Abstract
Effective obesity prevention and treatment interventions targeting children and their families are needed to help curb the obesity epidemic. Pediatric primary care is a promising setting for these interventions, and a growing number of studies are set in this context. This review aims to identify randomized controlled trials of pediatric primary care-based obesity interventions. A literature search of 3 databases retrieved 2947 publications, of which 2899 publications were excluded after abstract (n=2722) and full-text review (n=177). Forty-eight publications, representing 31 studies, were included in the review. Eight studies demonstrated a significant intervention effect on child weight outcomes (e.g., BMI z-score, weight-for-length percentile). Effective interventions were mainly treatment interventions, and tended to focus on multiple behaviors, contain weight management components, and include monitoring of weight-related behaviors (e.g., dietary intake, physical activity, or sedentary behaviors). Overall, results demonstrate modest support for the efficacy of obesity treatment interventions set in primary care.
Keywords: Childhood obesity, Prevention, Treatment, Primary care
Introduction
As obesity has emerged as a significant public health concern across the globe, the importance of early prevention and treatment cannot be overstated. Overweight and obesity in childhood tends to track into adulthood, with overweight and obese children at a greater risk for obesity in adulthood [1, 2]. Health conditions associated with obesity, such as type 2 diabetes [3] and hypertension [4], can emerge in childhood. Furthermore, there is increasing evidence that childhood adiposity is associated with poor health outcomes in adulthood [5, 6].
Pediatric primary care is a promising setting for behavioral obesity prevention and treatment interventions. Despite differences in the organization and delivery of primary care services around the globe [7–9], pediatric primary care is regarded as an important setting for obesity treatment and prevention efforts [10]. Clinical guidelines and recommendations for pediatric primary care providers have been issued by leading health organizations and expert committees in the USA [11] and internationally, including in Australia [12], Canada [13], and the UK [14]. Primary care settings provide high access to both children and their primary caregivers, given that large numbers of children in the USA and in many industrialized countries are seen in primary care settings. Primary care providers are trusted sources of health information, and interventions can build off of the existing provider relationship with the family. Additionally, primary care providers can link children and families to community resources that provide further support for building and maintaining healthy weight-related behaviors. Despite the fact that primary care is an appealing context for both prevention and treatment interventions, it has been a less frequently adopted setting for obesity interventions. A smaller number of obesity interventions have been conducted in health care settings and have primarily been treatment interventions carried out in specialty care settings.
The advantages of conducting obesity prevention and treatment interventions in primary care are counterbalanced by several major challenges. There are time and space constraints associated with conducting interventions in primary care settings (e.g., availability of clinic rooms, short clinic appointments), and making extra trips to the primary care clinic may be burdensome to families and may create participation barriers. Primary care providers across multiple countries cite time constraints as limiting their implementation of obesity prevention and treatment activities [15–17]. In the USA, there are additional barriers related to the relatively high cost of primary care providers’ services and poor reimbursement for provider activities related to obesity monitoring, prevention, and treatment services. Beyond logistical and cost barriers associated with conducting obesity interventions in primary care settings, primary care providers in the USA, Canada, Europe, Australia, and elsewhere have described barriers related to primary care provider training, knowledge and skills, and attitudes about obesity prevention and treatment [17–26]. While recent studies suggest increased provider comfort in screening and counseling for obesity [27, 28], rates of obesity prevention and treatment activities in primary care remain low in many countries, including the USA Israel, Australia, and several European countries [25, 29, 30•, 31–34]. Furthermore, primary care providers have expressed reservations about the effectiveness of provider-delivered obesity prevention and treatment strategies, citing concerns regarding the obesogenic environment, lack of parent motivation to make weight-related behavioral changes for themselves and their family, and low parent concern about child weight [16, 20, 22, 24, 27, 32, 34–36].
Although few behavioral obesity prevention and treatment interventions have been set in primary care, relative to other settings, a growing number of studies have been published that test the efficacy of primary care-based obesity interventions. These studies provide valuable findings on the efficacy of interventions set in primary care, as well as insight into strategies to minimize the barriers of conducting research in this setting and maximize the advantages. Sargent, Pilotto, and Baur’s [37] 2011 systematic review identified 17 obesity treatment interventions set in primary care, 12 of which demonstrated a significant intervention effect on child weight-related outcomes, including body mass index (BMI), dietary intake, and physical activity level. Of the 12 effective interventions identified in this review, 7 were randomized controlled trials (58.3 %). The current review builds upon this prior review article by examining both prevention and treatment intervention, limiting included studies to only randomized controlled trials, and focusing on the impact of interventions on child weight outcomes.
This review paper aims to identify randomized controlled trials (RCTs) focused on obesity treatment or prevention conducted in a primary care setting and to 1) describe the characteristics (e.g., sample, intervention participation, and retention) of behavioral pediatric obesity prevention and treatment interventions set in primary care; 2) assess the efficacy of behavioral pediatric obesity prevention and treatment interventions conducted in primary care; and 3) discuss the implications of these findings for future directions in obesity treatment and prevention in primary care.
Methods
A comprehensive search of PubMed (Web based), Cumulative Index to Nursing and Allied Health Literature (CINAHL— EBSCO platform), and PsycINFO (Ovid platform) databases was performed to identify original RCTs or intervention studies on pediatric/childhood obesity in English. Relevant systematic reviews and meta-analyses were also reviewed for background information but not included in this study. Dissertations, books, book chapters, and conference proceedings were excluded.
In PubMed, the Medical Subject Headings (MeSH) terms defined the concepts of obesity, overweight, or body mass index; children, childhood, adolescents, or pediatric; and RCTs or intervention studies. The intervention studies search set was further refined with primary health care terms to eliminate intervention studies done in specialty care, schools, or research settings. For optimal retrieval with all terms, medical subject headings were supplemented with relevant title and text words. Search parameters are available on request.
The search strategies for CINAHL and PsycINFO were adjusted for the syntax appropriate for each database using a combination of thesauri and text/title words. Published reports in the peer-reviewed literature from 1990 to Oct 2013 were identified and retrieved. Bibliographies from identified review articles, meta-analyses, and key original articles were also scanned for potentially relevant papers to include in this study.
Studies included in the review were selected using the following inclusion criteria: 1) randomized controlled trial; 2) completed pilot or full trial study; 3) behavioral obesity prevention and/or treatment interventions; 4) intervention delivered in or connected to primary care setting; 5) health care provider participates in intervention; 6) intervention for children between the ages of 0 and 18 and/or parents of children in this age range; 7) outcome measures reported include child weight outcome (e.g., BMI, weight for height); and 8) article available in English and published after 1990. For the purpose of this review, studies with published outcomes were considered complete. Studies were excluded if published intervention descriptions did not include specific information about the intervention setting or the role of the health care provider in intervention delivery. Studies in which health care providers participated only in study recruitment were excluded. Studies with an active control condition set in primary care were included, regardless of the intervention condition setting. Active control conditions were defined as conditions providing an alternative intervention with one or more components prescribed by the study.
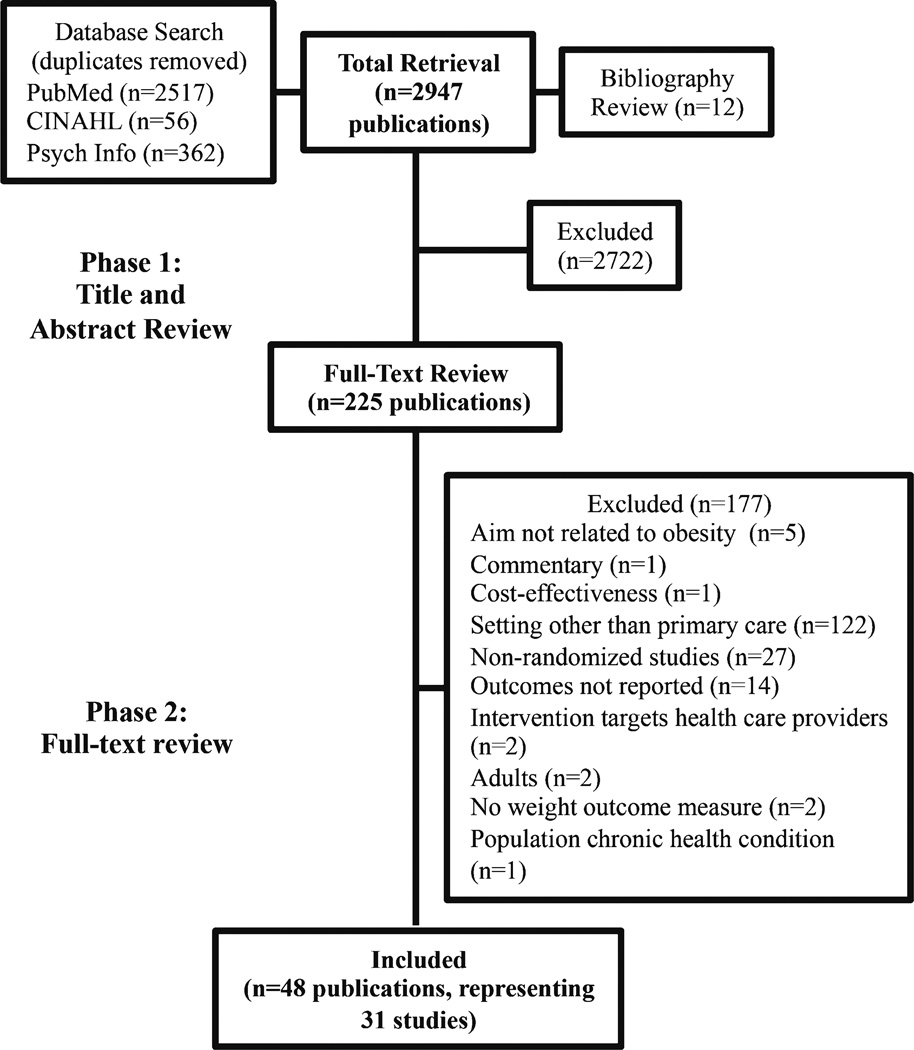
Figure 1 displays the flow of articles through the search process. The database search and bibliography review yielded 2947 publications after removal of duplicates. The first author (EMS) reviewed the title and abstract for all publications and excluded 2722 publications. Common exclusion reasons were a setting other than primary care, surgical or pharmacological intervention, or adult population. Four reviewers conducted a full-text review of the 225 potentially eligible publications. Each publication was independently reviewed by two authors and classified as eligible or ineligible. Inclusion decisions were compared for each publication, and coding disagreements were discussed by all authors to determine final inclusion in this review. Forty-eight publications, representing 31 studies, met our inclusion criteria and were included in this systematic review.
Fig. 1.
Selection process. This figure illustrates the selection process for publications included in this review
Data extraction was completed independently by four reviewers. Two reviewers performed a cross-check of data extraction to assure accuracy and completeness of data. For studies with multiple publications, all publications identified in the review were used for data extraction. Data were extracted for all study conditions that included primary care involvement. Intervention effectiveness was evaluated, and interventions were considered effective if there was a significant difference in child weight outcomes (e.g., BMI z-score, weight-for-length percentile) between study groups. Measures of child body composition (e.g., waist circumference) were not considered in the assessment of intervention effect. Additionally, interventions were not classified as effective if a significant intervention effect was observed only for a participant subgroup (e.g., boys vs. girls). To aid in interpretation, studies were classified by participant age group (e.g., infant, preschool age, elementary school age, and adolescent) and by prevention or treatment focus. Study samples that spanned multiple age groups were categorized by the mean age of participants at baseline.
Results
Summary of Study Characteristics
Appendix 1 provides information on the study characteristics of the 31 studies included in this review. The majority of studies were conducted in the USA (n=17). Of the international studies, studies were conducted in Australia (n=4), Belarus (n=1), Canada (n=1), Finland (n=1), Germany (n=1), Israel (n=1), Italy (n=1), Mexico (n=1), Sweden (n=1), the Netherlands (n=1), and the UK (n=1). By review design, all studies were RCTs, the majority of the trials were individually randomized trials (n=25), and six were cluster-randomized trials. One study included three non-randomly allocated comparison groups, in addition to two randomized intervention conditions [38]. Studies were primarily treatment studies targeting children who were overweight or obese (n=24). Five studies targeted infants; 4 studies were conducted with preschool-age children; 18 studies focused on elementary school-age children; and 4 studies were directed toward adolescents. All studies included a child weight outcome, and this was a primary outcome for most studies (n=27). Follow-up duration ranged from 3 months to 20 years, with most follow-up periods lasting 1 year or less (n= 23). While the majority of studies had relatively short follow-up periods, a small number followed participants for 5 years or longer (n=3). Study retention rates ranged from 52 to 100 %, and the average retention rate was 77.5 %.
Summary of Intervention Features
Table 1 displays study intervention features, grouped by child age group and prevention or treatment focus. Twelve studies had active control conditions in primary care settings, three of which did not have primary care involvement in the intervention condition [69, 71, 88]. Five studies targeted parents only and 26 studies targeted both parents and children. All studies had at least one study component that was delivered in person to participants in individual sessions. Eight studies included in-person, group intervention activities. Eight studies included a phone component. Fourteen studies had intervention components delivered across two or more modalities. For example, Taveras et al. [58] included in-person clinician visits, clinician telephone calls, and changes to the primary care system. In another study [61], intervention components were group sessions delivered by a team of health care providers (primary care provider, health educator, nutritionist, and physical therapist) and individual coaching sessions with a health coach conducted in person or by telephone.
Table 1.
Intervention characteristics by participant age and type of study (prevention or treatment)
| Author, year | Condition | Target |
Modality |
Provider type |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parent | Child | Individual, phone |
Individual, in-person |
Group, in person |
MD, NP, PA | Nurse | Dietitian/ nutritionist |
Psychologist | ||
| Infant, prevention (n=5) | ||||||||||
| French, 2012 [39, 40] | I1 and I2 | x | x | x | x | |||||
| Hakanen, 2010 [41–47] | I | x | x | x | x | x | ||||
| Martin, 2013 [48, 49] | I | x | x | x | x | |||||
| Paul, 2011 [50, 51] | I | x | x | x | ||||||
| Wake, 2011 [52–54] | I | x | x | x | ||||||
| Preschool, prevention (n=1) | ||||||||||
| Birken, 2012 [55] | I | x | x | x | x | x | ||||
| Preschool, treatment (n=3) | ||||||||||
| Quattrin, 2012 [56] | I | x | x | x | x | x | x | |||
| AC | x | x | x | x | x | x | ||||
| Stark, 2011 [57] | I | x | x | x | x | x | ||||
| AC | x | x | x | x | ||||||
| Taveras, 2011 [58–60] | I | x | x | x | x | x | ||||
| Elementary, treatment (n=18) | ||||||||||
| Arauz Boudreau, 2013 [61] | I | x | x | xb | xb | x | x | x | ||
| Banks, 2011c [62–65] | I | x | x | x | x | x | ||||
| Barkin, 2011 [66–68] | I | x | x | x | x | x | ||||
| AC | x | x | x | x | x | |||||
| Davis, 2013 [69, 70] | AC | x | x | x | x | |||||
| Davis, 2011 [71] | AC | x | x | x | x | |||||
| Diaz, 2010c [72] | I | x | x | x | x | x | x | |||
| AC | x | x | x | x | ||||||
| Duggins, 2010c [73] | I | x | x | x | x | x | x | |||
| AC | x | x | x | x | x | x | ||||
| Gillis, 2007c [74] | I | x | x | x | x | x | ||||
| AC | x | x | x | x | ||||||
| Mårild, 2013 [38] | I1 | x | x | x | x | x | x | |||
| I2 | x | x | x | x | x | x | ||||
| McCallum, 2007 [75–78] | I | x | x | x | x | |||||
| Nova, 2001c [79] | I | x | x | x | x | |||||
| O’Connor, 2011 [80, 81] | I | x | x | x | ||||||
| Small, 2014c [82] | I | x | x | x | x | |||||
| van Grieken, 2013 [83, 84] | I | x | x | x | x | |||||
| Wake, 2009 [85] | I | x | x | x | x | |||||
| Wake, 2013 [86, 87] | I | x | x | x | x | x | ||||
| Weigel, 2008c [88] | AC | x | x | x | x | |||||
| Wright, 2013 [89] | I | x | x | x | x | x | ||||
| Adolescent, prevention (n=1) | ||||||||||
| Patrick, 2006 [90] | I | x | x | x | x | x | ||||
| Adolescent, treatment (n=3) | ||||||||||
| DeBar, 2012 [91] | I | x | x | x | x | x | x | |||
| AC | x | x | x | x | ||||||
| MacDonell, 2012 [92] | I | x | x | x | x | |||||
| AC | x | x | x | x | ||||||
| Saelens, 2002 [93] | I | x | x | x | x | x | ||||
| AC | x | x | x | x | ||||||
| Author, year | Condition | Provider type | Intervention participation | Intervention dose (length and contacts) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PT/exercise processional |
Health coach/ counselor |
Medical assistant |
IVR | ≤3 months | 4 months– 1 year |
>1 year | Contacts | |||
| Infant, prevention (n=5) | ||||||||||
| French, 2012 [39, 40] | I1 and I2 | x | Unknown | x | 5 | |||||
| Hakanen, 2010 [41–47] | I | Unclear | x | 46–53a | ||||||
| Martin, 2013 [48, 49] | I | Unknown | x | Unknown | ||||||
| Paul, 2011 [50, 51] | I | Unknown | x | 2 | ||||||
| Wake, 2011 [52–54] | I | 57.5 % | x | 1–3 | ||||||
| Preschool, prevention (n=3) | ||||||||||
| Birken, 2012 [55] | I | 100 % | x | 1 | ||||||
| Preschool, treatment (n=3) | ||||||||||
| Quattrin, 2012 [56] | I | x | 88.5 % | x | 20 | |||||
| AC | x | 94.3 % | 20 | |||||||
| Stark, 2011 [57] | I | x | Unknown | x | 18 | |||||
| AC | Unknown | 1 | ||||||||
| Taveras, 2011 [58–60] | I | 56 %≥2 sess. | x | Year 1, 7; year 2, unknown | ||||||
| Elementary, treatment (n=18) | ||||||||||
| Arauz Boudreau, 2013 [61] |
I | x | x | 100 %≥1 sess.; sess. attendance, M= 78 % | x | 12 | ||||
| Banks, 2011c [62–65] | I | x | Did not attend rated=22 % | x | 5 | |||||
| Barkin, 2011 [66–68] | I | x | Unknown | x | 6 | |||||
| AC | x | Unknown | x | 2 | ||||||
| Davis, 2013 [69, 70] | AC | 96.3 % | x | 1 | ||||||
| Davis, 2011 [71] | AC | 100 % | x | 1 | ||||||
| Diaz, 2010c [72] | I | Child sess., M=9.3; parent sess., M=4.0 | x | 45 | ||||||
| AC | sess., M=4.7 | x | 12 | |||||||
| Duggins, 2010c [73] | I | x | 67 %≥1 nutrition sess.; nutrition sess., Mdn=3; YMCA sess., Mdn=5 |
x | 11 + 1 year YMCA membership |
|||||
| AC | 30 %≥1 nutrition sess.; nutrition sess., Mdn=2 | x | 10 | |||||||
| Gillis, 2007c [74] | I | Unknown | x | Unclear | ||||||
| AC | Unknown | x | 2 | |||||||
| Mårild, 2013 [38] | I1 | x | Unknown | x | 12 | |||||
| I2 | Unknown | x | 12 | |||||||
| McCallum, 2007 [75–78] | I | 41 % 4 sess., 21 % 3 sess., 17 % 2 sess., 14 % 1 sess. |
x | 4 | ||||||
| Nova, 2001c [79] | I | 12-month sess., 69.4 % | x | 10 | ||||||
| O’Connor, 2011 [80, 81] | I | x | Sess., M=4.7c 80 %≥4 sess. | x | 12 | |||||
| Small, 2014c [82] | I | x | Unknown | x | 7 | |||||
| van Grieken, 2013[83, 84] | I | x | 100 % sess. 1, 76.7 % sess. 2, 53.9 % sess. 3, 30.6 % all sess. |
x | 1–4 | |||||
| Wake, 2009 [85] | I | 37 % 4 sess., 22 % 3 sess., 21 % 2 sess., 12 % 1 sess., sess., M=2.7 |
x | 4 | ||||||
| Wake, 2013 [86, 87] | I | Sess., M= 2.4 | x | 12 | ||||||
| Weigel, 2008° [88] | AC | Unknown | x | 2 | ||||||
| Wright, 2013 [89] | I | x | Child, 81 %≥ 1 sess., child sess., M=9.0; parent, 76 %≥1 sess.; parent sess., M=9.1 |
x | 12 | |||||
| Adolescent, prevention (n= 1) | ||||||||||
| Patrick, 2006 [90] | I | x | 36 % 0–8 call, 64 % 9–11 call | x | 12 | |||||
| Adolescent, treatment (n=3) | ||||||||||
|
DeBar, 2012 [91] |
I | x | x | Teen sess., M=10.3; parent sess., M=7.9 | x | 18 (teens) 12 (parents) | ||||
| AC | 100 % | x | 1 | |||||||
| MacDonell, 2012 [92] |
I | 27 % all sess., 68 %≥2 sess. | x | 4 | ||||||
| AC | 36 % all sess., 82 %≥2 sess. | x | 4 | |||||||
| Saelens, 2002 [93] | I | x | 70 %≥9 call; calls, Mdn=9 100 % physician visit |
x | 13 | |||||
| AC | x | 100 % physician visit | x | 1 | ||||||
Studies in italics found significant effect on measure of child weight
I intervention, C no treatment control, AC active control receiving alternative intervention, IVR interactive voice technology, PT physical therapist, sess sessions, M mean, Mdn median
Children ages 7 months to 2 years: intervention sessions every 1–3 months. Children ages 2 to 20 years: biannual intervention sessions
Individual sessions completed by phone or in person
Mean age of participants between the ages of 5 and 12 years
Did not attend rate=total did not attend/total sessions
Authors considered the six in-person visits as sessions; six follow-up phone calls were not counted as sessions
Information regarding intervention intensity and delivery is also included in Table 1. Intervention intensity varied, ranging from brief, low-intensity interventions, such as one 10-min intervention session [55], to moderately intensive interventions involving regular intervention contacts over a period of time, like a 12-month program that involved monthly physician sessions, 12 weekly nutrition sessions followed by monthly nutrition sessions, and 6 parent education sessions [72]. Health care provider involvement in interventions varied, in terms of the type of provider and their role in the intervention. Seventeen studies involved more than one type of provider. Most studies had at least some involvement by a primary care physician, nurse practitioner, or physician assistant (n=23), with this type of provider the sole interventionist in eight studies. Other types of providers involved in intervention delivery were health coaches (n=10), nutritionists or dietitians (n=10), nurses (n= 7), exercise professionals (n=5), psychologists (n=1), and medical assistants (n=1). One study used interactive voice technology (IVR) to deliver a telephone counseling program to children and their parents, in conjunction with behavioral counseling from the child’s primary care provider [89].
As shown in Table 1, there was heterogeneity in the reporting of intervention participation data. Ten studies did not provide information about intervention participation. Among those studies that reported intervention participation data, the amount and type of information provided varied considerably, limiting comparisons of intervention dose across studies.
Effective Interventions
Obesity-related study outcomes and study results are summarized in Appendix 1. Eight of the 31 studies identified in this review had significant intervention effects on a child weight outcome. For one study, intervention effect was assessed for the two randomized intervention groups compared to a non-randomly allocated, age-, sex-, and BMI-matched control group [38]. Effective interventions targeted infants (n=1 of 5 studies in this age group), preschool-age children (n= 2 of 4 studies), elementary school-age children (n=3 of 18 studies), and adolescents (n=2 of 4 studies). Among the effective interventions, only one study, SLeeping and Intake Methods Taught to Infant and Mothers Early in life (SLIMTIME) [50], was a prevention study. Five of the seven effective treatment interventions targeted obese youth. These studies had relatively short follow-up periods; seven had a follow-up period of 1 year or less [38, 50, 56, 57, 72, 91, 93] and Nova, Russo, and Sala [79] had a 2-year follow-up, though results were reported for only the 6- and 12-month measurement points. High participant dropout rates were a concern in several studies [50, 72, 79]; however, despite a high dropout rate, Diaz et al. [72] collected primary outcome measures from 87% of the baseline sample. Most studies reported high retention rates (≥83 %) [38, 56, 57, 91, 93].
All studies demonstrating a significant intervention effect included parent-targeted intervention components; however, the two effective interventions conducted with adolescents focused on the adolescent as the agent of change and had a more limited role for parents [91, 93]. All effective interventions targeted multiple weight-related behaviors, and they tended to use multiple approaches and delivery modes. For example, the behavioral weight control intervention for adolescents by Saelens et al. [93] included 1) computer-guided behavior change plan and behavioral assessment for the adolescent; 2) in-person physician visit to discuss the adolescent’s physical activity, nutrition, and sedentary behaviors and their behavior change plan; 3) adolescent and parent session with study PI to learn food self-monitoring; 4) adolescent phone coaching sessions with a study counselor; and 5) informational materials for the adolescent and parent. Another study found that an intervention that was comprised of in-person, clinic-based group education sessions and in-person, home-based individual sessions was more effective than a single intervention session with a pediatrician [57].
Of the eight studies that demonstrated a significant intervention effect, six included daily child calorie goals or dietary plans [56, 57, 72, 79, 91, 93] and six had physical activity goals or plans [38, 56, 57, 72, 91, 93]. Five interventions also incorporated regular assessment of child weight, either at intervention sessions [57, 72, 91] or through self- or parent-weighing [56,93]. Three effective interventions, two targeting preschoolers [56, 57] and one directed at elementary school age children [72], had intervention components targeting parent weight or weight-related behaviors.
Discussion
This review paper identified primary care-based obesity prevention and treatment interventions and assessed the efficacy of these interventions on child weight outcomes. Our search yielded 31 RCTs set in primary care, 8 of which demonstrated a significant effect on a child weight outcome. We found modest evidence supporting the efficacy of treatment interventions conducted in primary care settings. There was little evidence demonstrating the efficacy of prevention interventions set in this context, though given the small number of prevention studies identified, it is clear that further research is needed before drawing conclusions on the efficacy of obesity prevention in primary care.
Characteristics of Effective Interventions
A common theme across effective interventions was an explicit intervention focus on weight management and/or regular monitoring of weight and weight-related behaviors, such as dietary intake and physical activity. For example, an obesity treatment study for adolescents incorporated self-monitoring activities, such as weekly weighing and calorie intake, and focused on decreasing calorie intake and increasing physical activity to meet individualized calorie and physical activity goals [93]. Another obesity treatment study targeting adolescent girls placed less emphasis on weight and calorie tracking, but included weight measurements at each intervention session, self-monitoring of dietary intake and physical activity, and guidelines related to daily calorie intake, physical activity, and screen time [91].
The two effective interventions targeting preschool-age children included parent behavior change targets and weight loss goals [56, 57]. Both studies emphasized parent modeling of healthy behaviors, and these interventions included physical activity and calorie goals for the parent and child, as well as parent monitoring of their own weight, dietary intake, and physical activity level. Stark et al. [57] also included parent monitoring of child and parent sedentary activities. One of the effective interventions for elementary-age children also targeted parent weight loss, but no specific intervention components directed at parent weight loss were described [72].
Effective interventions tended to be at least moderately intensive (≥10 intervention sessions), with the exception of the obesity prevention study focused on infants and their parents, which involved a relatively brief intervention of two sessions [50]. For example, one study found that brief pediatrician counseling was not as effective compared to a more intensive, multi-component, and multi-setting intervention in decreasing child BMI [57]. This finding is in line with a prior review article of pediatric obesity treatment interventions, which concluded that greater intervention intensity was associated with greater effectiveness [94].
Challenges Associated with Obesity Prevention
Of the 31 studies identified in our review, only 7 were focused on obesity prevention, of which 1 found a significant effect on child growth, over a relatively short follow-up period [50]. Our findings underscore the challenges associated with preventing childhood obesity, as well as the need for a greater number of RCTs assessing obesity prevention interventions delivered in primary care. It is well established that parents are often inaccurate in their perception of their child’s weight status and risk for obesity [95], which makes it challenging to motivate parents to engage in behavior change, as many do not view their child as at risk for obesity. Strategies to increase the salience of obesity prevention messages for parents and increase motivation for behavior change include using behavioral counseling techniques, such as motivational interviewing, to sensitively work through barriers to behavior change [96]. Another potential strategy is to help parents understand BMI and their child’s own weight status through discussions of BMI trajectories and obesity risk. Further studies are needed to rigorously evaluate strategies to motivate parents to engage in obesity prevention efforts and make changes to weight-related behaviors.
Role for Primary Care Providers in Obesity Interventions
This review demonstrated considerable heterogeneity in the role of primary care providers in obesity interventions set in primary care, as well as in the level of detail reported about the nature of this involvement. In effective interventions, provider contact with participants varied from brief encounters during routine or supplementary clinic visits augmented by other intervention activities [50, 56, 72] to intervention sessions delivered exclusively by the primary care provider [79]. Analysis of interventions with and without a significant intervention effect on child weight outcomes did not yield consistent themes about the optimal role of providers in obesity prevention and treatment interventions. Relatively little is known about the content and process of the provider role in obesity treatment and prevention counseling, which is a limitation of the existing literature. The quality with which primary care providers engage families around these issues could be important to the effectiveness of their efforts. Findings from a pilot study suggest that physician use of motivational interviewing techniques was significantly associated with adolescent weight loss [96], and a full-scale trial is underway to further evaluate these findings [97•]. Despite the limitations of the existing literature, results indicate the feasibility of engaging primary care providers in efforts to prevent and treat childhood obesity. Future research is needed to assess how interventions can optimize primary care provider involvement in such efforts to develop effective and sustainable obesity prevention and treatment strategies that are feasible in primary care settings.
Linking Primary Care to Community Settings and Resources
While more intensive interventions have demonstrated promise in the treatment of pediatric obesity, primary care obesity interventions with greater participant contacts are resource intensive, in terms of provider time, staff time, cost of services, and participant burden. It is critical to find ways to translate these interventions into sustainable models of obesity treatment that are feasible for implementation in routine primary care. One potential strategy is to leverage the primary care provider’s influence and relationship with families by linking primary care interventions to other potential intervention settings (e.g., community based, home based, and phone based) and existing community resources. A recent study by Ariza et al. [98] demonstrated the feasibility of identifying overweight children in pediatric practices and then linking families to existing community-based programs. Linking children and families to community resources is a practice in line with American Academy of Pediatrics recommendations for pediatricians, which highlight the importance of pediatrician community connectedness in the prevention and treatment of public health issues, such as obesity [99, 100].
Two studies in progress are using this approach to childhood obesity prevention and treatment [101, 102]. The Minnesota NET-Works study (Now Everybody Together for Amazing and Healthful Kids) is a multi-component obesity prevention intervention targeting low-income preschool-age children and their families [101], which aims to prevent obesity through a multi-setting intervention that links primary care-, community-, neighborhood-, and home-based intervention strategies. The Stanford GOALS study uses a similar approach in an obesity treatment intervention for preschool-age children and their families [102]. This study links a counseling intervention delivered by primary care providers to home- and community-based intervention activities, such as an after-school sports program and a home-based health education and behavioral counseling. These studies provide a model for linking primary care-based obesity treatment and prevention strategies to other settings, and results will help shed light on the feasibility and effectiveness of this approach.
Tailoring Interventions to the Developmental Needs of Children
This review of interventions directed at children and parents across infancy, childhood, and adolescence underscores the need for obesity prevention and treatment interventions that are targeted to the developmental needs of the child. As children become more independent and make more decisions that can influence weight, it is necessary for interventions to account for these changes and craft intervention strategies tailored for children and families throughout childhood. It is clear that obesity prevention and treatment interventions have moved in this direction, as most studies identified in this review included intervention components tailored to the developmental stage of the child. For example, adolescent-focused studies described a greater emphasis on the adolescent as the agent of change, through strategies such as adolescent-targeted intervention activities and increased focus on adolescent self-management of weight-related behaviors. These intervention strategies align with what is developmentally appropriate for adolescents [103] and recommendations for adolescent health care [94, 104].
Results of this review also identify areas for improvement. Several studies enrolled wide age ranges of children, without sufficient acknowledgement of the different strategies that may be needed to reach children of different ages enrolled in the study, and the changing parenting experiences and role of parents in weight-related behaviors as children age. Future studies should increase their consideration of children’s developmental needs, and one potential avenue for doing this is through the primary care provider. Primary care providers are particularly well equipped to provide this type of individualized intervention tailoring to children and families.
Conclusions
This review of obesity treatment and prevention interventions found modest support for the efficacy of behavioral treatment interventions set in primary care. We identified only a small number of prevention studies, limiting our ability to draw conclusions on the efficacy of prevention efforts in this context. Heterogeneity in the amount and type of information reported about provider involvement was observed. Examination of studies with a significant intervention effect did not reveal any discernable trends in the role and scope of providers. Further research is needed before making recommendations on the optimal role for providers in obesity prevention and treatment interventions. In addition, there is the need for future research on obesity prevention interventions in primary care settings, as this is a gap in the current evidence base.
Acknowledgments
The project was supported by Grant Numbers 1R01DK084475 (PI: Sherwood), P30DK050456 (PI: Levine), and P30DK092924 (PI: Schmittdiel) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Appendix 1
Table 2.
Study characteristics and relevant significant findings by participant age and type of study (prevention or treatment)
| Study author, year | Study design | Study sample | Obesity-related study outcomes | Relevant significant results, comparison of intervention group to control groupa |
Study length | Intervention length |
Retention rate |
|---|---|---|---|---|---|---|---|
| Infant, Prevention (n=5) | |||||||
| French, 2012 [39, 40] | 3 (Condition; I1, I2, UC) × 3 (Time: baseline, 6-, 12-month) cluster RCT |
Sample size: N=292 mother/infant dyads (I1, n=101; I2, n=101; C, n=104); N=3 clinics (I1, n=1; I2, n=1; C, n=1) Sample characteristics: infant≤2 months old; healthy full-term |
Primary: Infant weight for height Secondary: Maternal eating behaviors (breakfast; family meals; location of meals) and maternal feeding behaviors (child intake of milk, fruit, vegetables, and juice; child drinks from cup; and child self feeds) |
12-month: Less juice (intervention 1 vs. control, p<.05; intervention 2 vs. control, p<.05); more fruit (intervention 1 vs. control, p<.05); intervention 2 vs. control, p<.05 and more vegetables (intervention 1 vs. control, p<.05) |
12 months | 12 months | 64% |
| Hakanen, 2010 [41–47] | 2 (Condition: I, C) × Many (20 year study) RCT |
Sample size: N=1062 infants (I, n=540; C, n=522) Sample characteristics: infant 7 months at randomization; no severe illness |
Primary: Serum lipid and lipoprotein concentrations, growth (infancy: weight- for-height; childhood: BMI); nutrient intake; physical activity; NO-induced vasodilatation. Secondary: Parental eating attitudes |
10-year: Greater interest in healthy eating among parents (p<.001) and greater interest in light products among parents (p<.001) Over time (baseline, 13-month, 2-, 3-, 4-, 5 6-, 7-, 8-, 9-, 10-, 11-, 12-, 13-, 14-year): Lower serum total cholesterol (p<.001); lower low-density lipoprotein cholesterol (p<.001); lower fat intake (p<.001); lower saturated fat intake (p<.001) 15-year: Lower diastolic blood pressure (p=0.005); lower proportion with cardiometabolic risk factor cluster (p=0.046) |
Ongoing (20 years) |
Ongoing (20 years) | 52.3 % |
| Martin, 2013 [48, 49] | 2 (Condition: I, C) × 5 (Time: Baseline, 12-month, 6.5 year, 11.5 year, 15.5 year) cluster RCT |
Sample size: N= 17046 mother/infant dyads (I, 8865; C, 8181); N=31 clinics (I, n=16; C, n=15) Sample characteristics: full-term infant (≥37 weeks); birth weight≥2500 g; Apgar score≥5 at 5 minutes; mother planning to breastfeed |
Primary: Gastrointestinal tract infection; breastfeeding duration; exclusivity breastfeeding Secondary: BMI, fat and fat-free mass indices; % body fat; waist circumference, triceps; subscapular skinfold thicknesses; child eating attitudes; maternal adiposity; insulin-like growth factor 1; adiponectin, apolipoprotein A1; glucose; insulin; apolipoprotein B; child blood pressure; maternal blood pressure; child metabolic syndrome |
12-month: Lower risk≥1 gastrointestinal tract infection (adjusted OR, 0.60; 95 % CI, 0.40- 0.91); greater odds any breastfeeding (adjusted OR, 0.47, 95 % CI, 0.32–0.69) 11.5-year: Greater odds BMI≥ 85th percentile (adjusted OR, 1.18; 95 %CI, 1.01–1.39) |
16 years | 12–16 months | 81.4% |
| Paul, 2011 [50, 51] | 4 (Condition: I1 & C, I2 & C, I1 & I2, C) × 4 (Time: Baseline, 3 week, 16 week, 1 year) pilot RCT |
Sample size: N=160 mother/infant dyads (I1 & C, n=38; I2 & C, n=39; I1 & I2, n=2; C, n=41). Sample characteristics: gestational age≥34 weeks; singleton; primiparous mother; English-speaking mother; mother planning to breastfeed and follow-up with University-affiliated primary care provider. |
Primary: Weight-for-length percentile Secondary: Infant sleep (total daily sleep and nocturnal sleep); Maternal feeding behaviors (total daily feeds; nocturnal feeds; introduction of solid foods; and repeated exposure of vegetables) |
12-month: Lower weight-for-length percentile (I1 & I2 vs. other 3 groups, p=.009) |
12 months | 4–6 months | 68.8 % |
| Wake, 2011 [52–54] | 2 (Condition: I, C) × 5 (Time: Baseline, 2-, 4-, 16-month, 5-year) cluster RCT |
Sample size: N=328 children (I, n=174; C, n=154); N=49 centers. Sample characteristics: children with sleep problems, 7–8 months old from a population-based sample of infants recruited at 4 months; ≥ 32 weeks gestation; English-speaking mother. |
Primary: BMI z-score, percentage overweight/obese Secondary: Waist circumference; infant sleep; child sleep |
10-month: Lower odds infant sleep issues (adjusted OR, 0.58; 95 % CI, 0.36–0.94) 12-month: Lower odds infant sleep issues (adjusted OR, 0.50; 95 % CI, 0.31–0.80) 5-year: NS |
5 years | 1–3 sessions | 58.8 % |
| Preschool, Prevention (n=1) | |||||||
| Birken, 2012 [55] | 2 (Condition: I, C) × 2 (Time: Baseline, 1-year) RCT |
Sample size: N=132 (I, n=64; C, n=68). Sample characteristics: children age 3 years and their parents; child receives care at participating primary care practices. |
Primary: Total child screen time previous weekday and weekend day Secondary: BMI; TV in child bedroom; meals in front of TV on the last weekday and weekend day |
12-month: Decrease in weekday meals in front of TV (p=.03) |
12 months | 1 session | 82.5 % |
| Preschool, Treatment (n=3) | |||||||
| Quattrin, 2012 [56] | 2 (Condition: I, AC) X 3 (Time: Baseline, 3-, 6-month) RCT |
Sample size: N=105 (I, n=52; IC, n=53). Sample characteristics: children ages 2–5 years; BMI≥ 85 percentile; 1 parent/guardian with BMI≥27; normal developmental milestones; parent English/Spanish reading level≥5th grade; continue care at current primary care practice. |
Primary: Change in %0BMIb, parents BMI change score Secondary: Child intake of sugared drinks, high energy foods, fruits, and vegetables; child sedentary activities; child physical activity |
6-month: Greater decrease in BMI z-score (p<.001); greater decrease in %0BMI (p<.001) |
6 months | 6 months | 91.4% |
| Stark, 2011 [57] | 2 (Condition: I, AC) X 3 (Time: Baseline, 6-, 12-month) pilot RCT |
Sample size: N=18 (I, n=8; AC, n=10). Sample characteristics: Children ages 2–5 years; BMI≥95th percentile but not> 100 % above =the mean BMI; 1+ parent with BMI≥25; physician approval. |
Primary: BMI z-score; BMI percentile; Parent BMI Secondary: Child average caloric intake; child physical activity; home food environment |
6-month: Greater decrease in BMI z-score (p=.003); Greater decrease in BMI percentile (p=.03); Greater decrease in weight gain (p=.004) 12-month: Greater decrease in BMI z-score (p=.005); Greater decrease in BMI percentile (p=.04); Greater decrease in weight gain (p=.005) |
12 months | 6 months | 88.9 % |
| Taveras, 2011 [58–60] | 2 (Condition: I, UC) X 3 (Time: Baseline, 1-, 2-year) cluster RCT |
Sample size: N=475 (I, n=271; UC, n= 204); N=10 primary care practices. Sample characteristics: Children ages 2.0–6.9 years; BMI≥95th %tile OR 85th%tile<BMI <95th%tile and 1+ parent overweight; parent English-speaking. |
Primary: Change in BMI Secondary: TV viewing behaviors; SSB intake; and fast food intake |
1-year: Greater decrease in media (TV & video) use (p=.01) |
2 years | 2 years | 93.7% |
| Elementary, Treatment (n= 18) | |||||||
| Arauz Boudreau, 2013 [61] |
2 (Condition: I, WC) × 2 (Time: Baseline, 6-month) pilot RCT |
Sample size: N=41 (I, n=23; WC, n=18). Sample characteristics: children ages 9–12 years; BMI≥ 85th percentile; Latino |
Primary: BMI; child health-related quality of life; child metabolic indicators of obesity; child physical activity. |
6-month: NS | 6 months | 6 months | 63.4 % |
| Banks, 2011 [62–65] | 2 (Condition: I, AC) × 2 (Time: Baseline, 12-month) pilot RCT |
Sample size: N=76 children (I, n=45; AC, n=31). Sample characteristics: Children ages 5 to 16 years (mean age: I, 11.5; AC, 11.4); BMI≥98th percentile. |
Primary: Change in BMI SDS (standard deviation scores) Secondary: Treatment adherence |
12-month: NS | 12 months | 12 months | 68.4 % |
| Barkin, 2011 [66–68] | 2 (Condition: I, AC) X 3 (Baseline, 6-month, 12- month) RCT |
Sample size: N=159 (I, n=80; AC, n=79). Sample characteristics: Children ages 8 to 11 years; BMI≥85th percentile; Latino. |
Primary: Change in BMI Secondary: Perceived body image; parent BMI |
6-month: Greater decrease in absolute BMI (AC vs. I, p=0.03) |
6 months | 12 months | 68 %c |
| Davis, 2013 [69, 70] | 2 (Condition: I, AC) × 2 (Time: Baseline, ≈8-month) RCT |
Sample size: N=58 (I, n=31; AC, n=27). Sample characteristics: Children in Kindergarten thru 5th grade; live in rural Kansas; BMI≥85th percentile; English-speaking. |
Primary: BMI z-score Secondary: Child dietary intake; child physical activity (accelerometer data); child mealtime behavior problems |
8-month: NS | ≈ 8 months | 8 months | 72.4 % |
| Davis, 2011 [71] | 2 (Condition: I, AC) × 3 (Time: Baseline, 2-month post- treatment, 12-month post treatment) pilot RCT |
Sample size: N=17 families (I, n not reported; AC, n not reported). Sample characteristics: Children in 5th grade; BMI≥85th %ile; no major developmental problems. |
Primary: BMI percentile; child physical activity; child eating behaviors |
Post-intervention: NS 12-month post-intervention: NS |
≈ 14 months | 2 months | 100 % |
| Diaz, 2010 [72] | 2 (Condition: I, AC) × 3(Time: Baseline, 6-, 12-month) RCT |
Sample size: N=76 children; Intention-to- treat analysis, N=66 (I, n=33; AC, n=33). Sample characteristics: Children ages 9–17 years (mean age 11.6); BMI ≥95th percentile or BMI≥90th percentile and waist circumference ≥ 90th percentile. |
Primary: BMI and body weight Secondary: Body composition; blood pressure; biochemical parameters; other obesity parameters |
12-month: Lower body weight (p=.02); lower BMI (p=02) |
12 months | 12 months | 57 %d |
| Duggins, 2010 [73] | 2 (Condition: I, AC) × 6 (Time: Baseline, 2-, 4-, 6-, 9-, and 12-month) RCT |
Sample size:: N=83 children (I, n=44; AC, n=39) Sample characteristics: Children ages 5–17 (mean age: I, 10.6; AC, 10.6); BMI≥85th percentile. |
Primary: Change in BMI percentile Secondary: Meeting AMA Expert Committee weight loss targets; eating habits, nutrition class attendance, YMCA attendance |
12-month: NS | 12 months | AC, 9 months I, 12 months |
79.5 % |
| Gillis, 2007 [74] | 2 (Condition: I, AC) × 2 (Time: Baseline, 6-month) pilot RCT |
Sample size: N=27 (I, n=14, AC, n=13). Sample characteristics: Children ages 7–16 years Mean age: I, 11.2; AC, 9.9); BMI≥90th percentile. |
Primary: BMI SDS Secondary: Change in obesity-related attitudes; adverse metabolic effects of obesity |
6-month: NS | 6 months | 6 months | 66.7 % |
| Mårild, 2013 [38] | 2 e (Condition: I1, I2) × 2 (Time: Baseline, 12-month) RCT |
Sample size: N=64 (I, n=32; C, n=32). Other comparison groups (not- randomized): normal weight n=34; overweight n=29; obese n=138. Sample characteristics: children ages 9–13 years; obese according to IOTF criteria and no ongoing or previous treatment for obesity. |
Primary: Change in BMI SDS Secondary: BMI; proportion overweight or obese; neck circumference; waist circumference; waist/height ratio; change in biochemical markers related to metabolic syndrome |
12-month: Greater decrease in BMI (I1 vs. non-randomly allocated obese control, p=.0007; I2 vs. non-randomly allocated obese control, p=.002); greater decrease in BMI SDS (I1 vs. non-randomly allocated obese control, p=.0005; I2 vs. non-randomly allocated obese control, p=.002) |
12 months | 12 months | 85.9 % |
| McCallum, 2007 [75–78] |
2 (Condition: I, C) × 3 (Time: baseline, 9-, 15-month) RCT |
Sample size: N=163 (intervention=82, control=81). Sample characteristics: Children ages 5–9 years; overweight or mild obesity; BMI z-score <3.0; not currently receiving weight-management services; no medical condition affecting growth. |
Primary: BMI and BMI z scores Secondary: Waist circumference; general quality of life; health-related quality of life; physical activity; nutrition; sedentary behaviors; body satisfaction |
9-month: Improved nutrition score (p<.001) 15-month: Improved nutrition score (p<.001) |
15 months | 3 months | 89.6 % |
| Nova, 2001 [79] | 2 (Condition: I, UC) × 4 (Time: Baseline, 6-, 12-, 24-month) RCT, randomized at physician-level |
Sample size: N=186 (I=72, UC=114). N= 13 family pediatrician offices Sample characteristics: Children: ages 3–12 years (mean age 8.6); obese (≥20 % of ideal body weight). |
Primary: Change % overweight; dietary behavior; physical activity; computer and television use; attendance at follow-up visit |
6-month: Greater decrease in percentage overweight defined by EID Index (p=0.0001) 12-month: Greater decrease percentage overweight defined by EID Index (p=.002) |
2 years | 2 years | 69.9 %f |
| O’Connor, 2013 [80, 81] |
2 (Condition: I, WC) × 2 (Time: Baseline, 7–8 month) pilot RCT |
Sample size: N=40 (I, n=20; WC, n=20). Sample characteristics: Children 5–8 years old; BMI 85–99 th percentile |
Primary: Feasibility measures of intervention Secondary: BMI and BMI percentile; physical activity; dietary intake; TV viewing; parenting practices to promote fruit and vegetable intake; TV parenting practices; and physical activity parenting practices |
7-month: Less TV time (p<.05); greater parental support for physical activity (p < .05); greater parental practices (non-directive control) related to intake of fruit and vegetables (p <.05) |
7 months |
6 months |
85% |
| Small, 2014 [82] | 2 (Condition: I, C) × 4 (Baseline, immediately post-I, 3-month post-I, 6-month post-I) pilot RCT |
Sample size: N=67 parent-child dyads. Analysis sample N=60 (I, n=33, C, n= 27), 7 families did not complete baseline measures and were excluded from analyses. Sample characteristics: Children: ages 4–8 years (mean age 5.6), overweight or obese. |
Primary: BMI; Waist circumference, waist- by-height ratio |
Over time (Baseline, immediately post- intervention): Decrease in waist circumference (p=.03); decrease in waist-by- height ratio (p=.02) |
10 months | 4 months |
62% |
| van Grieken, 2013 [83, 84] |
2 (Condition: I, C) × 3 (Time: baseline, 12-, 24-month) cluster RCT |
Sample size: N=637 (I, n=349, C n=288); N=44 youth health care teams (I, n=22; C, n=22) Sample characteristics: Children age 5 years; overweight using international BMI cut points; no chronic medical condition; Dutch-speaking child and parent. |
Primary: BMI and waist circumference Secondary: Levels of overweight; inducing/ reducing behaviors; parenting practices; health related quality of life |
2-year: NS | 2 years |
Up to 6 months |
79.6 % |
| Wake, 2009 [85] | 2 (Condition: I, C) X 3 (Time: Baseline, 6-, 12-month) RCT |
Sample size: N=258 (I, n=139, C, n=119 Sample characteristics: Children ages 5–9 years; overweight or obese using International Obesity Taskforce cut points and BMI z- score<3.0. |
Primary: BMI Secondary: Mean activity count/minute; nutrition score; health related quality of life |
6-month: NS 12-month: NS |
12 months | 3 months |
94.9 % |
| Wake, 2013 [86, 87] | 2 (Condition: I, UC) × 2 (Time: Baseline, 15-month) RCT |
Sample size: N=118 (I, n=62; C, n=56) Sample characteristics: Children ages 3–10 years (mean age: I, 7.2; C, 7.4); BMI > 95th percentile. |
Primary: BMI z score Secondary: waist circumference; body fat percentage; body satisfaction; physical appearance and self-worth; health related quality of life; blood pressure; diet quality; physical activity; behavior; parent BMI; parent readiness to change |
15-month: NS | 15 months | 12 months |
90.7 % |
| Weigel, 2008 [88] | 2 (Condition: I, AC) × 3 (Time: Baseline, 6-, 12-month) RCT |
Sample size: N=73 children (I, n=37; AC, n=36). Sample characteristics: children ages 7 to 15 years (mean age 11.2); BMI>90th percentile |
Primary: BMI z score |
12 month
g: Decrease BMI z-score (p<.01); decrease BMI (p<.001); decrease fat mass (p<.001); increase lean mass (p<.001); decrease systolic BP (p<.01) |
12 months | 12 months | Not reported |
| Wright, 2013 [89] | 2 (Condition: I, WC) × 2 (Time: Baseline, 3-month) pilot RCT |
Sample size: N=50 (I, n=24; WC, n=26) Sample characteristics: children ages 9–12 years; obese; BMI >95th percentile |
Primary: BMI; dietary intake; screen time; parent BMI; parent dietary intake; parent TV time |
3-month: Increase fruit consumption parents (p=.046); decrease vegetable consumption parents (p=.012) |
3 months |
3 months |
86% |
| Adolescent, prevention (n= 1) | |||||||
| Patrick, 2006 [90] | 2 (Condition: I, C) by 3 (Time: Baseline, 6-, 12-month) RCT |
Sample size: N=819 (I, n=424; C, n=395) Sample characteristics: Adolescents ages 11–15 years; no medical condition affecting PA or nutrition |
Primary: Minutes MVPA; days/week physical activity and sedentary behaviors; percent of energy from fat and daily servings of fruits and vegetables Secondary: BMI |
12 month: Decrease sedentary behaviors (p<.001) |
12 months | 12 months | 84.2 % |
| Adolescent, treatment (n=3) | |||||||
| DeBar, 2012 [91] | 2 (Condition: I, AC) × 3 (Time: Baseline, 6-, 12-month) RCT |
Sample size: N=208 (I, n=105; UC, n=103) Sample characteristics: Girls; ages 12–17 years; BMI ≥90th percentile |
Primary: BMI z score Secondary: Disordered eating; screen time; physical activity; team sports participation; eating breakfast; family meals; fast food; dietary intake; dieting in previous 6 months; use of professional weight management services |
Over time (baseline, post-I, follow-up): Decrease in BMI z-score (p=.012); increase in body satisfaction (p=.026); decrease in internalization of appearance attitudes (p=.019); decrease in fast food frequency (p=.021); smaller decrease in family meals (p=.028) |
12 months | 5 months (teens) 3 months (parents) |
83% |
| MacDonell, 2012 [92] | 2 (Condition: I, AC) × 2 (Time: Baseline, 3-month) pilot RCT |
Sample size: N=44 adolescents (I, n not reported; AC, n not reported) Sample characteristics: Adolescents ages 13–17 years old; BMI≥ 85th percentile; African American |
Primary: BMI; fast food intake; soft drink intake; fruit intake; vegetable intake; intrinsic motivation for nutrition; physical activity; intrinsic motivation for exercise |
3-month: Decrease in fast food frequency (p=.03) |
3 months | 10 weeks |
70.5 % |
| Saelens, 2002 [93] | 2 (Condition: I, AC) X 3 (Time: Baseline, 4-, 7-month) RCT |
Sample size: N=44 (I, n=23; UC, n=21). Sample characteristics: Adolescents ages 12–16 years old; 20 % to 50 % above median BMI %ile (50th %ile) |
Primary: BMI z score Secondary: Percentage of overweight; weight; height; total energy intake; percent energy from fat; physical activity; sedentary behavior; problematic eating behaviors; weight-related behaviors or beliefs |
4 month: Decrease in BMI z-score (p<.02) Overtime (Baseline, 4-month, 7-month) Decrease in BMI z-score (p<.03) |
7 months | 4 months |
84.1 % |
Note. Studies in italics found significant effect on measure of child adiposity.
Abbreviations: I=Intervention; C=No treatment control; UC=Usual Care; AC=Active control receiving alternative intervention; WC=Waitlist control; BMI=body mass index.
For studies with>2 study conditions, comparison groups are specified.
%0BMI=child’s actual BMI minus BMI at the 50th %ile/BMI at the 50th %ile multiplied by 100.
Retention rate at 6-months.
Overall retention rate at 12-months. Primary outcome retention rate = 87%.
1 group of obese children randomized to Intervention 1 or Intervention 2; 3 other comparison groups, not random allocation, comprised of 1) normal weight children; 2) overweight children; 3) age-, sex-, and BMI-matched obese children.
Retention rate at 12-months.
Intervention did not include primary care involvement; active control group received in-person and written therapeutic advice from pediatrician.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Elisabeth M. Seburg, Barbara A. Olson-Bullis, Dani M. Bredeson, Marcia G. Hayes, and Nancy E. Sherwood declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Elisabeth M. Seburg, Email: Elisabeth.M.Seburg@HealthPartners.com.
Barbara A. Olson-Bullis, Email: Barbara.A.OlsonBullis@HealthPartners.com.
Dani M. Bredeson, Email: Dani.M.Bredeson@HealthPartners.com.
Marcia G. Hayes, Email: Marcia.G.Hayes@HealthPartners.com.
Nancy E. Sherwood, Email: Nancy.E.Sherwood@HealthPartners.com.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 2.Singh AS, Mulder C, Twisk JW, Van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 3.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 4.Sorof J, Daniels S. Obesity hypertension in children a problem of epidemic proportions. Hypertension. 2002;40(4):441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60(3):222–228. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 6.Magnussen CG, Smith KJ, Juonala M. What the long term cohort studies that began in childhood have taught us about the origins of coronary heart disease. Current Cardiovascular Risk Reports. 2014;8(2):1–10. [Google Scholar]
- 7.Katz M, Rubino A, Collier J, Rosen J, Ehrich JH. Demography of pediatric primary care in Europe: delivery of care and training. Pediatrics. 2002;109(5):788–796. doi: 10.1542/peds.109.5.788. [DOI] [PubMed] [Google Scholar]
- 8.Kuo AA, Inkelas M, Lotstein DS, Samson KM, Schor EL, Halfon N. Rethinking well-child care in the United States: an international comparison. Pediatrics. 2006;118(4):1692–1702. doi: 10.1542/peds.2006-0620. [DOI] [PubMed] [Google Scholar]
- 9.van Esso D, del Torso S, Hadjipanayis A, et al. Paediatric primary care in Europe: variation between countries. Arch Dis Child. 2010;95(10):791–795. doi: 10.1136/adc.2009.178459. [DOI] [PubMed] [Google Scholar]
- 10.Stettler N. Comment: The global epidemic of childhood obesity: is there a role for the paediatrician? Obes Rev. 2004;5(s1):1–3. doi: 10.1111/j.1467-789X.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 11.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 12.National Health and Medical Research Council. Summary guide for the management of overweight and obesity in primary care. Melbourne: National Health and Medical Research Council; 2013. [Google Scholar]
- 13.Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] Can Med Assoc J. 2007;176(8):S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Clinical Excellence. Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. London, United Kingdom: National Institute for Health and Clinical Excellence; 2006. www.nice.org.uk/guidance/cg43. [PubMed] [Google Scholar]
- 15.King LA, Loss JH, Wilkenfeld RL, Pagnini DL, Booth ML, Booth SL. Australian GPs’ perceptions about child and adolescent overweight and obesity: the Weight of Opinion study. Brit J Gen Pract. 2007;57(535):124–129. [PMC free article] [PubMed] [Google Scholar]
- 16.Walker O, Strong M, Atchinson R, Saunders J, Abbott J. A qualitative study of primary care clinicians’ views of treating childhood obesity. BMC Fam Pract. 2007;8(1):50. doi: 10.1186/1471-2296-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics. 2002;110(1):210–204. [PubMed] [Google Scholar]
- 18.Jelalian E, Boergers J, Alday CS, Frank R. Survey of physician attitudes and practices related to pediatric obesity. Clin Pediatr (Phila) 2003;42(3):235–245. doi: 10.1177/000992280304200307. [DOI] [PubMed] [Google Scholar]
- 19.Kolagotla L, Adams W. Ambulatory management of childhood obesity. Obes Res. 2004;12(2):275–283. doi: 10.1038/oby.2004.35. [DOI] [PubMed] [Google Scholar]
- 20.Yarborough BJH, DeBar LL, Wu P, Pearson J, Stevens VJ. Responding to pediatric providers’ perceived barriers to adolescent weight management. Clin Pediatr (Phila) 2012;51(11):1063–1070. doi: 10.1177/0009922812459269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin EM, Flower KB, Garrett J, Ammerman AS. Preventing and treating obesity: pediatricians’ self-efficacy, barriers, resources, and advocacy. Ambul Pediatr. 2005;5(3):150–156. doi: 10.1367/A04-104R.1. [DOI] [PubMed] [Google Scholar]
- 22.Spivack JG, Swietlik M, Alessandrini E, Faith MS. Primary care providers’ knowledge, practices, and perceived barriers to the treatment and prevention of childhood obesity. Obesity (Silver Spring) 2010;18(7):1341–1347. doi: 10.1038/oby.2009.410. [DOI] [PubMed] [Google Scholar]
- 23.Sivertsen LM, Woolfenden SR, Woodhead HJ, Lewis D. Diagnosis and management of childhood obesity: a survey of general practitioners in South West Sydney. J Paediatr Child H. 2008;44(11):622–629. doi: 10.1111/j.1440-1754.2008.01370.x. [DOI] [PubMed] [Google Scholar]
- 24.Franc C, Van Gerwen M, Le Vaillant M, Rosman S, Pelletier-Fleury N. French pediatricians’ knowledge, attitudes, beliefs towards and practices in the management of weight problems in children. Health Policy. 2009;91(2):195–203. doi: 10.1016/j.healthpol.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Mazur A, Matusik P, Revert K, et al. Childhood obesity: knowledge, attitudes, and practices of European pediatric care providers. Pediatrics. 2013;132(1):e100–e108. doi: 10.1542/peds.2012-3239. [DOI] [PubMed] [Google Scholar]
- 26.He M, Piché L, Clarson CL, Callaghan C, Harris SB. Childhood overweight and obesity management: a national perspective of primary health care providers’ views, practices, perceived barriers and needs. Paediatr Child Healt. 2010;15(7):419. doi: 10.1093/pch/15.7.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rausch JC, Perito ER, Hametz P. Obesity prevention, screening, and treatment: practices of pediatric providers since the 2007 expert committee recommendations. Clin Pediatr (Phila) 2011;50(5):434–441. doi: 10.1177/0009922810394833. [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein LM, Perrin EM, Campbell MK, Tate DF, Cai J, Ammerman AS. Primary care providers’ self-efficacy and outcome expectations for childhood obesity counseling. Childhood Obesity. 2013;9(3):208–215. doi: 10.1089/chi.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifi M, Rifas-Shiman SL, Marshall R, et al. Evaluating the implementation of expert committee recommendations for obesity assessment. Clin Pediatr (Phila) 2013;52(2):131–138. doi: 10.1177/0009922812471712. [DOI] [PubMed] [Google Scholar]
- 30. Liang L, Meyerhoefer C, Wang J. Obesity counseling by pediatric health professionals: an assessment using nationally representative data. Pediatrics. 2012;130(1):67–77. doi: 10.1542/peds.2011-0596. This article uses the Medical Expenditure Panel Survey (2001–2007) to calculate nationally representative rates of parent-reported health care provider screening and counseling of childhood obesity and weight-related behaviors.
- 31.Tanda R, Salsberry P. The impact of the 2007 expert committee recommendations on childhood obesity preventive care in primary care settings in the United States. J Pediatr Health Car. 2014;28(3):241–250. doi: 10.1016/j.pedhc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rurik I, Torzsa P, Ilyés I, et al. Primary care obesity management in Hungary: evaluation of the knowledge, practice and attitudes of family physicians. BMC Fam Pract. 2013;14(1):156. doi: 10.1186/1471-2296-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiscock H, Roberts G, Efron D, et al. Children Attending Paediatricians Study: a national prospective audit of outpatient practice from the Australian Paediatric Research Network—what conditions are paediatricians seeing in outpatient settings? Med J Australia. 2011;194(8):392. doi: 10.5694/j.1326-5377.2011.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldman RD, Modan-Moses D, Bujanover Y, Glasser S, Meyerovitch J. Physicians’ attitude toward identification and management of childhood obesity in Israel. Clin Pediatr (Phila) 2004;43(8):737–741. doi: 10.1177/000992280404300808. [DOI] [PubMed] [Google Scholar]
- 35.Klein JD, Sesselberg TS, Johnson MS, et al. Adoption of body mass index guidelines for screening and counseling in pediatric practice. Pediatrics. 2010;125(2):265–272. doi: 10.1542/peds.2008-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isma GE, Bramhagen A-C, Ahlstrom G, Östman M, Dykes A-K. Obstacles to the prevention of overweight and obesity in the context of child health care in Sweden. BMC Fam Pract. 2013;14(1):143. doi: 10.1186/1471-2296-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent G, Pilotto L, Baur L. Components of primary care interventions to treat childhood overweight and obesity: a systematic review of effect. Obes Rev. 2011;12(5):e219–e235. doi: 10.1111/j.1467-789X.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 38.Marild S, Gronowitz E, Forsell C, Dahlgren J, Friberg P. A controlled study of lifestyle treatment in primary care for children with obesity. Pediatr Obes. 2013;8(3):207–217. doi: 10.1111/j.2047-6310.2012.00105.x. [DOI] [PubMed] [Google Scholar]
- 39.French SA, Mitchell NR, Hannan PJ. Decrease in television viewing predicts lower body mass index at 1-year follow-up in adolescents, but not adults. J Nutr Educ Behav. 2012;44(5):415–422. doi: 10.1016/j.jneb.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholson LM, Schwirian PM, Klein EG, et al. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials. 2011;32(3):353–362. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakanen M, Lagstrom H, Pahkala K, et al. Dietary and lifestyle counselling reduces the clustering of overweight-related cardio-metabolic risk factors in adolescents. Acta Paediatr. 2010;99(6):888–895. doi: 10.1111/j.1651-2227.2009.01636.x. [DOI] [PubMed] [Google Scholar]
- 42.Lapinleimu H, Salo P, Routi T, et al. Prospective randomised trial in 1062 infants of diet low in saturated fat and cholesterol. Lancet. 1995;345(8948):471–476. doi: 10.1016/s0140-6736(95)90580-4. [DOI] [PubMed] [Google Scholar]
- 43.Lagstrom H, Hakanen M, Niinikoski H, et al. Growth patterns and obesity development in overweight or normal-weight 13-year-old adolescents: the STRIP study. Pediatrics. 2008;122(4):e876–e883. doi: 10.1542/peds.2007-2354. [DOI] [PubMed] [Google Scholar]
- 44.Talvia S, Rasanen L, Lagstrom H, et al. Parental eating attitudes and indicators of healthy eating in a longitudinal randomized dietary intervention trial (the STRIP study) Public Health Nutr. 2011;14(11):2065–2073. doi: 10.1017/S1368980011000905. [DOI] [PubMed] [Google Scholar]
- 45.Niinikoski H, Lagstrom H, Jokinen E, et al. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation. 2007;116(9):1032–1040. doi: 10.1161/CIRCULATIONAHA.107.699447. [DOI] [PubMed] [Google Scholar]
- 46.Pahkala K, Heinonen OJ, Lagstrom H, et al. Clustered metabolic risk and leisure-time physical activity in adolescents: effect of dose? Br J Sports Med. 2012;46(2):131–137. doi: 10.1136/bjsm.2010.073239. [DOI] [PubMed] [Google Scholar]
- 47.Hakanen M, Lagström H, Kaitosaari T, et al. Development of overweight in an atherosclerosis prevention trial starting in early childhood. STRIP study Int J Obesity. 2006;30(4):618–626. doi: 10.1038/sj.ijo.0803249. [DOI] [PubMed] [Google Scholar]
- 48.Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309(10):1005–1013. doi: 10.1001/jama.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 50.Paul IM, Savage JS, Anzman SL, et al. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring) 2011;19(2):353–361. doi: 10.1038/oby.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anzman-Frasca S, Stifter CA, Paul IM, Birch LL. Infant temperament and maternal parenting self-efficacy predict child weight outcomes. Infant Behav Dev. 2013;36(4):494–497. doi: 10.1016/j.infbeh.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wake M, Price A, Clifford S, Ukoumunne OC, Hiscock H. Does an intervention that improves infant sleep also improve overweight at age 6? Follow-up of a randomised trial. Arch Dis Child. 2011;96(6):526–532. doi: 10.1136/adc.2010.196832. [DOI] [PubMed] [Google Scholar]
- 53.Price AM, Wake M, Ukoumunne OC, Hiscock H. Five-year follow-up of harms and benefits of behavioral infant sleep intervention: randomized trial. Pediatrics. 2012;130(4):643–651. doi: 10.1542/peds.2011-3467. [DOI] [PubMed] [Google Scholar]
- 54.Hiscock H, Bayer J, Gold L, Hampton A, Ukoumunne O, Wake M. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child. 2006 doi: 10.1136/adc.2006.099812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birken CS, Maguire J, Mekky M, et al. Office-based randomized controlled trial to reduce screen time in preschool children. Pediatrics. 2012;130(6):1110–1115. doi: 10.1542/peds.2011-3088. [DOI] [PubMed] [Google Scholar]
- 56.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Efficacy of family-based weight control program for preschool children in primary care. Pediatrics. 2012;130(4):660–666. doi: 10.1542/peds.2012-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stark LJ, Spear S, Boles R, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) 2011;19(1):134–141. doi: 10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taveras EM, Gortmaker SL, Hohman KH, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165(8):714–722. doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taveras EM, Hohman KH, Price SN, et al. Correlates of participation in a pediatric primary care-based obesity prevention intervention. Obesity (Silver Spring) 2011;19(2):449–452. doi: 10.1038/oby.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo Baidal JA, Price SN, Gonzalez-Suarez E, et al. Parental perceptions of a motivational interviewing-based pediatric obesity prevention intervention. Clin Pediatr (Phila) 2013;52(6):540–548. doi: 10.1177/0009922813483170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arauz Boudreau AD, Kurowski DS, Gonzalez WI, Dimond MA, Oreskovic NM. Latino families, primary care, and childhood obesity: a randomized controlled trial. Am J Prev Med. 2013;44(3):S247–S257. doi: 10.1016/j.amepre.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banks J, Sharp DJ, Hunt LP, Shield JP. Evaluating the transfer-ability of a hospital-based childhood obesity clinic to primary care: a randomised controlled trial. Br J Gen Pract. 2012;62(594):e6–e12. doi: 10.3399/bjgp12X616319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banks J, Shield JP, Sharp D. Barriers engaging families and GPs in childhood weight management strategies. Br J Gen Pract. 2011;61(589):e492–e497. doi: 10.3399/bjgp11X588466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banks J, Williams J, Cumberlidge T, Cimonetti T, Sharp DJ, Shield JP. Is healthy eating for obese children necessarily more costly for families? Br J Gen Pract. 2012;62(594):e1–e5. doi: 10.3399/bjgp12X616300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollinghurst S, Hunt LP, Banks J, Sharp DJ, Shield JP. Cost and effectiveness of treatment options for childhood obesity. Pediatr Obes. 2014;9(1):e26–e34. doi: 10.1111/j.2047-6310.2013.00150.x. [DOI] [PubMed] [Google Scholar]
- 66.Barkin SL, Gesell SB, Poe EK, Ip EH. Changing overweight Latino preadolescent body mass index: the effect of the parent–child dyad. Clin Pediatr (Phila) 2011;50(1):29–36. doi: 10.1177/0009922810379039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gesell SB, Scott TA, Barkin SL. Accuracy of perception of body size among overweight Latino preadolescents after a 6-month physical activity skills building intervention. Clin Pediatr (Phila) 2010;49(4):323–329. doi: 10.1177/0009922809339386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzmn A, Richardson IM, Gesell S, Barkin SL. Recruitment and retention of Latino children in a lifestyle intervention. Am J Health Behav. 2009;33(5):581–586. [PMC free article] [PubMed] [Google Scholar]
- 69.Davis AM, Sampilo M, Gallagher KS, Landrum Y, Malone B. Treating rural pediatric obesity through telemedicine: outcomes from a small randomized controlled trial. J Pediatr Psychol. 2013;38(9):932–943. doi: 10.1093/jpepsy/jst005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallagher KS, Davis AM, Malone B, Landrum Y, Black W. Treating rural pediatric obesity through telemedicine: baseline data from a randomized controlled trial. J Pediatr Psychol. 2011;36(6):687–695. doi: 10.1093/jpepsy/jsr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis AM, James RL, Boles RE, Goetz JR, Belmont J, Malone B. The use of TeleMedicine in the treatment of paediatric obesity: feasibility and acceptability. Matern Child Nutr. 2011;7(1):71–79. doi: 10.1111/j.1740-8709.2010.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diaz RG, Esparza-Romero J, Moya-Camarena SY, Robles-Sardin AE, Valencia ME. Lifestyle intervention in primary care settings improves obesity parameters among Mexican youth. J Am Diet Assoc. 2010;110(2):285–290. doi: 10.1016/j.jada.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 73.Duggins M, Cherven P, Carrithers J, Messamore J, Harvey A. Impact of family YMCA membership on childhood obesity: a randomized controlled effectiveness trial. J Am Board Fam Med. 2010;23(3):323–333. doi: 10.3122/jabfm.2010.03.080266. [DOI] [PubMed] [Google Scholar]
- 74.Gillis D, Brauner M, Granot E. A community-based behavior modification intervention for childhood obesity. J Pediatr Endocrinol Metab. 2007;20(2):197–203. doi: 10.1515/jpem.2007.20.2.197. [DOI] [PubMed] [Google Scholar]
- 75.McCallum Z, Wake M, Gerner B, et al. Outcome data from the LEAP (Live, Eat and Play) trial: a randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obesity. 2007;31(4):630–636. doi: 10.1038/sj.ijo.0803509. [DOI] [PubMed] [Google Scholar]
- 76.McCallum Z, Wake M, Gerner B, et al. Can Australian general practitioners tackle childhood overweight/obesity? Methods and processes from the LEAP (Live, Eat and Play) randomized controlled trial. J Paediatr Child H. 2005;41(9–10):488–494. doi: 10.1111/j.1440-1754.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 77.Wake M, Gold L, McCallum Z, Gerner B, Waters E. Economic evaluation of a primary care trial to reduce weight gain in overweight/obese children: the LEAP trial. Ambul Pediatr. 2008;8(5):336–341. doi: 10.1016/j.ambp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Rogers L, Gerner B, Wake M. LEAP trial. Aust Fam Physician. 2007;36(11):887–888. [PubMed] [Google Scholar]
- 79.Nova A, Russo A, Sala E. Long-term management of obesity in paediatric office practice: experimental evaluation of two different types of intervention. Ambulatory Child Health. 2001;7(3–4):239–247. [Google Scholar]
- 80.O’Connor TM, Hilmers A, Watson K, Baranowski T, Giardino AP. Feasibility of an obesity intervention for paediatric primary care targeting parenting and children: Helping HAND. Child Care Health Dev. 2013;39(1):141–149. doi: 10.1111/j.1365-2214.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 81.Ledoux T, Hilmers A, Watson K, Baranowski T, O’Connor TM. Development and feasibility of an objective measure of patient-centered communication fidelity in a pediatric obesity intervention. J Nutr Educ Behav. 2013;45(4):349–354. doi: 10.1016/j.jneb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Small L, Bonds-McClain D, Melnyk B, Vaughan L, Gannon AM. The preliminary effects of a primary care-based randomized treatment trial with overweight and obese young children and their parents. J Pediatr Health Car. 2014;28(3):198–207. doi: 10.1016/j.pedhc.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Grieken A, Veldhuis L, Renders CM, et al. Population-based childhood overweight prevention: outcomes of the ‘Be active, eat right’ study. PLoS One. 2013;8(5):e65376. doi: 10.1371/journal.pone.0065376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veldhuis L, Struijk MK, Kroeze W, et al. ‘Be active, eat right’, evaluation of an overweight prevention protocol among 5-year-old children: design of a cluster randomised controlled trial. BMC Public Health. 2009;9:177. doi: 10.1186/1471-2458-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wake M, Baur LA, Gerner B, et al. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ. 2009;339:b3308. doi: 10.1136/bmj.b3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wake M, Lycett K, Clifford SA, et al. Shared care obesity management in 3–10 year old children: 12 month outcomes of HopSCOTCH randomised trial. BMJ. 2013;346:f3092. doi: 10.1136/bmj.f3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wake M, Lycett K, Sabin MA, et al. A shared-care model of obesity treatment for 3–10 year old children: protocol for the HopSCOTCH randomised controlled trial. BMC Pediatr. 2012;12:39. doi: 10.1186/1471-2431-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weigel C, Kokocinski K, Lederer P, Dotsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav. 2008;40(6):369–373. doi: 10.1016/j.jneb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Wright JA, Phillips BD, Watson BL, Newby PK, Norman GJ, Adams WG. Randomized trial of a family-based, automated, conversational obesity treatment program for underserved populations. Obesity (Silver Spring) 2013;21(9):E369–E378. doi: 10.1002/oby.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patrick K, Calfas KJ, Norman GJ, et al. Randomized controlled trial of a primary care and home-based intervention for physical activity and nutrition behaviors: PACE+ for adolescents. Arch Pediatr Adolesc Med. 2006;160(2):128–136. doi: 10.1001/archpedi.160.2.128. [DOI] [PubMed] [Google Scholar]
- 91.DeBar LL, Stevens VJ, Perrin N, et al. A primary care-based, multicomponent lifestyle intervention for overweight adolescent females. Pediatrics. 2012;129(3):e611–e620. doi: 10.1542/peds.2011-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macdonell K, Brogan K, Naar-King S, Ellis D, Marshall S. A pilot study of motivational interviewing targeting weight-related behaviors in overweight or obese African American adolescents. J Adolesc Health. 2012;50(2):201–203. doi: 10.1016/j.jadohealth.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 93.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10(1):22–32. doi: 10.1038/oby.2002.4. [DOI] [PubMed] [Google Scholar]
- 94.Force UPST. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125(2):361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 95.Doolen J, Alpert PT, Miller SK. Parental disconnect between perceived and actual weight status of children: a metasynthesis of the current research. J Am Acad Nurse Pract. 2009;21(3):160–166. doi: 10.1111/j.1745-7599.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 96.Pollak KI, Alexander SC, Østbye T, et al. Primary care physicians’ discussions of weight-related topics with overweight and obese adolescents: results from the Teen CHAT Pilot study. J Adolescent Health. 2009;45(2):205–207. doi: 10.1016/j.jadohealth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bravender T, Tulsky JA, Farrell D, et al. Teen CHAT: development and utilization of a web-based intervention to improve physician communication with adolescents about healthy weight. Patient Educ Couns. 2013;93(3):525–531. doi: 10.1016/j.pec.2013.08.017. This article describes a promising online training for pediatric health care providers on motivational interviewing skills for counseling patients on obesity and weight-related behaviors.
- 98.Ariza AJ, Hartman J, Grodecki J, et al. Linking pediatric primary care obesity management to community programs. J Health Care Poor U. 2013;24(2):158–167. doi: 10.1353/hpu.2013.0112. [DOI] [PubMed] [Google Scholar]
- 99.DuPlessis HM, Boulter CSC, Cora-Bramble D, et al. The pediatrician’s role in community pediatrics. Pediatrics. 2005;115:1092–4. doi: 10.1542/peds.2004-2680. [DOI] [PubMed] [Google Scholar]
- 100.Pediatrics AAO. AAP publications reaffirmed and retired. Pediatrics. 2010;126(1):177. [Google Scholar]
- 101.Sherwood NE, French SA, Veblen-Mortenson S, et al. NET-Works: linking families, communities and primary care to prevent obesity in preschool-age children. Contemp Clin Trials. 2013;36(2):544–554. doi: 10.1016/j.cct.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robinson TN, Matheson D, Desai M, et al. Family, community and clinic collaboration to treat overweight and obese children: Stanford GOALS-A randomized controlled trial of a three-year, multi-component, multi-level, multi-setting intervention. Contemp Clin Trials. 2013;36(2):421–435. doi: 10.1016/j.cct.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 104.Shrewsbury VA, Nguyen B, O’Connor J, et al. Short-term outcomes of community-based adolescent weight management: The Loozit(R) Study. BMC Pediatr. 2011;11:13. doi: 10.1186/1471-2431-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]