Abstract
Tremendous progress has recently been made in both molecular subgrouping, and the establishment of prognostic biomarkers for embryonal brain tumors, particularly medulloblastoma. Several prognostic biomarkers that were initially identified in retrospective cohorts of medulloblastoma, including MYC and MYCN amplification, nuclear β-catenin accumulation, and chromosome 17 aberrations have now been validated in clinical trials. Moreover, molecular subgroups based on distinct transcriptome profiles have been consistently reported from various groups on different platforms demonstrating that the concept of distinct medulloblastoma subgroups is very robust. Well-described subgroups of medulloblastomas include tumors showing wingless signaling pathway (Wnt) activation, and another characterized by sonic hedgehog pathway activity. Two or more additional subgroups were consistently reported to contain the vast majority of high-risk tumors, including most tumors with metastatic disease at diagnosis and/or large cell/anaplastic histology. Several years ago, atypical teratoid rhabdoid tumor (AT/RT) was recognized as a separate entity based on its distinct biology and particularly aggressive clinical behavior. These tumors may occur supra or infratentorially and are usually found to have genetic alterations of SMARCB1 (INI1/hSNF5), a tumor suppressor gene located on chromosome 22q. Subsequent loss of SMARCB1 protein expression comprises a relatively specific and sensitive diagnostic marker for AT/RT. For CNS primitive neuroectodermal tumors (CNS PNETs), a consistent finding has been that they are molecularly distinct from medulloblastoma. Furthermore, a distinct fraction of CNS PNETs with particularly poor prognosis only occurring in young children was delineated, which was previously labeled ependymoblastoma or embryonal tumor with abundant neuropil and true rosettes (ETANTR) and which is morphologically characterized by the presence of multilayered “ependymoblastic” rosettes. This group of tumors shows a unique cytogenetic abnormality not seen in other brain tumors: focal amplification of a micro-RNA cluster at chromosome 19q13.42, which has never been found to be amplified in other CNS PNETs, medulloblastoma or AT/RT. In summary, these consistent findings have significantly contributed to our ability to sub-classify embryonal brain tumors into clinically and biologically meaningful strata and, for some of the subgroups, have led to the identification of specific targets for future development of molecularly targeted therapies.
Keywords: Embryonal brain tumors, Medulloblastoma, AT/RT, ETANTR, ETMR, Molecular marker, Prognostic marker, Diagnostic marker
Introduction
Tumors of embryonal origin comprise by far the largest group of malignant brain tumors in childhood and are still associated with a comparably high mortality and with significant long-term morbidity for survivors. Although our understanding of the molecular biology of these tumors has enormously improved in recent years, little of this knowledge has been translated into clinical applications to date. Consistent results across different studies using different platforms and methods of detection, however, very well justify the routine use of specific diagnostic and prognostic biomarkers in clinical trials. Thus, this review focuses on selected molecular biomarkers that have shown promise to be useful for diagnostic purposes and/or patient stratification into different risk groups and molecular subgroups.
Medulloblastoma
Clinical markers
The annual incidence of medulloblastoma ranges from 0.48 in girls to 0.75 in boys per 100,000 children [12] with a peak age at presentation of 7 years. More than 80% of childhood medulloblastomas arise as midline tumors of the vermis of the cerebellum, whereas involvement of cerebellar hemispheres increases with age to reach around 50% in adult patients [79]. Approximately 30% of children show evidence of disseminated disease at diagnosis. Metastatic disease at diagnosis, either macroscopically as assessed by MRI of the brain and total spine, or microscopically as detected by cerebrospinal fluid cytology, has consistently been found to be the strongest clinical predictor of poor patient outcome [114]. Furthermore, the level of surgical resection (macroscopic complete vs. incomplete resection as evaluated by post-operative imaging) has been demonstrated to be a valuable prognostic indicator in the majority of studies [1, 114]. Apart from these two markers (metastatic stage at diagnosis and level of surgical resection), no other clinical parameter has consistently been reported to be of prognostic value for medulloblastoma.
Histopathological markers
According to the 2007 WHO [67] classification, medulloblastoma is defined as a grade IV malignant embryonal neoplasm arising in the cerebellum. They are multipotent tumors, but are predominantly undifferentiated, or neuronally differentiated. Neuronal immunohistochemical markers such as synaptophysin are typically at least focally positive, and are commonly used in routine diagnostics.
Five histological varieties are recognized in the 2007 WHO classification (Fig. 1): (1) classic medulloblastoma with densely packed and primarily undifferentiated cells surrounded by scanty cytoplasm, as well as numerous Homer-Wright rosettes. (2) Anaplastic tumors with enlarged, tightly packed pleomorphic nuclei showing angulation, moulding and wrapping. Nucleoli are also sometimes prominent in this subtype, and mitotic and apoptotic activity is often more pronounced than in classic medulloblastoma [7, 19]. (3) Large cell medulloblastoma are composed of enlarged round cells with prominent nucleoli [39]. A high mitotic rate, extensive apoptosis and wrapping of one tumor cell about another are also all characteristic features. Tumors with both large cell and anaplastic regions may be encountered [7]. It should be emphasized that large cell or anaplastic changes can be present in only a fraction of the overall tumor area, but because embryonal neoplasms are inherently pleomorphic, it is recommended that these aggressive features be severe or widespread before diagnosing these two variants [67]. (4) The desmoplastic/nodular variant, in which round or elongated zones of predominantly neurocytic neuronal differentiation are observed between more cellular tumor regions associated with prominent desmoplasia. The distinctive lucent differentiated areas known as “pale islands” are also characterized by reduced proliferation, while the intervening areas with abundant inter-nodular reticulin are quite proliferative [67]. Biphasic medulloblastoma with nodules but no inter-nodular desmoplasia have also been described, but this variant appears to be genetically and clinically distinct from the desmoplastic/nodular variant [71]. Desmoplasia alone, which is often seen in the context of leptomeningeal invasion by the tumor, is not sufficient for a diagnosis of desmoplastic/nodular medulloblastoma. (5) Medulloblastoma with extensive nodularity (formerly known as “cerebellar neuroblastoma”) denotes a tumor almost entirely composed of long, streaming nodules with large areas of fibrillar neuropil and little or no desmoplastic inter-nodular tissue [38].
Fig. 1.
Medulloblastoma
Associations have been made between histopathological subtype, specific genetic changes, and clinical outcome. Patients with classical tumors tend to have average clinical outcomes, while in some studies desmoplastic/nodular or extensively nodular medulloblastoma are associated with improved survival [19, 38, 71]. In contrast, most investigators have found that large cell and anaplastic histologies portend a shorter survival [19, 39, 71, 83].
Isochromosome 17
Isochromosome 17q (i17q) is the most frequent structural aberration in medulloblastoma, found in 30–40% of cases [17, 77, 78, 83]. This abnormality consists of a chromosome with two centromeres, two copies of the ‘q’ arm of chromosome 17, and two copies of very centromeric ‘17p’ that are fused together. This results in a very long chromosome, which is usually found in the presence of an additional, structurally normal copy of chromosome 17. Medulloblastomas with an isochromosome 17q therefore usually have one copy of ‘17p’ and three copies of ‘17q’. The existence of tumor suppressor gene(s) on 17p, and oncogenes(s) on 17q have therefore been hypothesized for some time, but specific genes that are recurrently mutated in i17q cases have not yet been identified. Specifically, the TP53 gene located at 17p13.1 does not appear to be mutated more commonly in medulloblastomas with i17q than those without i17q [98]. Rarely, medulloblastomas are seen with either isolated ‘17p’ loss, or isolated ‘17q’ gain [78, 83]. Whether these latter cases have a similar biology to classic i17q, or some distinct biology is unclear. Large re-sequencing projects should soon determine the presence or absence of recurrently mutated genes on 17p and 17q in medulloblastomas with i17q. Large cohorts of medulloblastoma studied by DNA copy-number array techniques have not identified recurrent regions of high-level amplification on chromosome 17q [78, 83]. It is certainly possible that the ‘second hit’ on 17p is epigenetic in nature and will therefore be missed by re-sequencing techniques focusing on genetic mutations. The disruption of a specific gene at the 17p breakpoint is unlikely because the breakpoint has some variability, and occurs in a gene poor region. A further possibility is that i17q drives clonal selection through haploinsufficiency for genes on 17p, and a modest copy-number driven increase of expression of genes on chromosome 17q [13]. Determination of the specific genes/pathways driving clonal selection of cells carrying an i17q would allow the development of targeted therapeutics effective against in a broad swathe of medulloblastoma patients.
The breakpoints for i17q are located in the pericentromeric region of 17p, in the so-called ‘Smith–Magenis’ region that contains a pair of head-to-head inverted DNA sequence repeats. Current thinking is that non-allelic homologous recombination between these repeat sequences leads to the formation of an i17q [9, 72]. More recent fine mapping of the 17p breakpoint has identified a small number of poorly characterized genes whose disruption could play a role in the pathogenesis of medulloblastoma [70]. Intriguingly, recurrent medulloblastomas show increased levels of 17q gain as compared to the initial tumor, suggesting an important role for i17q in the progression of medulloblastoma [62].
While i17q is an enticing target for the therapy of medulloblastoma due to its high frequency, ultimately the specific genes/pathways driving clonal selection of cells carrying an i17q must be identified, and their specific role in tumor initiation versus maintenance versus progression must be determined before therapy can be appropriately targeted against this most common of abnormalities.
MYC/MYCN aberrations
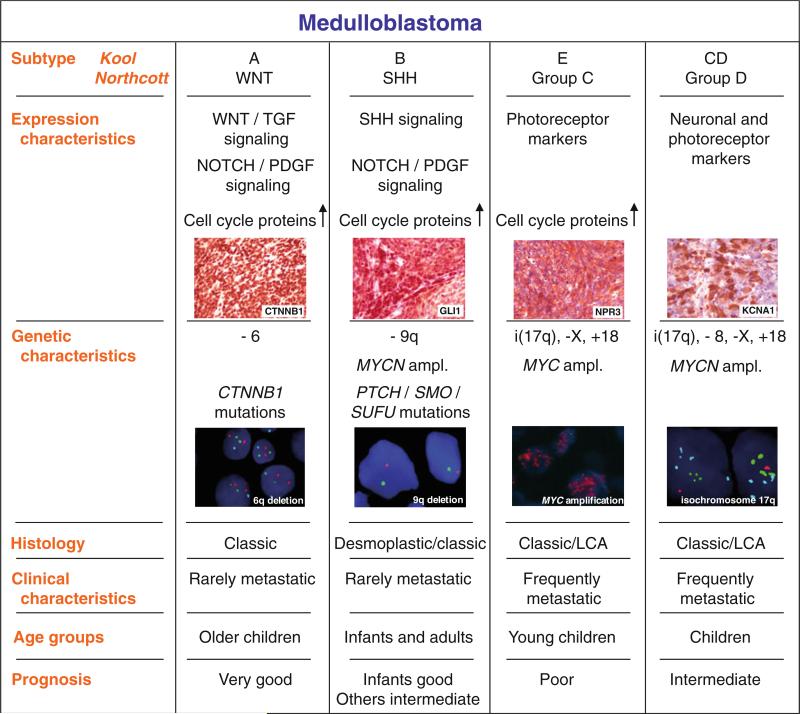
Increased MYC gene dosage in medulloblastoma was initially documented in 1988 in the cell line D341 Med [31]. Subsequently, a number of other groups have used various techniques to identify an elevated DNA copy number at the MYC locus [2, 3, 60, 65, 78, 100]. While the percentage of amplified cases varies between series, few exceed 10%, and one large analysis of 260 cases using FISH, identified high-level amplification of MYC copy number (>4 copies/cell on average) in 4% [83]. Early on, it was recognized that amplification of the MYC locus was significantly associated with poor clinical outcome and with the large cell and anaplastic medulloblastoma phenotype, which has been confirmed in multiple subsequent studies [2, 7, 21, 65, 83, 94, 100]. Indeed, it has recently been suggested that MYC amplification may be a more powerful prognostic marker than large cell/anaplastic change, as histologically aggressive cases lacking increased MYC gene dosage were not significantly associated with worse outcome [109] (Fig. 1).
The biological impact of MYC mRNA levels has also been extensively analyzed in medulloblastoma. Investigators have used in situ hybridization [20, 47] and quantitative PCR [41, 47, 96] to link increased transcript levels to the large cell/anaplastic subtype or worse clinical outcomes. However, this data is conflicting, since comparably high levels of MYC RNA have been observed in Wnt subgroup medulloblastomas, which are known to have a particularly favorable prognosis [60, 76, 105]. In vitro experiments have shown that increased expression of MYC can promote proliferation of cerebellar granule cells in rodents, and an “anaplastic” phenotype in medulloblastoma cell lines [35, 96].
MYCN gene amplification has also been identified in up to 10% of medulloblastoma specimens and, like MYC, is often found in tumors with large cell/anaplastic features [2, 3, 21, 78, 87, 106]. In 260 cases studied by FISH, Pfister and colleagues [83] found MYCN gene amplification in 7%. As with MYC, increased MYCN gene dosage is prognostic of worse clinical outcome, although patients with MYCN amplified MB are clinically much more heterogeneous [83] (Fig. 1). The causal role of MYCN in the initiation and progression of medulloblastoma is highlighted by the fact that increased MYCN expression is sufficient to drive the formation of metastatic medulloblastoma in transgenic mice [97]. Effective, non-toxic agents targeting MYC transcription factors have been difficult to develop, which is unfortunate in light of the therapeutic effectiveness of withdrawing Mycn expression in this mouse model [97].
As for MYC transcripts, the prognostic role of increased MYCN mRNA levels is less clear [20]. This may be in part due to positive regulation of MYCN expression and protein stability by sonic hedgehog (SHH) in the developing cerebellum and in SHH-driven medulloblastoma [45, 57, 104]. Because SHH and MYCN associated tumors are often of the clinically less aggressive desmoplastic/nodular subtype, this fraction of the overall medulloblastoma population would be predicted to have high MYCN levels but still good survival.
Genetic aberrations in the WNT signaling pathway
Medulloblastoma were first associated with WNT signaling as they arise in Turcot syndrome patients with germline defects in APC, a tumor suppressor gene which keeps the WNT signaling pathway in check [43]. It was subsequently shown that activating point mutations in CTNNB1, the gene encoding β-catenin, a downstream effector of WNT signaling, were present in 4–10% of sporadic medulloblastoma [26, 64, 105]. Activating mutations of CTNNB1 result in an inability of GSK3-β to phosphorylate β-catenin, thereby rendering it resistant to degradation, whereupon it relocates to the nucleus and activates canonical WNT signaling. When nuclear translocation of β-catenin protein is used as an immunohistochemical marker of WNT signaling, between 18 and 27% of tumors show signs of pathway activation [23, 26, 64, 91]. Some medulloblastomas may also activate WNT signaling through epigenetic silencing of the SFRP family of secreted WNT signaling inhibitors [59]. Expression microarray analyses of medulloblastoma cohorts have also identified a subgroup of 13–15% defined by WNT pathway markers [60, 76, 105], typically in combination with monosomy 6 as the only large cytogenetic aberration in these tumors [11, 22, 48, 58, 116]. As patients with WNT subgroup medulloblastomas have an excellent prognosis, they are excellent candidates for which to develop therapy sparing targeted agents (Fig. 1).
Genetic aberrations in sonic hedgehog signaling
Individuals with germline mutations in the sonic hedgehog (SHH) receptor PATCHED (PTCH) have Gorlin syndrome, in which affected individuals develop basal cell carcinoma, have a number of developmental abnormalities, and have a greatly increased incidence of medulloblastoma [107]. A downstream element in the SHH signaling pathway, SUFU is mutated in the germline of some infants with medulloblastoma [102]. Mice with a heterozygous mutation in the SHH receptor Patched develop medulloblastoma due to uncontrolled proliferation of progenitor cells in the external granule cell layer of the cerebellum [40, 110].
Similarly, somatic mutations in medulloblastoma of SHH pathway genes including PTCH, PTCH2, SMO, and SUFU have been demonstrated [102, 103, 115]. Northcott and colleagues [78] have recently demonstrated high-level amplification of the SHH effector transcription factors GLI1 and GLI2 in a subset of medulloblastomas. It seems highly likely that additional genes in the SHH signaling cascade will be identified as amplified or mutated in medulloblastoma, as the signaling pathway is better understood and our catalogue of medulloblastoma mutations nears completion. It is currently unclear, whether or not mutations of SHH pathway genes are restricted to tumors that belong to the ‘SHH group’ of medulloblastomas as identified through clustering of transcriptional profiles, as detailed below [60, 77] (Fig. 1). Transcriptional targets of the SHH pathway including the mir17-92 complex, and YAP1 have been demonstrated to be amplified in medulloblastoma where they are acting as oncogenes [27, 28, 77]. Recently, small molecules that inhibit SMO have been demonstrated to be an effective, albeit temporary, therapy for human and mouse medulloblastoma [92, 113]. As SMO inhibitors function upstream at the level of the membrane, the identification of downstream mutations/amplifications (i.e., SUFU, GLI1, GLI2) becomes critical when assessing the suitability of an individual patient for therapy. Currently in clinical trials, SHH pathway inhibitors seem poised to become the first approved targeted therapies for effective treatment of medulloblastoma.
Other genetic aberrations
The TP53 pathway
Individuals with germline mutations of TP53 have Li–Fraumeni Syndrome that includes an increased predisposition to a number of cancers, including medulloblastoma. Similarly, Tp53 deficiency has been reported as an enhancer in a large number of mouse models of medulloblastoma [111]. While some early reports suggested that the TP53 pathway was only rarely affected in medulloblastoma, more recent reports suggest that up to 15% of medulloblastomas carry mutations in TP53 or, less frequently in other genes in the TP53 pathway, making it the most frequently mutated tumor suppressor gene in medulloblastoma identified to date [30, 98].
Histone lysine methylation
Post-translational modification of histone lysine moieties is an epigenetic mechanism to increase or decrease the accessibility of chromatin for transcription. Specifically, dimethylation of histone 3, lysine 9 (H3K9) is a silencing mark that is necessary for differentiation of a number of tissues, including embryonic stem cells. Recurrent homozygous deletions (EHMT1) and amplifications (JMJD2C, JMJD2B, and MYST3) found in medulloblastoma target H3K9 methylation with resultant hypomethylation at that locus [78]. As a number of pharma and academic consortia are developing compounds that target methylation of H3K9, this may constitute a future avenue for targeted therapy.
Miscellaneous medulloblastoma oncogenes
NOTCH2 is over-expressed, and rarely amplified in a subset of medulloblastomas [24]. Notch signaling may also be activated in medulloblastoma through silencing of mir199b-5p [36]. OTX2 is commonly amplified in non-SHH, non-WNT medulloblastomas, where it likely plays a role in both tumor maintenance and progression [15, 16]. The cell cycle progression factor CDK6 is recurrently amplified in medulloblastoma, particularly in adults [64, 73].
Subclassification based on molecular profiling
The first mRNA expression profiling studies of medulloblastoma series used supervised approaches to identify differentially expressed genes after grouping for metastatic status, histology or survival [68, 75, 86]. MacDonald et al. [68] analyzed 23 primary medulloblastomas designated as either M+ or M0 using Affymetrix G110 cancer arrays and identified 85 genes as differentially expressed between the two classes. PDGFR and members of the RAS/MAPK signaling pathway were found to be more highly expressed in metastatic as compared to non-metastatic cases. Pomeroy et al. [86] used Affymetrix HuGeneFl arrays representing ~6,000 genes to compare the expression profiles in a set of 34 medulloblastomas between tumors with classic (25 cases) or desmoplastic (9 cases) histology. Genes identified as over-expressed in desmoplastic medulloblastoma included PTCH, GLI, MYCN, and IGF2, all targets of SHH signaling. These analyses demonstrated for the first time that sporadic desmoplastic medulloblastoma, like tumors associated with Gorlin's syndrome, was characterized by aberrant SHH signaling.
More recently, unsupervised approaches have been used to identify distinct molecular subgroups in medulloblastoma using gene expression data [60, 76, 105]. Thompson et al. [105], who analyzed 46 medulloblastomas using Affymetrix 133A arrays, were the first to show that WNT-and SHH-driven medulloblastomas comprise two very distinct biological subgroups based on their transcriptome (Fig. 1). As one might expect, these distinct expression signatures were strongly associated with specific genetic events described above (i.e., CTNNB1 mutation and monosomy 6 for WNT-driven tumors, and PTCH/SUFU mutation as well as 9q deletion in tumors showing SHH activation).
More insight into the non-WNT/SHH tumors came from a study by Kool et al. [60] who used mRNA expression data of 62 medulloblastomas generated with Affymetrix 133plus2 arrays to identify five molecular subtypes. Sub-types characterized by WNT signaling or by SHH signaling form the two most distinct subtypes. The other three sub-types are more related to each other and show overlapping gene signatures. Kool type C and Kool type D are characterized by elevated expression of neuronal differentiation genes, whereas photoreceptor genes are specifically expressed both in Kool type D and E tumors (Fig. 1). In the most recent study, Northcott et al. [76] analyzed gene expression profiles of 103 medulloblastomas using Affymetrix Exon 1.0 ST arrays. The authors identified the same molecular subgroups except that the two related C and D tumors, described as two distinct subtypes in the study of Kool et al. [60], were now seen as one subgroup, called Northcott group D [76]. Importantly, Northcott and colleagues identified specific protein markers for each subgroup: DKK1 (or CTNNB1) for WNT tumors, SFRP1 (or GLI1) for SHH tumors, NPR3 for Northcott group C tumors, and KCNA1 for group D tumors that can be used for immunohistochemistry-based classification of formalinfixed and paraffin embedded medulloblastoma tissues with very high accuracy (Fig. 1). Staining TMAs with specific antibodies for these markers and linking the staining to clinical follow-up data demonstrated that patients with Northcott group C tumors have the worst prognosis, regardless of M-stage. The best outcome was found for WNT tumors as anticipated from previous studies.
Both Kool et al. and Northcott et al. [60, 76] also determined DNA copy-number alterations in their medulloblastoma series using a-CGH or high density SNP arrays, respectively. Several specific genetic aberrations were identified in these studies that were associated with the distinct subgroups. For instance, monosomy 6 was found in almost every WNT tumor, but not in any of the other tumors, confirming previous studies [11, 26, 64, 105]. Loss of 9q was only found in SHH tumors. Chromosome 17 aberrations were strongly associated with non-WNT/SHH tumors, as were gain of chromosome 18, and loss of the X chromosome in females [60, 76] (Fig. 1).
Clinico-pathological features also significantly differed between the molecular subtypes identified in these profiling studies [60, 76, 105]. Most (27/43) desmoplastic cases were found in the SHH group, never in the WNT group, but also sometimes among the non-WNT/SHH tumors (16/119). WNT tumors demonstrate classic histology in all reported series. Large cell or anaplastic histology, associated with poor outcome [7], was found in all subtypes except WNT tumors. Medulloblastomas in infants are treated with surgery and chemotherapy alone. For this group of patients it is therefore even more important to understand the underlying genetics and biology. Most (29/47) medulloblastomas in infants in these three profiling studies were classified as SHH tumors and 21/29 had desmoplastic histology. For non-SHH tumors in infants only 2/15 had desmoplastic histology. Medulloblastomas in infants not classified as SHH tumors, were in most cases classified as Kool type E/Northcott group C [60, 76]. SHH tumors were not only seen in infants but also in older children and particularly adults, whereas WNT and Northcott group D (subtype CD in Kool et al. [60]) tumors typically occur in older children. It is well known that medulloblastomas occur more often in males than in females (M:F = 1.5:1) and several studies have suggested that males with medulloblastoma have a worse outcome than females [14, 99, 105]. These recent profiling studies demonstrate, however, that males with medulloblastomas are more often classified as non-WNT/SHH tumors, while there is an almost equal distribution of SHH tumors among males and females, and a predominance of females among WNT tumors [60, 76, 105]. Interestingly, metastatic disease at diagnosis is also significantly associated with non-WNT/SHH tumors and most strongly with Kool sub-type E/Northcott group C tumors [60, 76, 105]. Whether the increased occurrence of metastasis in non-WNT/SHH tumors also explains the unfavorable outcome in males is not yet known.
In summary, these recent microarray studies clearly demonstrate that medulloblastoma is not just one disease, but comprises different subtypes that are demographically, clinically, transcriptionally and genetically distinct. Future clinical trials should consider distinguishing these different subtypes, and targeted therapies may need to be developed for each subtype separately.
Medulloblastoma in adults
Genomic profiles of adult and pediatric MB demonstrate significant differences in terms of DNA copy-number aberrations [64]. Approximately 25% of adult MB show no genomic imbalances in comparison to 5% within the pediatric cohort, suggesting that genomic instability is more critical for tumorigenesis in childhood medulloblastoma. CDK6 was the frequent focal amplification in adult MB detected to date, whereas focal amplifications of MYC/MYCN are far more common in pediatric tumors [64]. Gain of chromosomes 3q, 4, and 19 are more common in adult MB, whereas gain of chromosomes 1q, 2, 7, and 17q, as well as loss of 16q are more frequent in pediatric MB. All chromosome 6 deletions were monosomy 6 in pediatric tumors, whereas they were mostly partial deletions in adult MB cases. In contrast to pediatric tumors, adult MB cases with CTNNB1 mutation did not always show a monosomy 6. For adult MB, shortened survival was found for tumors with CDK6 amplification, 17q gain, and 10q loss. Adult MB with WNT signaling pathway activation, however, did not share the excellent prognosis seen in childhood MB [64].
Atypical teratoid/rhabdoid tumor
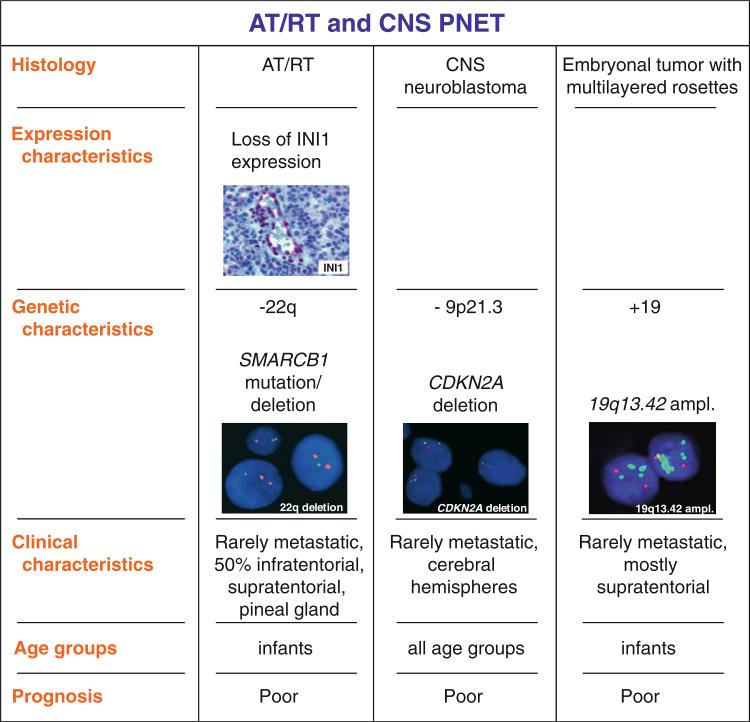
Atypical teratoid/rhabdoid tumors (AT/RT) (WHO grade IV) are highly malignant tumors predominantly occurring in very young children [55]. Since the diagnosis of AT/RT usually implies a dismal prognosis, and also a more aggressive therapeutic approach [10, 29, 61], distinction from other embryonal tumors is of paramount clinical importance. Unlike most other central nervous system tumors, genetic alterations encountered in AT/RT are remarkably uniform, the majority of cases showing genetic alterations affecting the SMARCB1 (INI1/hSNF5) locus on 22q11 resulting in loss of SMARCB1 protein expression [89, 108] (Fig. 1). Genetic alterations include homozygous deletions, heterozygous deletions as well as copy-number neutral loss of heterozygosity (LOH) and mutations affecting each of all nine exons of SMARCB1; a higher frequency of mutations for exons 5 and 9 has been reported [4, 51].
In recent years, immunohistochemistry using an antibody directed against SMARCB1 has evolved as a convenient first line diagnostic tool in neuropathology laboratories for the diagnosis of AT/RT. Loss of SMARCB1 protein expression is quite characteristic for AT/RT and routine screening of all malignant pediatric CNS tumors has been advocated [6, 42, 56, 112]. This is especially relevant in small biopsy specimens, where rhabdoid tumor cells can be missed. Indeed, in this setting SMARCB1 protein loss has been shown to be associated with an aggressive clinical course and poor therapeutic response, even in the absence of rhabdoid tumor cells [6, 42].
Subsequently, molecular genetic analyses are performed to confirm underlying genetic alterations affecting the SMARCB1 locus. Using a combination of FISH and genomic sequencing, genetic alterations can be demonstrated in about 75% of AT/RTs [61]. The diagnostic yield can be increased substantially by high-resolution methods. Adding MLPA [52] and SNP-based oligonucleotide arrays to the diagnostic armamentarium, biallelic alterations involving the SMARCB1 locus could be demonstrated in 36/36 AT/RT examined [51]. An important reason for molecular genetic testing is the need to screen for germline mutations, which can be identified in up to 25% of patients, including familial cases described as rhabdoid tumor predisposition syndrome (OMIM #609322). Since children harboring germline mutations are younger and usually have a fatal course, this finding is also of prognostic importance. Most mutations or deletions occur de novo and parents are unaffected, but germline mutations have also been described in unaffected adult carriers, suggesting possible incomplete penetrance and/or a critical time window for the tumorigenesis of AT/RT early in life [53, 101].
Careful molecular characterization and staining for SMARCB1 protein expression has resulted in many pediatric brain tumors initially categorized as CNS primitive neuroectodermal tumor (CNS PNET), medulloblastoma, or choroid plexus carcinoma to be reclassified as AT/RT [42, 54]. Conversely, it has become evident that extracranial non-rhabdoid tumors such as epithelioid sarcoma [74] and schwannoma in the context of familial schwannomatosis [49, 80] may also carry genetic alterations of SMARCB1 resulting in SMARCB1 protein loss. In the central nervous system, the vast majority of non-rhabdoid, as well as composite rhabdoid tumors (e.g. rhabdoid meningioma) [82] show retained SMARCB1 staining. Recently, however, two children with unusual intracranial non-rhabdoid neuroectodermal tumors within, and around the third or fourth ventricle have been reported [44]. These unusual tumors were histopathologically characterized by cribriform strands and trabeculae, well-defined epithelial membrane antigen-immunopositive surfaces and loss of SMARCB1 protein expression. Molecular genetic analyses by FISH and sequencing disclosed a homozygous 4-bp duplication in exon 4 (492duplCCTT) as well as deletions affecting the SMARCB1 locus in two further yet unpublished cases. The term cribriform neuroepithelial tumor (CRINET) has been coined for these rare non-rhabdoid ventricular tumors. Recognition and better characterization of CRINET could be of prognostic importance, since limited evidence suggests that these tumors might respond favorably to conventional chemotherapy regimens, thereby expanding the histological and clinical spectrum of SMARCB1-deficient central nervous system tumors.
To further complicate matters, some biologically aggressive tumors in small children show the characteristic histopathology and immunohistochemical staining profile of AT/RT, but retain SMARCB1 protein staining, and do not have alterations of the SMARCB1 locus on genetic analyses [32]. These rare tumors (representing approximately 2% of AT/RT) still comprise a diagnostic challenge and some uncertainty had remained if they indeed represented AT/RT. However, the SMARCB1 gene codes for only one member of the large SWI/SNF ATP-dependent chromatin remodeling complex [90], raising the possibility that genetic alterations of other members of this complex could be involved in the pathogenesis of AT/RT lacking SMARCB1 alterations. Recently, inactivation of the ATPase subunit SMARCA4 (also known as BRG1) located on 19p13.2 was demonstrated in the tumor cells of two sisters with rhabdoid tumors (one AT/RT, and one malignant rhabdoid tumor of the kidney). In this family, genetic alterations of SMARCB1 were lacking, but a SMARCA4 germline mutation, and LOH by uniparental disomy was detected [95]. A further child harboring an AT/RT showing retained SMARCB1 staining, but loss of SMARCA4 protein expression associated with a homozygous nonsense SMARCA4 mutation has been described [33]. Whether genetic alterations of other members of the SWI/SNF ATP-dependent chromatin remodeling complex such as SMARCA2 (BRM), SMARCC1 (BAF155) or SMARCC2 (BAF170) might play a role in those very rare cases of AT/RT showing both retained SMARCB1 and SMARCA4 staining, remains to be determined. Furthermore, the diagnostic value of antibodies directed against claudin-6, a structural protein found to be highly expressed in AT/RT [5] awaits confirmation.
CNS primitive neuroectodermal tumors (PNETS)
As medulloblastoma and AT/RT, CNS primitive neuroectodermal tumors (PNETs) are malignant embryonal neoplasms of WHO grade IV [67]. Although CNS PNETs are less frequently metastatic at the time of primary diagnosis when compared to medulloblastoma, they represent a particularly unfavorable group of embryonal brain tumors and patients frequently fail to respond to standard therapies, especially in early childhood when cranio-spinal radiotherapy is avoided because of the resulting pronounced neurocognitive deficits. Differences in outcome could be due to different anatomic localizations, but could also be based on distinct molecular pathomechanisms.
This heterogeneous group of tumors according to the WHO classification may be subdivided into CNS (ganglio-) neuroblastoma, medulloepithelioma, and ependymoblastoma.
CNS neuroblastoma
CNS PNET may display divergent degrees of differentiation along with neuronal, astrocytic, muscular, or melanocytic lines. Tumors with neuronal differentiation are designated CNS neuroblastoma, or, if ganglion cells are present, ganglioneuroblastoma. CNS neuroblastomas are composed of undifferentiated and poorly differentiated neuroepithelial cells. Homer-Wright rosettes may be found, but vary in frequency [67]. Ganglioneuroblastoma shows a combination of primitive-appearing and terminally differentiated cells. The most consistent genetic finding has been that chromosome 17 alterations (loss of 17p or isochromosome 17q (i17q) are very rare in CNS neuroblastoma in comparison to medulloblastoma [8, 50, 69, 85, 93]. Genomic aberrations that occur more frequently in CNS neuroblastoma include 13q telomeric deletion, 14q deletion, homozygous deletion of 9p21.3 spanning the CDKN2A and CDKN2B loci, and 19q gain [69, 85, 93] (Fig. 2). Other reported genomic abnormalities in CNS neuroblastoma include RASSF1A promoter methylation, transcriptional silencing of the DLC1 gene, and expression of Neuro D family genes. TP53 mutations were not observed in these tumors, although a few samples of adult CNS neuroblastoma revealed mutation of IDH1 [46]. Recent studies of global gene expression signatures have revealed the absence of external granular cell gene and proneuronal transcripts in CNS PNETs [86]. Comparative transcriptome analysis of CNS PNETs and medulloblastomas revealed a high level of expression of SOX, NOTCH1, ID1 and ASCL-1 transcripts in supratentorial PNETs, whereas transcription of proneuronal factors was more pronounced in medulloblastomas. In addition, an activation of JAK/STAT3 signaling was found in CNS PNETs. Therefore, it has been hypothesized that CNS PNETs predominantly express glial molecular features, whereas medulloblastomas largely follow neuronal differentiation pattern. Thus, although the number of cytogenetic and molecular genetics studies of supratentorial CNS PNETs is obviously scarce, it appears that molecular events typical for these tumors are different from medulloblastomas.
Fig. 2.
AT/RT and CNS PNET
Medulloepithelioma
Medulloepithelioma is a rare, highly malignant tumor affecting young children. Histologically medulloepithelioma mimics the embryonic neural tube with external limited membrane. These tumors often display multiple lines of differentiation including neuronal, glial and mesenchymal elements. Molecular genetics studies of medulloepitheliomas are very scarce and only a few tumors were investigated up to date. Amplification of hTERT gene on 5p15 was found to be a frequent cytogenetic aberration [25]. In addition, gain on chromosomal arms 3p, 6p 14q, 15q, and 20q as well as losses on 4q, 5q, 13q and 18q were found.
Ependymoblastoma
Ependymoblastoma is a rare and very aggressive embryonal neoplasm characterized by the presence of true multilayered or “ependymoblastic” rosettes in association with small undifferentiated cells. Since its initial description, it has widely been discussed if ependymoblastoma should be regarded a distinct entity of embryonal CNS tumors. Some investigators have proposed that the designation ependymoblastoma should be eliminated from the current classifications arguing that “ependymoblastic” rosettes are not a specific pattern. Further Eberhart and coworkers more recently described a yet different pediatric embryonal brain tumor entity. Based on its characteristic histopathological findings, this tumor was designated “embryonal tumor with abundant neuropil and true rosettes (ETANTR)”. The microscopic appearance of this neoplasm includes both ependymoblastic rosettes and patterns of neuronal differentiation, including neurocytes, ganglion cells and neuropil-like background.
An increased frequency of chromosome 2 gain was observed in ependymoblastoma and ETANTR [18, 34, 37, 88]. More recently, a highly specific focal amplification at chromosome band 19q13.42 containing a cluster of mi-RNA-coding genes was found in virtually all embryonal brain tumors with true multilayered rosettes [63, 66, 84] (Fig. 2). 19q13.42 amplification is associated with up-regulation of mi-RNA clusters mir-371-373 and C19MC [65, 85]. These data indicate that (1) ETANTR and ependymoblastoma may comprise a single biological entity and (2) 19q13.42 focal amplification may serve as a highly specific and sensitive novel diagnostic marker to define this biologically distinct subgroup of CNS PNETs that seems to be restricted to young children, and is associated with a particularly unfavorable prognosis. Therefore, it was recently proposed to uniformly use the term embryonal tumor with multilayered rosettes (ETMR) for this novel entity [81].
In summary, extensive genetic investigations of embryo-nal brain tumors in recent years has significantly contributed to a refined classification of these tumors based on their distinct clinical, histopathological and molecular features. Consistently reported molecular prognostic markers will for the first time be exploited for risk stratification in upcoming clinical trials, especially in medulloblastoma, which is an important breakthrough in brain tumor research. Furthermore, molecular sub-classification will likely help in the near future to target treatment strategies at the underlying biology of distinct molecular subgroups, rather than uniformly treating morphologically indistinguishable tumors with similar clinical characteristics all in the same way.
Contributor Information
Stefan M. Pfister, Molecular Genetics of Pediatric Brain Tumors, German Cancer Research Center, Heidelberg, Germany Department of Pediatric Oncology, Hematology and Immunology, University of Heidelberg, Heidelberg, Germany.
Andrey Korshunov, Clinical Cooperation Unit Neuropathology, German Cancer Research Center, Heidelberg, Germany.
Marcel Kool, Department of Human Genetics, Academic Medical Center, Amsterdam, Netherlands.
Martin Hasselblatt, Institute of Neuropathology, University Hospital Münster, Münster, Germany.
Charles Eberhart, Departments of Pathology, Ophthalmology and Oncology, Johns Hopkins University School of Medicine, Baltimore, USA.
Michael D. Taylor, Sonia and Arthur Labatt Brain Tumor Research Center, Hospital For Sick Children, Toronto, Canada Division of Neurosurgery, Hospital for Sick Children, Toronto, Canada.
References
- 1.Albright A, Wisoff J, Zeltzer P, Boyett J, Rorke L, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neuro-surgery. 1996;38:265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Aldosari N, Bigner SH, Burger PC, Becker L, Kepner JL, Friedman HS, McLendon RE. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children's Oncology Group. Arch Pathol Lab Med. 2002;126:540–544. doi: 10.5858/2002-126-0540-MAMOAI. [DOI] [PubMed] [Google Scholar]
- 3.Bayani J, Zielenska M, Marrano P, Kwan Ng Y, Taylor MD, Jay V, Rutka JT, Squire JA. Molecular cytogenetic analysis of medulloblastomas and supratentorial primitive neuroectodermal tumors by using conventional banding, comparative genomic hybridization, and spectral karyotyping. J Neurosurg. 2000;93:437–448. doi: 10.3171/jns.2000.93.3.0437. [DOI] [PubMed] [Google Scholar]
- 4.Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extra-renal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–3467. [PubMed] [Google Scholar]
- 5.Birks DK, Kleinschmidt-DeMasters BK, Donson AM, Barton VN, McNatt SA, Foreman NK, Handler MH. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010;20:140–150. doi: 10.1111/j.1750-3639.2008.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourdeaut F, Freneaux P, Thuille B, Lellouch-Tubiana A, Nicolas A, Couturier J, Pierron G, Sainte-Rose C, Bergeron C, Bouvier R, Rialland X, Laurence V, Michon J, Sastre-Garau X, Delattre O. hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol. 2007;211:323–330. doi: 10.1002/path.2103. [DOI] [PubMed] [Google Scholar]
- 7.Brown HG, Kepner JL, Perlman EJ, Friedman HS, Strother DR, Duffner PK, Kun LE, Goldthwaite PT, Burger PC. “Large cell/anaplastic” medulloblastomas: a Pediatric Oncology Group Study. J Neuropathol Exp Neurol. 2000;59:857–865. doi: 10.1093/jnen/59.10.857. [DOI] [PubMed] [Google Scholar]
- 8.Burnett ME, White EC, Sih S, von Haken MS, Cogen PH. Chromosome arm 17p deletion analysis reveals molecular genetic heterogeneity in supratentorial and infratentorial primitive neuroectodermal tumors of the central nervous system. Cancer Genet Cytogenet. 1997;97:25–31. doi: 10.1016/s0165-4608(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho CM, Lupski JR. Copy number variation at the breakpoint region of isochromosome 17q. Genome Res. 2008;18:1724–1732. doi: 10.1101/gr.080697.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, Rorke-Adams LB, Fisher MJ, Janss A, Mazewski C, Goldman S, Manley PE, Bowers DC, Bendel A, Rubin J, Turner CD, Marcus KJ, Goumnerova L, Ullrich NJ, Kieran MW. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27:385–389. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifford S, Lusher M, Lindsey J, Langdon J, Gilbertson R, Straughton D, Ellison D. Wnt/wingless pathway activation and chromosome 6 loss characterise a distinct molecular sub-group of medulloblastomas associated with a favourable prognosis. Cell Cycle. 2006;5:2666–2670. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 12.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham SC, Gallmeier E, Hucl T, Dezentje DA, Abdelmohsen K, Gorospe M, Kern SE. Theoretical proposal: allele dosage of MAP2K4/MKK4 could rationalize frequent 17p loss in diverse human cancers. Cell Cycle. 2006;5:1090–1093. doi: 10.4161/cc.5.10.2805. [DOI] [PubMed] [Google Scholar]
- 14.Curran EK, Saisani KL, Le GM, Propp JM, Fisher PG. Gender affects survival for medulloblastoma only in older children and adults: a study from the Surveillance Epidemiology and End Results Registry. Pediatr Blood Cancer. 2009;52:60–64. doi: 10.1002/pbc.21832. [DOI] [PubMed] [Google Scholar]
- 15.de Haas T, Oussoren E, Grajkowska W, Perek-Polnik M, Popovic M, Zadravec-Zalatel L, Perera M, Corte G, Wirths O, van Sluis P, Pietsch T, Troost D, Baas F, Versteeg R, Kool M. OTX1 and OTX2 expression correlates with the clinicopathologic classification of medulloblastomas. J Neuropathol Exp Neurol. 2006;65:1–11. doi: 10.1097/01.jnen.0000199576.70923.8a. [DOI] [PubMed] [Google Scholar]
- 16.Di C, Liao S, Adamson DC, Parrett TJ, Broderick DK, Shi Q, Lengauer C, Cummins JM, Velculescu VE, Fults DW, McLendon RE, Bigner DD, Yan H. Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res. 2005;65:919–924. [PubMed] [Google Scholar]
- 17.Dubuc AM, Northcott PA, Mack S, Witt H, Pfister S, Taylor MD. The genetics of pediatric brain tumors. Curr Neurol Neurosci Rep. 2010;10:215–223. doi: 10.1007/s11910-010-0103-9. [DOI] [PubMed] [Google Scholar]
- 18.Dunham C, Sugo E, Tobias V, Wills E, Perry A. Embryonal tumor with abundant neuropil and true rosettes (ETANTR): report of a case with prominent neurocytic differentiation. J Neurooncol. 2007;84:91–98. doi: 10.1007/s11060-007-9346-y. [DOI] [PubMed] [Google Scholar]
- 19.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94:552–560. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 20.Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, Dang CV, Burger PC. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol. 2004;63:441–449. doi: 10.1093/jnen/63.5.441. [DOI] [PubMed] [Google Scholar]
- 21.Eberhart CG, Kratz JE, Schuster A, Goldthwaite P, Cohen KJ, Perlman EJ, Burger PC. Comparative genomic hybridization detects an increased number of chromosomal alterations in large cell/anaplastic medulloblastomas. Brain Pathol. 2002;12:36–44. doi: 10.1111/j.1750-3639.2002.tb00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol. 2000;59:333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- 23.Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC. β-Catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, Wang Y, Kratz J, Brat DJ, Robitaille Y, Moghrabi A, Perlman EJ, Dang CV, Burger PC, Eberhart CG. hTERT gene amplification and increased mRNA expression in central nervous system embryonal tumors. Am J Pathol. 2003;162:1763–1769. doi: 10.1016/S0002-9440(10)64311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S, Manie E, Raquin MA, Bours D, Carpentier S, Barillot E, Grill J, Doz F, Puget S, Janoueix-Lerosey I, Delattre O. Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol. 2009;218:86–94. doi: 10.1002/path.2514. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez LA, Northcott PA, Taylor MD, Kenney AM. Normal and oncogenic roles for microRNAs in the developing brain. Cell Cycle. 2009;8:4049–4054. doi: 10.4161/cc.8.24.10243. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein-Shechter T, Gassas A, Mabbott D, Huang A, Bartels U, Tabori U, Laura J, Hawkins C, Taylor M, Bouffet E. Atypical teratoid or rhabdoid tumors: improved outcome with high-dose chemotherapy. J Pediatr Hematol Oncol. 2010 doi: 10.1097/MPH.0b013e3181dce1a2. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Frank AJ, Hernan R, Hollander A, Lindsey JC, Lusher ME, Fuller CE, Clifford SC, Gilbertson RJ. The TP53-ARF tumor suppressor pathway is frequently disrupted in large/cell anaplastic medulloblastoma. Brain Res Mol Brain Res. 2004;121:137–140. doi: 10.1016/j.molbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Friedman HS, Burger PC, Bigner SH, Trojanowski JQ, Brodeur GM, He XM, Wikstrand CJ, Kurtzberg J, Berens ME, Halperin EC, et al. Phenotypic and genotypic analysis of a human medulloblastoma cell line and transplantable xenograft (D341 Med) demonstrating amplification of c-myc. Am J Pathol. 1988;130:472–484. [PMC free article] [PubMed] [Google Scholar]
- 32.Frühwald MC, Hasselblatt M, Wirth S, Kohler G, Schneppenheim R, Subero JI, Siebert R, Kordes U, Jurgens H, Vormoor J. Non-linkage of familial rhabdoid tumors to SMARCB1 implies a second locus for the rhabdoid tumor predisposition syndrome. Pediatr Blood Cancer. 2006;47:273–278. doi: 10.1002/pbc.20526. [DOI] [PubMed] [Google Scholar]
- 33.Frühwald MC, Schneppenheim R, Gesk S, Hasselblatt M, Vater I, Kordes U, Leuschner I, Subero JI, Obser T, Oyen F, Siebert R. Rhabdoid predispostion syndrome due to mutation of BRG1/SMARCA4. Neuro Oncol. 2010;12:ii63. (abstract) [Google Scholar]
- 34.Fuller C, Fouladi M, Gajjar A, Dalton J, Sanford R, Helton K. Chromosome 17 abnormalities in pediatric neuroblastic tumor with abundant neuropil and true rosettes. Am J Clin Pathol. 2006;126:277–283. doi: 10.1309/TFBX-1LWQ-93MX-QBAW. [DOI] [PubMed] [Google Scholar]
- 35.Fults D, Pedone C, Dai C, Holland EC. MYC expression promotes the proliferation of neural progenitor cells in culture and in vivo. Neoplasia. 2002;4:32–39. doi: 10.1038/sj.neo.7900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garzia L, Andolfo I, Cusanelli E, Marino N, Petrosino G, De Martino D, Esposito V, Galeone A, Navas L, Esposito S, Gargiulo S, Fattet S, Donofrio V, Cinalli G, Brunetti A, Vecchio LD, Northcott PA, Delattre O, Taylor MD, Iolascon A, Zollo M. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gessi M, Giangaspero F, Lauriola L, Gardiman M, Scheithauer BW, Halliday W, Hawkins C, Rosenblum MK, Burger PC, Eberhart CG. Embryonal tumors with abundant neuropil and true rosettes: a distinctive CNS primitive neuroectodermal tumor. Am J Surg Pathol. 2009;33:211–217. doi: 10.1097/PAS.0b013e318186235b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giangaspero F, Perilongo G, Fondelli MP, Brisigotti M, Carollo C, Burnelli R, Burger PC, Garre ML. Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg. 1999;91:971–977. doi: 10.3171/jns.1999.91.6.0971. [DOI] [PubMed] [Google Scholar]
- 39.Giangaspero F, Rigobello L, Badiali M, Loda M, Andreini L, Basso G, Zorzi F, Montaldi A. Large-cell medulloblastomas. A distinct variant with highly aggressive behavior. Am J Surg Pathol. 1992;16:687–693. [PubMed] [Google Scholar]
- 40.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 41.Grotzer MA, Hogarty MD, Janss AJ, Liu X, Zhao H, Eggert A, Sutton LN, Rorke LB, Brodeur GM, Phillips PC. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clin Cancer Res. 2001;7:2425–2433. [PubMed] [Google Scholar]
- 42.Haberler C, Laggner U, Slavc I, Czech T, Ambros IM, Ambros PF, Budka H, Hainfellner JA. Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol. 2006;30:1462–1468. doi: 10.1097/01.pas.0000213329.71745.ef. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, Krush AJ, Berk T, Cohen Z, Tetu B, et al. The molecular basis of Turcot's syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 44.Hasselblatt M, Oyen F, Gesk S, Kordes U, Wrede B, Bergmann M, Schmid H, Frühwald MC, Schneppenheim R, Siebert R, Paulus W. Cribriform neuroepithelial tumor (CRINET): a nonrhabdoid ventricular tumor with INI1 loss and relatively favorable prognosis. J Neuropathol Exp Neurol. 2009;68:1249–1255. doi: 10.1097/NEN.0b013e3181c06a51. [DOI] [PubMed] [Google Scholar]
- 45.Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, Eisenman RN. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–8661. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- 46.Hayden J, Frühwald M, Hasselblatt M, Ellison D, Bailey S, Clifford S. Frequent IDH1 mutations in supratentorial primitive neuroectodermal tumors (sPNET) of adults but not children. Cell Cycle. 2009;8:1806–1807. doi: 10.4161/cc.8.11.8594. [DOI] [PubMed] [Google Scholar]
- 47.Herms J, Neidt I, Luscher B, Sommer A, Schurmann P, Schroder T, Bergmann M, Wilken B, Probst-Cousin S, Hernaiz-Driever P, Behnke J, Hanefeld F, Pietsch T, Kretzschmar HA. CMYC expression in medulloblastoma and its prognostic value. Int J Cancer. 2000;89:395–402. [PubMed] [Google Scholar]
- 48.Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H. APC mutations in sporadic medulloblastomas. Am J Pathol. 2000;156:433–437. doi: 10.1016/S0002-9440(10)64747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inda MM, Perot C, Guillaud-Bataille M, Danglot G, Rey JA, Bello MJ, Fan X, Eberhart C, Zazpe I, Portillo E, Tunon T, Martinez-Penuela JM, Bernheim A, Castresana JS. Genetic heterogeneity in supratentorial and infratentorial primitive neuroectodermal tumours of the central nervous system. Histopathology. 2005;47:631–637. doi: 10.1111/j.1365-2559.2005.02304.x. [DOI] [PubMed] [Google Scholar]
- 51.Jackson EM, Sievert AJ, Gai X, Hakonarson H, Judkins AR, Tooke L, Perin JC, Xie H, Shaikh TH, Biegel JA. Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res. 2009;15:1923–1930. doi: 10.1158/1078-0432.CCR-08-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jalali GR, Vorstman JA, Errami A, Vijzelaar R, Biegel J, Shaikh T, Emanuel BS. Detailed analysis of 22q11.2 with a high density MLPA probe set. Hum Mutat. 2008;29:433–440. doi: 10.1002/humu.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janson K, Nedzi LA, David O, Schorin M, Walsh JW, Bhattacharjee M, Pridjian G, Tan L, Judkins AR, Biegel JA. Predisposition to atypical teratoid/rhabdoid tumor due to an inherited INI1 mutation. Pediatr Blood Cancer. 2006;47:279–284. doi: 10.1002/pbc.20622. [DOI] [PubMed] [Google Scholar]
- 54.Judkins AR, Burger PC, Hamilton RL, Kleinschmidt-DeMasters B, Perry A, Pomeroy SL, Rosenblum MK, Yachnis AT, Zhou H, Rorke LB, Biegel JA. INI1 protein expression distinguishes atypical teratoid/rhabdoid tumor from choroid plexus carcinoma. J Neuropathol Exp Neurol. 2005;64:391–397. doi: 10.1093/jnen/64.5.391. [DOI] [PubMed] [Google Scholar]
- 55.Judkins AR, Eberhart CG, Wesseling P. Atypical teratoid/rhabdoid tumour. In: Louis DN, Ohgaki H, Wiestler O, Cavenee WK, editors. World Health Organization classification of tumors. Pathology and genetics of tumours of the nervous system. IARC Press; Lyon: 2007. [Google Scholar]
- 56.Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28:644–650. doi: 10.1097/00000478-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 58.Koch A, Waha A, Tonn JC, Sorensen N, Berthold F, Wolter M, Reifenberger J, Hartmann W, Friedl W, Reifenberger G, Wiestler OD, Pietsch T. Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer. 2001;93:445–449. doi: 10.1002/ijc.1342. [DOI] [PubMed] [Google Scholar]
- 59.Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene. 2010;29:3017–3024. doi: 10.1038/onc.2010.32. [DOI] [PubMed] [Google Scholar]
- 60.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsic A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kordes U, Gesk S, Fruhwald MC, Graf N, Leuschner I, Hasselblatt M, Jeibmann A, Oyen F, Peters O, Pietsch T, Siebert R, Schneppenheim R. Clinical and molecular features in patients with atypical teratoid rhabdoid tumor or malignant rhabdoid tumor. Genes Chromosomes Cancer. 2010;49:176–181. doi: 10.1002/gcc.20729. [DOI] [PubMed] [Google Scholar]
- 62.Korshunov A, Benner A, Remke M, Lichter P, von Deimling A, Pfister S. Accumulation of genomic aberrations during clinical progression of medulloblastoma. Acta Neuropathol. 2008;116:383–390. doi: 10.1007/s00401-008-0422-y. [DOI] [PubMed] [Google Scholar]
- 63.Korshunov A, Remke M, Gessi M, Ryzhova M, Witt H, Tobias V, Buccoliero A, Gardiman M, Bonnin J, Scheithauer B, Kulozik A, Witt O, Mork S, von Deimling A, Giangaspero F, Rosenblum M, Pietsch T, Lichter P, Pfister S. Focal genomic amplification at 19q13.42 comprises a diagnostic marker for embryonal tumors with ependymoblastic rosettes. Acta Neuropathol. 2010;120:253–260. doi: 10.1007/s00401-010-0688-8. [DOI] [PubMed] [Google Scholar]
- 64.Korshunov A, Remke M, Werft W, Benner A, Ryzhova M, Witt H, Sturm D, Wittmann A, Schöttler A, Felsberg J, Reifenberger G, Rutkowski S, Scheurlen W, Kulozik A, von Deimling A, Lichter P, Pfister S. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28:3054–3060. doi: 10.1200/JCO.2009.25.7121. [DOI] [PubMed] [Google Scholar]
- 65.Lamont JM, McManamy CS, Pearson AD, Clifford SC, Ellison DW. Combined histopathological and molecular cytogenetic stratification of medulloblastoma patients. Clin Cancer Res. 2004;10:5482–5493. doi: 10.1158/1078-0432.CCR-03-0721. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Lee KF, Lu Y, Clarke I, Shih D, Eberhart C, Collins VP, Van Meter T, Picard D, Zhou L, Boutros PC, Modena P, Liang M-L, Scherer SW, Bouffet E, Rutka JT, Pomeroy SL, Lau CC, Taylor MD, Gajjar A, Dirks PB, Hawkins CE, Huang A. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell. 2009;16:533–546. doi: 10.1016/j.ccr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis D, Ohgaki H, Wiestler O, Cavenee W, Burger P, Jouvet A, Scheithauer B, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, Packer RJ, Cogen P, Stephan DA. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29:143–152. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- 69.McCabe M, Ichimura K, Liu L, Plant K, Backlund L, Pearson D, Collins V. High-resolution array-based comparative genomic hybridization of medulloblastomas and supratentorial primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 2006;65:549–561. doi: 10.1097/00005072-200606000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCabe MG, Ichimura K, Pearson DM, Liu L, Clifford SC, Ellison DW, Collins VP. Novel mechanisms of gene disruption at the medulloblastoma isodicentric 17p11 breakpoint. Genes Chromosomes Cancer. 2009;48:121–131. doi: 10.1002/gcc.20625. [DOI] [PubMed] [Google Scholar]
- 71.McManamy CS, Pears J, Weston CL, Hanzely Z, Ironside JW, Taylor RE, Grundy RG, Clifford SC, Ellison DW. Nodule formation and desmoplasia in medulloblastomas—defining the nodular/desmoplastic variant and its biological behavior. Brain Pathol. 2007;17:151–164. doi: 10.1111/j.1750-3639.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendrzyk F, Korshunov A, Toedt G, Schwarz F, Korn B, Joos S, Hochhaus A, Schoch C, Lichter P, Radlwimmer B. Isochromosome breakpoints on 17p in medulloblastoma are flanked by different classes of DNA sequence repeats. Genes Chromosomes Cancer. 2006;45:401–410. doi: 10.1002/gcc.20304. [DOI] [PubMed] [Google Scholar]
- 73.Mendrzyk F, Radlwimmer B, Joos S, Kokocinski F, Benner A, Stange DE, Neben K, Fiegler H, Carter NP, Reifenberger G, Korshunov A, Lichter P. Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J Clin Oncol. 2005;23:8853–8862. doi: 10.1200/JCO.2005.02.8589. [DOI] [PubMed] [Google Scholar]
- 74.Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, Sozzi G. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012–4019. doi: 10.1158/0008-5472.CAN-04-3050. [DOI] [PubMed] [Google Scholar]
- 75.Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, Golanov A, Joos S, Lichter P. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64:3103–3111. doi: 10.1158/0008-5472.can-03-3968. [DOI] [PubMed] [Google Scholar]
- 76.Northcott P, Korshunov A, Witt H, Hielscher T, Eberhart C, Mack S, Bouffet E, Clifford S, Hawkins C, French P, Rutka J, Pfister S, Taylor M. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.27.4324. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Northcott PA, Fernandez LA, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, Kenney AM, Taylor MD. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra YS, Zilberberg K, McLeod J, Scherer SW, Sunil Rao J, Eberhart CG, Grajkowska W, Gillespie Y, Lach B, Grundy R, Pollack IF, Hamilton RL, Van Meter T, Carlotti CG, Boop F, Bigner D, Gilbertson RJ, Rutka JT, Taylor MD. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–472. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padovani L, Sunyach M-P, Perol D, Mercier C, Alapetite C, Haie-Meder C, Hoffstetter S, Muracciole X, Kerr C, Wagner J-P, Lagrange J-L, Maire J-P, Cowen D, Frappaz D, Carrie C. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68:433–440. doi: 10.1016/j.ijrobp.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 80.Patil S, Perry A, Maccollin M, Dong S, Betensky RA, Yeh TH, Gutmann DH, Stemmer-Rachamimov AO. Immunohistochemical analysis supports a role for INI1/SMARCB1 in hereditary forms of schwannomas, but not in solitary, sporadic schwannomas. Brain Pathol. 2008;18:517–519. doi: 10.1111/j.1750-3639.2008.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paulus W, Kleihues P. Genetic profiling of CNS tumors extends histological classification. Acta Neuropathol. 2010;120:269–270. doi: 10.1007/s00401-010-0710-1. [DOI] [PubMed] [Google Scholar]
- 82.Perry A, Fuller CE, Judkins AR, Dehner LP, Biegel JA. INI1 expression is retained in composite rhabdoid tumors, including rhabdoid meningiomas. Mod Pathol. 2005;18:951–958. doi: 10.1038/modpathol.3800375. [DOI] [PubMed] [Google Scholar]
- 83.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, Kulozik A, Reifenberger G, Rutkowski S, Wiestler OD, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 84.Pfister S, Remke M, Castoldi M, Bai A, Muckenthaler M, Kulozik A, von Deimling A, Pscherer A, Lichter P, Korshunov A. Novel genomic amplification targeting the microRNA cluster at 19q13.42 in a pediatric embryonal tumor with abundant neuropil and true rosettes. Acta Neuropathol. 2009;117:457–464. doi: 10.1007/s00401-008-0467-y. [DOI] [PubMed] [Google Scholar]
- 85.Pfister S, Remke M, Toedt G, Werft W, Benner A, Mendrzyk F, Wittmann A, Devens F, von Hoff K, Rutkowski S, Kulozik A, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Supratentorial primitive neuroectodermal tumors of the central nervous system frequently harbor deletions of the CDKN2A locus and other genomic aberrations distinct from medulloblastomas. Genes Chromosomes Cancer. 2007;46:839–851. doi: 10.1002/gcc.20471. [DOI] [PubMed] [Google Scholar]
- 86.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 87.Reardon DA, Jenkins JJ, Sublett JE, Burger PC, Kun LK. Multiple genomic alterations including N-myc amplification in a primary large cell medulloblastoma. Pediatr Neurosurg. 2000;32:187–191. doi: 10.1159/000028932. [DOI] [PubMed] [Google Scholar]
- 88.Rickert C, Hasselblatt M. Cytogenetic features of ependymoblastomas. Acta Neuropathol. 2006;111:559–562. doi: 10.1007/s00401-006-0074-8. [DOI] [PubMed] [Google Scholar]
- 89.Roberts CW, Biegel JA. The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther. 2009;8:412–416. doi: 10.4161/cbt.8.5.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts CW, Orkin SH. The SWI/SNF complex—chromatin and cancer. Nat Rev Cancer. 2004;4:133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 91.Rogers HA, Miller S, Lowe J, Brundler MA, Coyle B, Grundy RG. An investigation of WNT pathway activation and association with survival in central nervous system primitive neuroectodermal tumours (CNS PNET). Br J Cancer. 2009;100:1292–1302. doi: 10.1038/sj.bjc.6604979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russo C, Pellarin M, Tingby O, Bollen A, Lamborn K, Mohapatra G, Collins V, Feuerstein B. Comparative genomic hybridization in patients with supratentorial and infratentorial primitive neuroectodermal tumors. Cancer. 1999;86:331–339. doi: 10.1002/(sici)1097-0142(19990715)86:2<331::aid-cncr18>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 94.Scheurlen WG, Schwabe GC, Joos S, Mollenhauer J, Sorensen N, Kuhl J. Molecular analysis of childhood primitive neuroectodermal tumors defines markers associated with poor outcome. J Clin Oncol. 1998;16:2478–2485. doi: 10.1200/JCO.1998.16.7.2478. [DOI] [PubMed] [Google Scholar]
- 95.Schneppenheim R, Fruhwald MC, Gesk S, Hasselblatt M, Jeibmann A, Kordes U, Kreuz M, Leuschner I, Martin Subero JI, Obser T, Oyen F, Vater I, Siebert R. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86:279–284. doi: 10.1016/j.ajhg.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG. c-Myc overexpression causes anaplasia in medulloblastoma. Cancer Res. 2006;66:673–681. doi: 10.1158/0008-5472.CAN-05-1580. [DOI] [PubMed] [Google Scholar]
- 97.Swartling FJ, Grimmer MR, Hackett CS, Northcott PA, Fan QW, Goldenberg DD, Lau J, Masic S, Nguyen K, Yakovenko S, Zhe XN, Gilmer HC, Collins R, Nagaoka M, Phillips JJ, Jenkins RB, Tihan T, Vandenberg SR, James CD, Tanaka K, Taylor MD, Weiss WA, Chesler L. Pleiotropic role for MYCN in medulloblastoma. Genes Dev. 2010;24:1059–1072. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tabori U, Baskin B, Shago M, Alon N, Taylor MD, Ray PN, Bouffet E, Malkin D, Hawkins C. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28:1345–1350. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
- 99.Tabori U, Sung L, Hukin J, Laperriere N, Crooks B, Carret AS, Silva M, Odame I, Mpofu C, Strother D, Wilson B, Samson Y, Bouffet E. Distinctive clinical course and pattern of relapse in adolescents with medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;64:402–407. doi: 10.1016/j.ijrobp.2005.07.962. [DOI] [PubMed] [Google Scholar]
- 100.Takei H, Nguyen Y, Mehta V, Chintagumpala M, Dauser RC, Adesina AM. Low-level copy gain versus amplification of myc oncogenes in medulloblastoma: utility in predicting prognosis and survival. Laboratory investigation. J Neurosurg Pediatr. 2009;3:61–65. doi: 10.3171/2008.10.PEDS08105. [DOI] [PubMed] [Google Scholar]
- 101.Taylor MD, Gokgoz N, Andrulis IL, Mainprize TG, Drake JM, Rutka JT. Familial posterior fossa brain tumors of infancy secondary to germline mutation of the hSNF5 gene. Am J Hum Genet. 2000;66:1403–1406. doi: 10.1086/302833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 103.Taylor MD, Mainprize TG, Rutka JT. Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: a review. Neurosurgery. 2000;47:888–901. doi: 10.1097/00006123-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 104.Thomas WD, Chen J, Gao YR, Cheung B, Koach J, Sekyere E, Norris MD, Haber M, Ellis T, Wainwright B, Marshall GM. Patched1 deletion increases N-Myc protein stability as a mechanism of medulloblastoma initiation and progression. Oncogene. 2009;28:1605–1615. doi: 10.1038/onc.2009.3. [DOI] [PubMed] [Google Scholar]
- 105.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 106.Tomlinson FH, Jenkins RB, Scheithauer BW, Keelan PA, Ritland S, Parisi JE, Cunningham J, Olsen KD. Aggressive medulloblastoma with high-level N-myc amplification. Mayo Clin Proc. 1994;69:359–365. doi: 10.1016/s0025-6196(12)62221-6. [DOI] [PubMed] [Google Scholar]
- 107.Unden AB, Holmberg E, Lundh-Rozell B, Stahle-Backdahl M, Zaphiropoulos PG, Toftgard R, Vorechovsky I. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: different in vivo mechanisms of PTCH inactivation. Cancer Res. 1996;56:4562–4565. [PubMed] [Google Scholar]
- 108.Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 109.von Hoff K, Hartmann W, von Bueren AO, Gerber NU, Grotzer MA, Pietsch T, Rutkowski S. Large cell/anaplastic medulloblastoma: outcome according to myc status, histopathological, and clinical risk factors. Pediatr Blood Cancer. 2010;54:369–376. doi: 10.1002/pbc.22339. [DOI] [PubMed] [Google Scholar]
- 110.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 111.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–516. [PubMed] [Google Scholar]
- 112.Wrede B, Hasselblatt M, Peters O, Thall PF, Kutluk T, Moghrabi A, Mahajan A, Rutkowski S, Diez B, Wang X, Pietsch T, Kortmann RD, Paulus W, Jeibmann A, Wolff JE. Atypical choroid plexus papilloma: clinical experience in the CPT-SIOP-2000 study. J Neurooncol. 2009;95:383–392. doi: 10.1007/s11060-009-9936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, Bazan JF, Kan Z, Seshagiri S, Hann CL, Gould SE, Low JA, Rudin CM, de Sauvage FJ. Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ. metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 Randomized Phase III Study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 115.Zurawel RH, Allen C, Chiappa S, Cato W, Biegel J, Cogen P, de Sauvage F, Raffel C. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27:44–51. doi: 10.1002/(sici)1098-2264(200001)27:1<44::aid-gcc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 116.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–899. [PubMed] [Google Scholar]