capable of maintaining performance while others are not. The fMRI data suggest one potential source of variability, indicating that the ability to engage subregions of the PRC may change with age. While both age groups engaged the posterior PRC bilaterally to a similar degree during the difficult object matching task, a more anterior region of the PRC showed group differences. Young adults engaged the anterior PRC bilaterally during complex object discrimination to a greater degree overall than older adults. In contrast, older adults showed asymmetric activation, with significant activation increase in the left, but not the right anterior PRC relative to the baseline condition. Further, the degree to which older adults engaged the left anterior PRC above baseline was related to individual differences in behavioral performance on complex objects discrimination. Activation in this region predicted more than 30% of the variance in false alarm rates and the resulting decrease in d prime scores for older adults. Taken together, the results are consistent with the growing literature from animal models and human neuropsychological studies suggesting that the PRC is involved in disambiguating complex objects that share overlapping features. The results are also consistent with recent research with both monkeys and rats suggesting that the PRC is changing functionally with age (Burke et al., 2010, 2011, 2012 this issue).
The specific location of the posterior PRC region identified in the present study is very consistent with other fMRI studies of complex object discrimination using fMRI (Barense et al., 2010a; 2011a). The anterior PRC region is not commonly reported. This region borders the most anterior portion of the PRC and the amygdala. Most studies to date have assessed PRC activation by applying a conservative mask and then averaging activation across the entire region. This procedure may result in increased power to detect activation, but it may also have resulted in the loss of information regarding activation differences within subregions of the PRC. In addition, to our knowledge the relationships between activation within the PRC (or subregions of the PRC) and individual differences in object discrimination performance have not been assessed previously. Whether functional differences exist across these PRC regions remains to be investigated. One speculation is that the posterior PRC region is utilized specifically for comparison of object features, while more anterior PRC regions are involved in evaluation and categorization processes based on feature comparisons. For example, Daselaar et al. (2002) identified a region of left anterior medial temporal lobe, which included the anterior PRC adjacent to the amygdala, that was specifically recruited when participants were engaging during a semantic classification task (living/nonliving judgments).
The present study is by no means the first to demonstrate age-related differences in complex object perception. For example, using fMRI, Park et al. (2004) showed age-related decreases in selectivity within ventral visual cortical regions to four categories of visually presented stimuli. In contrast to older adults, young adults showed more distinct activation patterns that differentiated each class of stimuli. Park and colleagues (2004) suggested that the reduced uniqueness of cortical stimulus representations could play an important role in age-related changes in perceptual speed and perceptual discrimination. Chee et al. (2006) also demonstrated age-related difference in object perception using an fMRI adaptation paradigm. While being scanned, older and younger adults were shown a series of novel objects, scenes, and objects embedded in scenes. Older adults, relative to young, showed a lack of adaptation to repeated objects presented in the context of changing backgrounds but not to repeated objects presented in isolation. Chee et al. (2006) interpret this result to suggest that older adults are deficient in concurrent visual processing of objects and scene backgrounds. Decreased adaptation has also been shown in older adults relative to young adults when observing faces (Goh et al., 2010). Chee et al. (2006) suggest that at least some of the age-related impairment observed during visual associative encoding (Sperling et al., 2003) might be explained by the tradeoff between attention to contextual information at the expense of object processing. Incomplete or inefficient processing of objects may result in insufficient information being available to bind objects and context into coherent representations. This could result in poor subsequent memory for objects and their contexts.
Such perceptual inefficiency for processing objects may arise in older adults, at least in part, because of functional changes to the PRC, as suggested by the results of the current study. While few human studies exist that utilize paradigms specifically designed to engage the PRC, supporting evidence is provided by a time-honored neuropsychological test that is virtually identical to the novel face matching tests used in many investigations of PRC function (Barense et al., 2010a, 2011b; Lee et al., 2005, 2008; Buckley et al., 2001). The Benton Test of Facial Recognition is more appropriately described as a face discrimination task requiring the identification of one or more unfamiliar faces in a match-to-sample multiple choice display (Hamsher et al., 1979). On each trial, the target picture is a black and white front-view photograph of an unfamiliar face. The match-to-sample choices include the identical front-view photograph, photos of the same face taken from off-angle viewpoints, and front-view photos taken under different lighting conditions. The matching conditions from differing viewpoints and different lighting conditions are very similar to the task used to assess the impact of lesions to the PRC in humans. Benton and colleagues (Eslinger and Benton, 1983) showed that matching performance in older adults (above age 65) declined by an average of 0.5 standard deviations per decade, with a regression coefficient between age and face matching performance of −0.51, p<.0001.
The present study raises several interesting questions. First, it will be important to understand how age-related differences on complex discrimination tasks relate to memory for visual displays, particularly those involving objects. Some older adults who cannot easily discriminate between similar objects may have difficulty on object recognition tasks, particularly when object lures and targets are similar. Alternatively, as several researchers have argued (for example, Park and Reuter-Lorenz, 2009), the additional processing resources required to deal with complex visual representations may impair the ability of older adults to encode the stimuli in the first place, thereby conferring a more general deficit in memory performance for visual stimuli.
Second, it is also interesting to note that older adults in the present study showed extensive activation of medial and lateral frontal regions during the difficult object discrimination condition that was not observed in young adults. Such age-related increases in frontal activation have been interpreted as compensation for increasing task difficulty, in this case, the perceptual discrimination difficulty imparted by increasing object feature overlap. Compensatory responses in older adults have been observed in an array of cognitive tasks that include working memory (Reuter-Lorenz, 2002), visual long term memory (Gutchess et al., 2005; Grady et al., 2006), visual attention (Madden et al., 1999; Cabeza et al., 2004), visual perception (Park et al., 2010; Davis et al., 2008), and word stem completion (Persson et al., 2006). A review by Park and Gutchess (2005) noted consistent age-related decreases in medial temporal lobe activation in the same fMRI studies where increased bilateral prefrontal activations were observed (Cabeza et al., 2004; Gutchess et al., 2005), although these studies focused primarily on hippocampus proper, rather than surrounding medial temporal lobe structures. A second suggestion is that older adults recruit more extensive frontal regions to compensate for sensory processing deficits (Grady, 1996; Davis et al., 2008), but the particular processing deficits remain unspecified. These two suggestions may not be totally independent of one another, and the paradigm utilized here may be useful in disentangling the relationship between perceptual inefficiency and frontal compensatory responses. In the current study, it may well be the case that older adults did not effectively allocate frontal resources to solve the difficult object discriminations, and inter-regional communication deficits between perirhinal cortex frontal regions contributed to the observed deficits. In summary, the present study adds to the growing literature demonstrating that perirhinal-dependent perceptual tasks are impaired with age. Recent experiments have revealed that both rats and monkeys have age-associated impairments in the ability to discriminate between similar complex objects (reviewed in Burke, Ryan, and Barnes, In press). The current study suggests that these findings apply to human aging as well. Older adults have difficulty in object discrimination when objects have a high level of feature overlap, suggesting that assessing perirhinal-cortical functions in elderly humans and their influence on memory functions is an important research avenue to pursue. Additionally, because of the close correspondence between paradigms that have been used to assess perirhinal function in animals and humans, this area of research lends itself particularly well to cross-species studies of age-related changes in object discrimination and the impact that such perceptual impairments may have on memory function.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Lee ACH, Graham KS. Medial temporal lobe activity during complex visual discrimination of faces, objects and scenes: The effect of viewpoint. Hippocampus. 2010a;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Rogers TT, Bussye TJ, Saksida LM, Graham KS. Influence of conceptual knowledge on visual object discrimination: Insights from semantic dementia and MTL amnesia. Cereb Cortex. 2010b;20:2568–2582. doi: 10.1093/cercor/bhq004. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Graham KS. Perception and conception: Temporal lobe activity during complex discriminations of familiar and novel faces and objects. J Cogn Neurosci. 2011a;23:3052–3067. doi: 10.1162/jocn_a_00010. [DOI] [PubMed] [Google Scholar]
- Barense MD, Ngo JK, Hung LH, Peterson MA. Interactions of Memory and Perception in Amnesia: The Figure-Ground Perspective. Cereb Cortex. 2011b doi: 10.1093/cercor/bhr347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Groen IIA, Lee ACH, Yeung L-K, Brady SM, Gregori M, Kapur N, Bussey TJ, Saksida LM, Henson RNA. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007;27:2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111:467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. J Neurosci. 2001;21:9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-Associated Deficits in Pattern Separation Functions of the Perirhinal Cortex: A Cross-species Consensus. Behav Neurosci. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Ryan L, Barnes CA. Characterizing cognitive aging of recognition memory and related processes in animal models and in humans. Front Aging Neurosci. doi: 10.3389/fnagi.2012.00015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Hartzell AL, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Representation of 3-dimensional objects by the rat perirhinal cortex. Hippocampus. doi: 10.1002/hipo.22060. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q J Exp Psychol B. 2005;58:269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. J Cogn Neurosci. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philos Trans Roy Soc Lond, Ser B. 1983;302:341–359. [Google Scholar]
- Craik FIM, Rose NS. Memory encoding and aging: A neurocognitive perspective. Neurosci Biobehav Rev. 2011 doi: 10.1016/i.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Lazeron RH, Jonker C. Medial temporal lobe activity during semantic classification using a flexible fMRI design. Behav Brain Res. 2002;136:399–404. doi: 10.1016/s0166-4328(02)00187-0. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Price CJ. Perirhinal contributions to human visual perception. Current Biology. 2007;17:1484–1488. doi: 10.1016/j.cub.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Benton AL. Visuoperceptual performances in aging and dementia: Clinical and theoretical implications. J Clin Neuropsychol. 1983;5:213–220. doi: 10.1080/01688638308401170. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Exploring structural dynamics within and between sensory and intellectual functioning in old and very old age: Longitudinal evidence from the Berlin Aging Study. Intelligence. 2005;33:555–597. [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR, reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. NeuroImage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Age-related changes in cortical blood flow activation during perception and memory. Ann N Y Acad Sci. 1996;777:14–21. doi: 10.1111/j.1749-6632.1996.tb34396.x. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hamsher K, Levin HS, Benton AL. Facial recognition in patients with focal brain lesions. Arch Neurol. 1979;36:837–839. doi: 10.1001/archneur.1979.00500490051008. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, et al. Perceptual deficits in amnesia: Challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 2005;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 2008;18:683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Lee AC, Rudebeck SR. Investigating the interaction between spatial perception and working memory in the human medial temporal lobe. J Cogn Neurosci. 2010;22:2823–2835. doi: 10.1162/jocn.2009.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson L-G, Markowitsch H, editors. Cognitive neuroscience of memory. Seattle: Hogrefe & Huber; 1999. pp. 103–146. [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age:cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol Aging. 2009;1:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Allen PA. Adult age differences in visual search accuracy: attentional guidance and target detectability. Psychol Aging. 1999;14:683–694. doi: 10.1037//0882-7974.14.4.683. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Paradoxical false memory for objects after brain damage. Science. 2010;330:1408–1410. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Newsome RN, Duarte A, Barense MD. Reducing perceptual interference improves visual discrimination in mild cognitive impairment: Implications for a model of perirhinal cortex function. Hippocampus. doi: 10.1002/hipo.22071. In this issue. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MGH, Holroyd CB, Kok A, Molen MW. A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cogn Affect Behav Neurosci. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- O’Neil EB, Cate AD, Kohler S. Perirhinal cortex contributes to accuracy in recognition memory and perceptual discriminations. J Neurosci. 2009;29:8329–8334. doi: 10.1523/JNEUROSCI.0374-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long-term memory and aging: A cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2005. pp. 218–245. [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Pieta Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimaraes M, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE. The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat. Neurobiol Aging. 2005;26:259–264. doi: 10.1016/j.neurobiolaging.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Sakellaridis N. Memantine and recognition memory: Possible facilitation of its behavioral effects by the nitric oxide (NO) donor molsidomine. Eur J Pharmacol. 2007;571:174–179. doi: 10.1016/j.ejphar.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cox C, Hayes SM, Nadel L. Hippocampal activation during episodic and semantic memory retrieval: Comparing category production and category cued recall. Neuropsychologia. 2008a;46:2109–2121. doi: 10.1016/j.neuropsychologia.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Hoscheidt S, Nadel L. Perspectives on episodic and semantic memory retrieval. In: Dere E, Easton A, Nadel L, Huston JP, editors. Handbook of Behavioral Neuroscience Series, Vol. 18: Handbook of Episodic Memory. Netherlands: Elsevier Sciences; 2008b. pp. 5–18. [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KJ, Henson RNA, Graham KS. Recognition memory for faces and scenes in amnesia: Dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2135–2146. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]


