Abstract
A mouse model that mediates temporal, specific, and efficient myocardial deletion with Cre-LoxP technology will be a valuable tool to determine the function of genes during heart formation. Mhy6 encodes a cardiac muscle specific protein: alpha-myosin heavy chain. Here, we generated a new Myh6-MerCreMer (Myh6MerCreMer/+) inducible Cre knock-in mouse by inserting a MerCreMer cassette into the Myh6 start codon. By crossing knock-in mice with Rosa26 reporter lines, we found the Myh6MerCreMer/+ mice mediate complete Cre-LoxP recombination in cardiomyocytes after tamoxifen induction. X-gal staining and immunohistochemistry analysis revealed that Myh6-driven Cre recombinase was specifically activated in cardiomyocytes at embryonic and adult stages. Furthermore, echocardiography showed that Myh6MerCreMer/+ mice maintained normal cardiac structure and function before and after tamoxifen administration. These results suggest that the new Myh6MerCreMer/+ mouse can serve as a robust tool to dissect the roles of genes in heart development and function. Additionally, myocardial progeny during heart development and after cardiac injury can be traced using this mouse line.
Introduction
Congenital heart disease (CHD) is the leading cause of birth defects in humans [1–3] with an incidence varying from 19 to 75 per 1,000 of live births [4]. Deletion of genes through Cre-LoxP technology in mice has facilitated the discovery of a number of genes critical for heart development and function (e.g., Nkx2.5, Hand2, Gata4, Mef2c, Tbx5 and Tbx20) [5–10]. To further understand the mechanisms underlying heart development and disease, several inducible Cre mouse lines with tTA/rtTA/TetO and MerCreMer (Cre recombinase fused to two mutated estrogen receptor (Mer) ligand binding domains) have been developed and allow myocardial specific deletion of genes of interest in a temporal manner [11–13]. However, the tTA/rtTA/TetO animal models do not mediate instant and complete Cre excision [14], and the transgenic α-MHC-MerCreMer mice are imperfect deleter since they display cardiac functional defects after tamoxifen treatment [15–21]. These unfavorable features may eventually lead to misinterpretation of data in cardiac studies.
Myh6 (α-MHC, MYHC and MYHCA) encodes the cardiac muscle specific protein alpha-myosin heavy chain and is critical for heart development [22–24]. MYH6 mutations in humans cause atrial septal defect as well as dilated and hypertrophic cardiomyopathy [22–24]. The alpha-myosin heavy chain is dynamically expressed in cardiomyocytes during heart formation [25]. In this study, we created Myh6 MerCreMer/+ knock-in mice by inserting the MerCreMer cassette into the Myh6 start codon. Myh6-driven Cre recombinase was specifically activated in cardiomyocytes after tamoxifen induction at embryonic and adult stages. Thus, the Myh6 MerCreMer/+ knock-in mouse model may be a useful instrument in the temporal genetic deletion of genes of interest in cardiomyocytes in addition to tracing myocardial lineage during development and after cardiac injury.
Materials and Methods
Animals
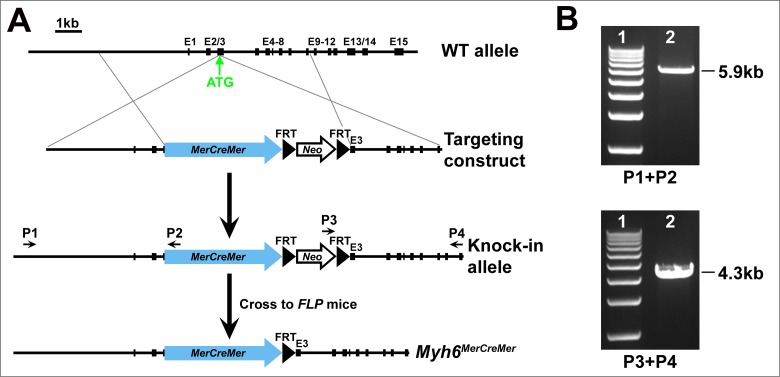
Myh6 MerCreMer/+ knock-in mice were generated by gene targeting. A MerCreMer-FRT-Neo-FRT cassette was inserted 6 bp upstream of the start codon of Myh6 (with disruption of endogenous ATG). The targeting construct contains a MerCreMer-FRT-Neo-FRT cassette flanked by 5' and 3' homologous arms (Fig 1). A linearized construct was transfected into mouse embryonic stem (ES) cells. Positive ES cells were identified by long-range PCR (Roche) with a primer external to the homologous arms and a primer located in the MerCreMer-FRT-Neo-FRT cassette. PCR fragments were verified by DNA sequencing.
Fig 1. Generation of Myh6 MerCreMer/+ knock-in mouse.
(A) Schematic diagram of gene targeting. The MerCreMer-FRT-Neo-FRT cassette was inserted into the Myh6 locus (6 bp upstream ATG). The Neo cassette was removed by crossing Myh6 MerCreMer-Neo/+ mice with the Flippase deleter (FLPe) mice. (B) Long-range PCR of genomic DNA from the positive ES cells generated a 5.9-kb recombinant fragment at 5' end and a 4.3-kb recombinant fragment at 3' end with primers P1/P2 and P3/P4, respectively.
Chimeric mice derived from the positive ES cells were crossed with Black Swiss wild type mice to obtain Myh6 MerCreMer-Neo/+ mice. The Myh6 MerCreMer/+ allele was obtained by crossing Myh6 MerCreMer-Neo/+ mice with FLPe mice [26]. R26R lacZ/+ and R26R tdTomato/+ mice were obtained from the Jackson Laboratory [27, 28]. Myh6 MerCreMer/+ mice were genotyped by PCR of DNA isolated from mouse tails using the following primers: GCAGGCACTTTACATAGAGTCCTG (Forward, 5'→3'); GTTCAGCATCCAACAAGGCACTGA (Reverse, 5'→3'). Mice were euthanized through cervical dislocation for collecting embryonic and postnatal tissues. Animal husbandry procedures were approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai (LA09-00494) and are in compliance with NIH guidelines (PHS Animal Welfare Assurance A3111-01).
Tamoxifen Administration
Myh6 MerCreMer/+ mice were crossed with R26R lacZ/+ or R26R tdTomato/+ reporter mice to get Myh6 MerCreMer/+;R26R lacZ/+ and Myh6 MerCreMer/+;R26R tdTomato/+ doubly heterozygous animals. Tamoxifen (Sigma-Aldrich) was prepared in sesame oil (Sigma-Aldrich) and was administered to the pregnant (0.05 mg/g body weight/day) and adult (0.1 mg/g body weight/day) mice by intraperitoneal injections for 2 (for embryonic) or 3 (for postnatal) consecutive days [12, 21, 29]. Tissues were harvested 24 hours after the final administration of Tamoxifen for X-gal staining or immunofluorescence.
X-Gal and Trichrome Staining
Whole mouse embryos and hearts were harvested at the indicated time points. β-galactosidase activity was assessed by X-gal staining as described previously [30]. Mouse embryos or hearts were dissected in PBS and fixed in 4% paraformaldehyde/PBS for 30 min at 4°C. The fixed samples were then washed twice with PBS, followed by staining in X-gal solution overnight at room temperature [31]. For cryosections, cardiac samples were embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek) on dry ice and were cut to 10 μm in thickness. Frozen sections were stained in X-gal solution at 37°C overnight. After two washes in PBS, cardiac sections were mounted in permount medium and the images were then captured under a Leica microscope. Cardiac fibrosis was examined by Masson’s trichrome Stain Kit (Sigma-Aldrich) using the manufacturer’s protocol.
Immunofluorescence
Mouse hearts were fixed in 4% paraformaldehyde/PBS on ice for 30 min. They were then embedded in OCT using standard procedure. Cryosections were cut to 6 μm in thickness for immunofluorescence. Tissue sections were washed with PBS and blocked with 10% goat normal serum for 30 min at room temperature. The tissues were subsequently incubated with either primary antibody anti-mouse cardiac Troponin T (1:200, Thermo scientific), anti-mouse αSMA (1:100, Sigma), or anti-mouse PECAM (CD31) (1:100, BD Biosciences), for 1 hour at room temperature. Slides were washed three times in PBS, followed by incubation with Alexa Flour 488 conjugated secondary antibodies (1:500; Invitrogen) for 45 min at room temperature. Sections were counterstained with diamidino-2-phenylindole (DAPI) and photographed under a fluorescence microscope.
Echocardiography Analysis
Echocardiography (Echo) was performed with Vevo2100 system (FujiFilm VisualSonics Inc.) with methods described previously [32]. Briefly, left ventricular cardiac function and structure were assessed from short axis B-mode and M-mode images at the level of the papillary muscles. M-mode recordings were used to measure ventricular wall thickness and chamber dimensions. VisualSonics Vevo 2100 V1.5.0 software (Visualsonics; Toronto, Canada) was used to measure echocardiographic parameters, including diastolic and systolic left ventricular internal diameter (LVID, short axis B-mode), anterior wall thickness (LVAW, short axis M-mode), posterior wall thickness (LVPW, short axis M-mode). Using left ventricular systolic and diastolic chamber dimensions, short-axis ejection fraction (LVEF) and fractional shortening (LVFS) were calculated within the software using standard formulas [32]. Heart rate (HR) and body temperature were monitored throughout image acquisition. All echocardiographic data acquisition and analysis were performed by two independent examiners blinded to the experimental groups. All quantitative data are expressed as mean ± SDEV. Differences between two groups were analyzed by Student’s t test using GraphPad Prism 6 software. P<0.05 was considered statistically significant.
Results
Generation of Myh6 MerCreMer/+ Knock-In Mouse Line
To achieve temporal inactivation of genes of interest in cardiomyocytes, we generated the Myh6 MerCreMer/+ knock-in mouse line by targeting the Myh6 locus. A targeting vector containing the MerCreMer-FRT-Neo-FRT cassette flanked by homologous arms was electroporated into mouse ES cells. After homologous recombination, MerCreMer-FRT-Neo-FRT was inserted into the start codon of Myh6 (Fig 1A). The recombinant bands 5.9-kb (5' end) and 4.3-kb (3' end) were amplified by long-range PCR with primers external to the arms and primers in the MerCreMer-FRT-Neo-FRT cassette (Fig 1B). Myh6 MerCreMer-Neo/+ mice derived from the positive ES cells were crossed with Flippase deleter mice [26] to obtain Myh6 MerCreMer/+ mice (Fig 1A). Myh6 MerCreMer/+ is a knock-in/knock-out allele for Myh6 and those mice developed normally without any evident defects in appearance or behavior in Black Swiss background.
Cardiac Cre Recombination Is Achieved in Myh6 MerCreMer/+ Mice After Tamoxifen Induction
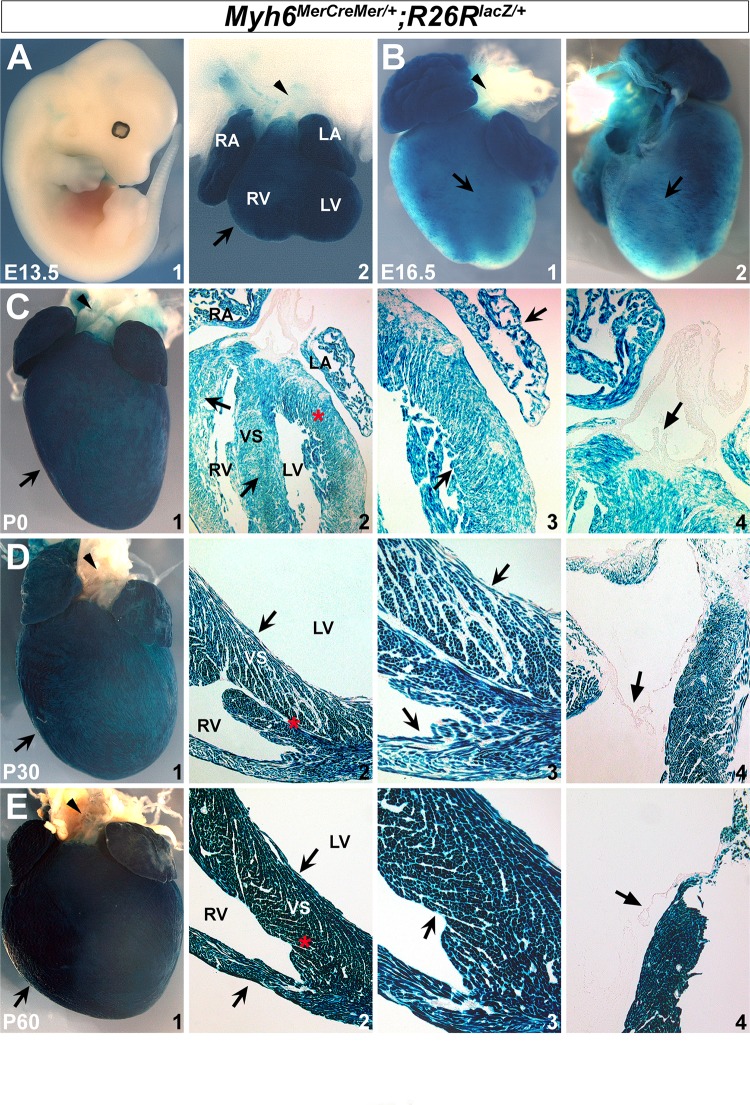
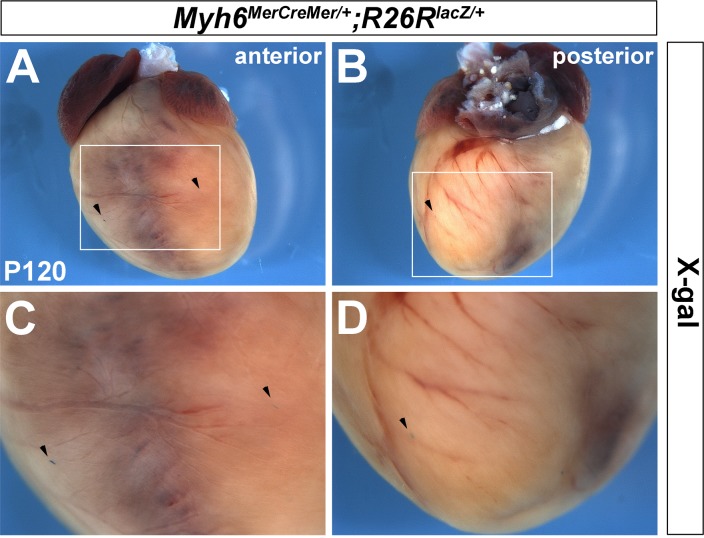
To determine the efficiency and the specificity of Cre recombination in Myh6 MerCreMer/+ mice, we crossed Myh6 MerCreMer/+ mice with the R26R lacZ/+ reporter line to generate Myh6 MerCreMer/+;R26R lacZ/+ double heterozygous animals. β-galactosidase is expressed when Cre translocates from the cytoplasm to the nucleus after tamoxifen induction. To examine Myh6-mediated inducible Cre activity during gestation, tamoxifen was administered to pregnant dams with dosage of 0.05 mg/g body weight/day at embryonic days (E) 11.5, E14.5, and E16.5 by intraperitoneal injection for two consecutive days [29]. Whole embryos or hearts were harvested at E13.5, E16.5, and neonate (P0) to evaluate β-galactosidase activity, respectively. We observed robust X-gal staining in the hearts at E13.5, E16.5 and P0, but no staining was detected in other regions or organs (Fig 2A–2C). This revealed that tamoxifen effectively induces recombination in the hearts at embryonic stages. Furthermore, with three consecutive days of tamoxifen injection at 0.1 mg/g body weight/day, we were also able to detect robust X-gal staining on the postnatal hearts at P30 and P60 (Fig 2D–2E). Of note, X-gal staining was not detected in the aorta or pulmonary artery on Myh6 MerCreMer/+;R26R lacZ/+ hearts at embryonic and adult stages (arrowheads in Fig 2A2,2B1,2C1,2D1 and 2E1). Further examination of cardiac sections revealed that β-galactosidase activity encompasses atrial and ventricular walls, as well as septal regions (notched arrows in Fig 2C2,2C3,2D2,2D3,2E2 and 2E3), but not valves (unnotched arrows in Fig 2C4,2D4 and 2E4). Moreover, very few X-gal positive cells were detected in Myh6 MerCreMer/+;R26R lacZ/+ hearts at the adult stage when tamoxifen was not injected (Fig 3), suggesting that Myh6 MerCreMer/+ mice have very minimal Cre leakiness. Collectively, these observations indicated that the Myh6 MerCreMer/+ allele mediates highly efficient Cre-LoxP recombination in embryonic and postnatal hearts after tamoxifen induction.
Fig 2. Myh6-driven Cre recombination is efficiently activated in heart after tamoxifen induction.
X-gal staining revealed robust β-galactosidase expression in the embryonic and postnatal hearts (R26R lacZ/+;Myh6 MerCreMer/+) after tamoxifen treatment. No X-gal positive cells were found in other regions on E13.5 embryos (A1). Notched arrows in A2/B1/C1/D1/E1 indicate positive X-gal staining in hearts. Arrowheads in A2/B1/C1/D1/E1 indicate pulmonary artery and aorta. Unnotched arrows in C4/D4/E4 indicate valves. C3/D3/E3 are high magnification images for C2/D2/E2 in the areas labeled by red asterisks. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; VS, ventricular septum.
Fig 3. Recombination on Myh6 MerCreMer/+ hearts without tamoxifen.
Very few X-gal positive cells were detected on Myh6 MerCreMer/+;R26R lacZ/+ hearts without tamoxifen treatment. A is anterior view and B is posterior view of a 4-month-old heart (P120). C and D are high magnification of A and B in the square area, respectively. Arrowheads indicate X-gal positive cell.
Myh6 MerCreMer/+ Introduces Specific Recombination in Cardiomyocytes
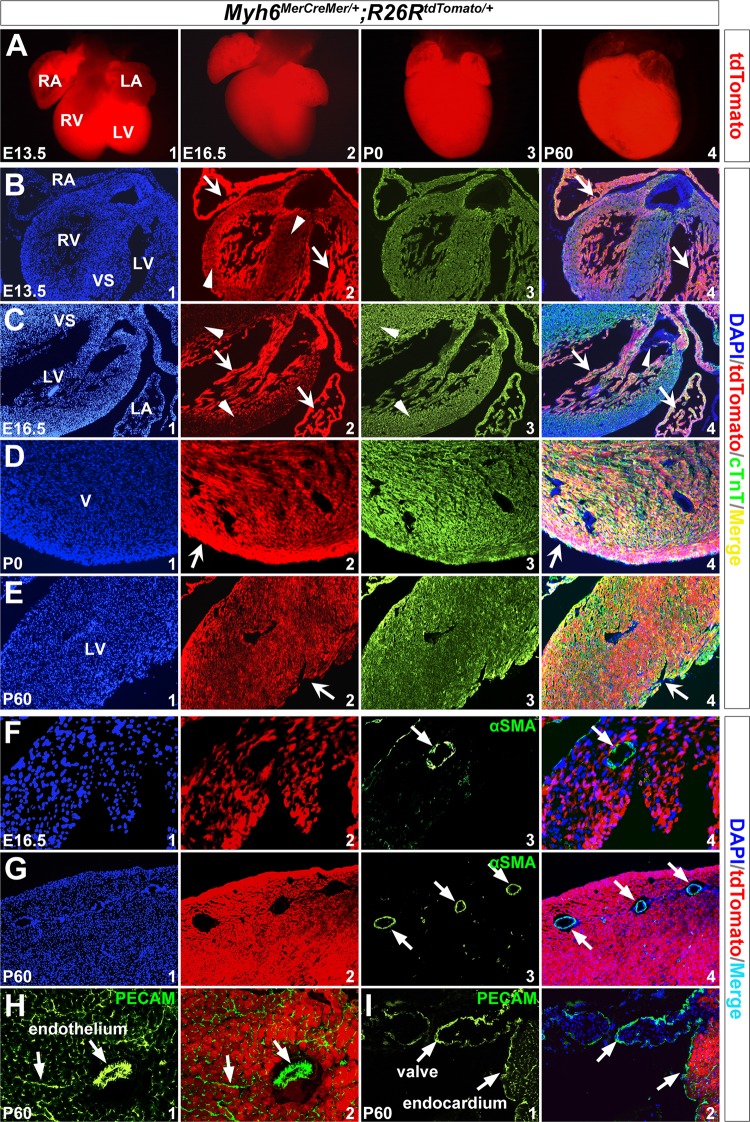
Next, we attempted to determine whether recombination mediated by Myh6 MerCreMer/+ is limited to cardiomyocytes. R26R tdTomato/+ reporter mice were crossed with Myh6 MerCreMer/+ mice to obtain Myh6 MerCreMer/+;R26R tdTomato/+ double heterozygous animals. Consistent with the X-gal staining results, high levels of tdTomato expression were observed on Myh6 MerCreMer/+;R26R tdTomato/+ hearts at E13.5, E16.5, P0, and P60 following tamoxifen induction (Fig 4A). To examine whether the tdTomato-positive cells were cardiomyocytes, cardiac sections were made for immunofluorescence. We observed that at E13.5 and E16.5, even though robust tdTomato expression was detected in both atrial and ventricular walls, atrial fluorescent signals appear much stronger than that of ventricles. The trabeculated myocardium also displayed stronger fluorescence than the compact myocardium in the ventricles (arrows and arrowheads in Fig 4B2 and 4C2). This may reflect differential Myh6 expression level in cardiac compartments at embryonic stages as previously described [33]. From birth to adulthood, tdTomato signals were evenly distributed in the ventricle (notched arrows in Fig 4D2 and 4E2). We further performed immuostaining with anti-cardiac troponin T (cTnT/Tnnt2, marker for cardiomyocytes), anti-alpha smooth muscle actin (αSMA, marker for vascular smooth muscle cells), and anti-PECAM (marker for endothelial cells) [34–37]. The results showed that tdTomato was fully co-localized with cTnT in the heart at E16.5-P60 (notched arrows in Fig 4B4,4C4,4D4 and 4E4), but not with αSMA or PECAM in the coronary smooth muscle cells (unnotched arrows in Fig 4F4 and 4G4) or endothelial/endocardial cells (unnotched arrows in Fig 4H2 and 4I2). Additionally, tdTomato was not detected in valves (arrowheads in Fig 4C4 and 4I2). This indicates that Myh6 MerCreMer/+ specifically drives Cre recombination in cardiomyocytes.
Fig 4. Myh6 MerCreMer/+ introduces specific recombination in cardiomyocytes.
R26R tdTomato/+ reporter line was used to trace Myh6 lineage after tamoxifen induction. (A) tdTomato expression on the whole-mount hearts at E13.5-P60. (B-I) Immunostaining on transverse sections of hearts revealed tdTomato expression was co-localized with Tnnt2 (B-E), but was not co-localized with αSMA (F,G) or PECAM (H,I). Notched arrows in B2/C2/D2/E2 indicate tdTomato expression. Arrowheads in B2/C2 indicate lower level expression of tdTomato in VS and ventricular wall. Unnotched arrows in F,G indicate αSMA staining, and in H,I indicate PECAM staining. Arrowheads in C4 indicate valves. B4-G4 are merged images for B1/2/3-G1/2/3, respectively. H2 and I2 are merged images of H1 and I1 with corresponding tdTomato/DAPI images.
Myh6 MerCreMer/+ Hearts Display Normal Structure and Function before and after Tamoxifen Induction
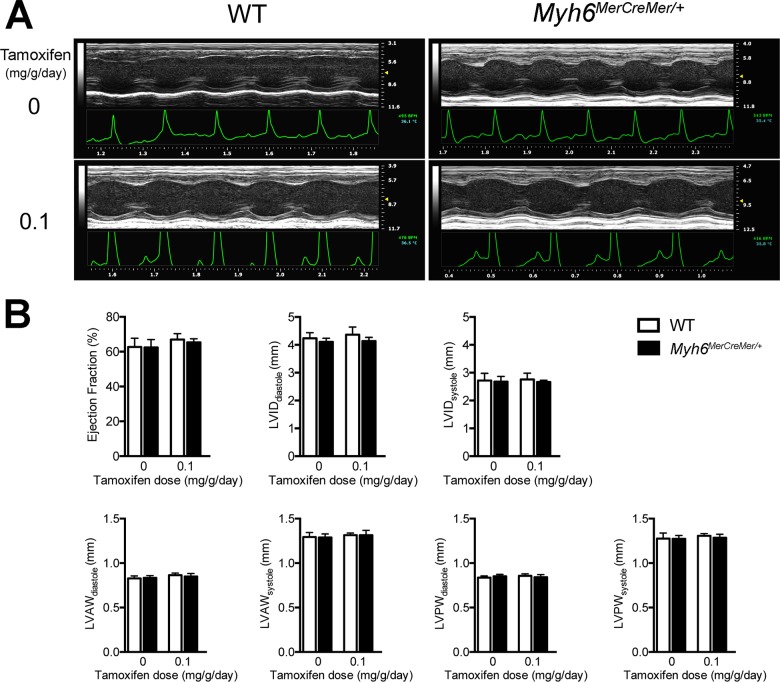
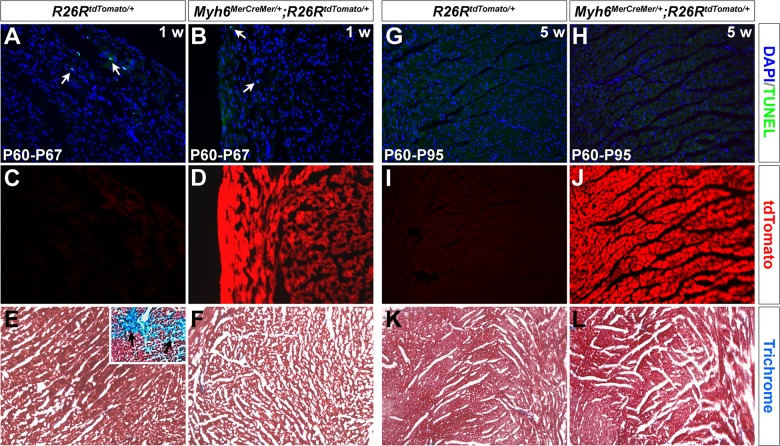
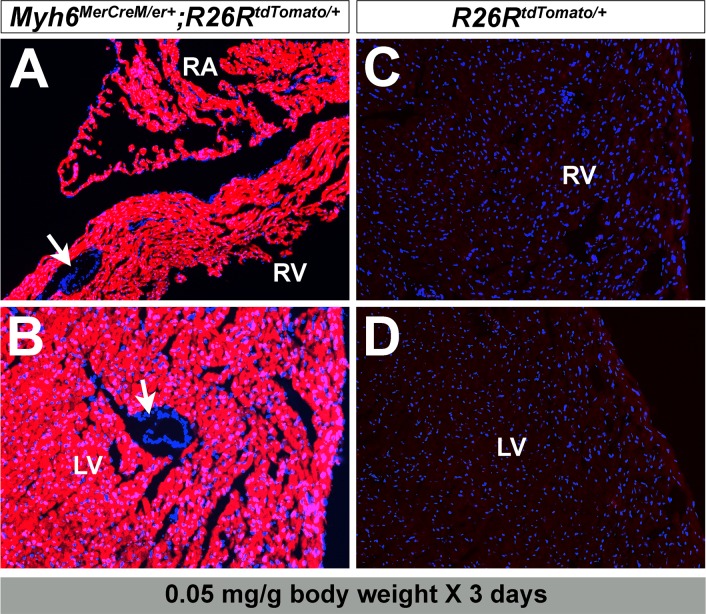
Given that Myh6 MerCreMer/+ is a knock-in/knock-out allele for Myh6, it is important to know whether the cardiac function and structure were adversely affected by MerCreMer insertion. We performed transthoracic echocardiography on Myh6 MerCreMer/+ and wild type littermate mice at P60-90 (n = 10 for each group). Left ventricular short-axis measurements showed no change in cardiac structure and function between Myh6 MerCreMer/+ and wild type littermate mice (Fig 5A and S1 and S2 Videos). There was no significant difference in cardiac chamber dimensions, wall thicknesses, fractional shortening, or ejection fraction in Myh6 MerCreMer/+ mice when compared to their wild type littermates (Fig 5B, Table 1), suggesting MerCreMer insertion into the Myh6 start codon had no effect on cardiac development and function. Furthermore, to determine whether tamoxifen administration had any impact on cardiac function, we performed echocardiography on Myh6 MerCreMer/+ and wild type littermates five weeks after the final injection (0.1 mg/g body weight/day for 3 days). No significant difference was found between Myh6 MerCreMer/+ and littermate controls (n = 6 for each group, Fig 5, Table 1 and S3 and S4 Videos). Subsequent TUNEL and trichrome staining demonstrated that tamoxifen does not lead to myocardial apoptosis or fibrosis on Myh6 MerCreMer/+ hearts one and five weeks after injection (Fig 6). In addition, we attempted to determine the minimum effective tamoxifen dosage to minimize any potential cardiac toxicity. With 0.05 mg/g body weight/day for three days, the adult Myh6 MerCreMer/+ hearts still exhibited sufficient recombination one month after tamoxifen injection (Fig 7).
Fig 5. Normal cardiac function and dimensions after tamoxifen induction in Myh6 MerCreMer/+ mice.
(A) M-mode echocardiography of the same Myh6 MerCreMer/+ and wild type littermate mouse at baseline and five weeks after tamoxifen administration. (B) Quantified indices of left ventricular function and structure before and after Cre induction. Tamoxifen was administered at 0.1 mg/g body weight/day. Ejection fraction, EF; left ventricular internal diameter, LVID; left ventricular anterior wall, LVAW; left ventricular posterior wall, LVPW. All values were plotted as mean ± STDEV.
Table 1. Transthoracic echocardiography of Myh6 MerCreMer/+ mice.
| TMX dose (mg/g/day) | Genotype | Number (n) | HR (BPM) | LVID,s (mm) | LVID,d (mm) | FS (%) | LVAW,d (mm) | LVAW,s (mm) | LVPW,d (mm) | LVPW,s (mm) | EF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Wild type | 10 | 465 ± 48 | 2.72 ± 0.26 | 4.24 ± 0.20 | 35.8 ± 4.6 | 0.83 ± 0.03 | 1.29 ± 0.05 | 0.84 ± 0.02 | 1.28 ± 0.06 | 62.7 ± 5.0 |
| Myh6 MerCreMer/+ | 10 | 482 ± 45 | 2.68 ± 0.19 | 4.11 ± 0.13 | 34.8 ± 4.7 | 0.83 ± 0.03 | 1.29 ± 0.04 | 0.85 ± 0.02 | 1.27 ± 0.04 | 62.4 ± 4.6 | |
| p-value | 0.41 | 0.67 | 0.10 | 0.62 | 0.59 | 0.79 | 0.09 | 0.92 | 0.89 | ||
| 0.1 | Wild type | 6 | 441 ± 44 | 2.75 ± 0.23 | 4.37 ± 0.27 | 36.9 ± 2.6 | 0.86 ± 0.02 | 1.31 ± 0.02 | 0.86 ± 0.02 | 1.31 ± 0.02 | 67.0 ± 3.4 |
| Myh6 MerCreMer/+ | 6 | 421 ± 22 | 2.66 ± 0.06 | 4.14 ± 0.13 | 35.5 ± 1.6 | 0.85 ± 0.03 | 1.31 ± 0.05 | 0.84 ± 0.03 | 1.29 ± 0.04 | 65.4 ± 2.0 | |
| p-value | 0.36 | 0.37 | 0.09 | 0.29 | 0.40 | 1.00 | 0.34 | 0.27 | 0.35 | ||
Normal left ventricular dimensions and function of Myh6 MerCreMer/+ mice in comparison to wild type controls. TMX, Tamoxifen; Heart rate, HR; left ventricular internal diameter, diastole, LVID,d; LVID, systole, LVID,s; fractional shortening, FS; left ventricular anterior wall, systole, LVAW,s; LVAW, diastole, LVAW,d; left ventricular posterior wall, diastole, LVPW,d; LVPW, systole, LVPW,s; ejection fraction, EF. Groups were compared using Student’s t-test. P < 0.05 was considered significant. All values were expressed as mean ± STDEV.
Fig 6. Tamoxifen has little effect on Myh6 MerCreMer/+ hearts.
Apoptosis and fibrosis assays on Myh6 MerCreMer/+ mouse hearts in one week (A-F) and five weeks (G-L) after tamoxifen injection (0.01 mg/g body weight for 3 days). A few apoptotic cells were observed on both R26R tdTomato/+ and Myh6 MerCreMer/+;R26R tdTomato/+ hearts after one week (arrows in A,B, P60→P67), and very few detected after five weeks (G,H, P60→P95). Tamoxifen mediates robust recombination on Myh6 MerCreMer/+;R26R tdTomato/+ hearts (D,J), but not on R26R tdTomato/+ control hearts (C,I). Myocardial fibrosis was not detected on R26R tdTomato/+ or Myh6 MerCreMer/+;R26R tdTomato/+ hearts by trichrome staining after one (E,F) and five weeks (K,L). Image in the upright corner of E is from an unrelated study, showing positive trichrome staining and cardiac fibrosis (arrows). This staining was performed in parallel with sample in E.
Fig 7. Lower dosage of tamoxifen mediates sufficient recombination on adult Myh6 MerCreMer/+ hearts.
Administration of tamoxifen at 0.05 mg/g body weight for 3 days also introduces robust tdTomato expression in Myh6 MerCreMer/+;R26R tdTomato/+ mouse hearts (A,B). No recombination occurs in the control hearts (R26R tdTomato/+, C,D). Arrows in A,B indicate coronary vessels.
Discussion
In this study, we described generation and characterization of a new Myh6 MerCreMer/+ knock-in mouse model. Myh6 MerCreMer/+ mice develop normally without cardiac functional defects. Short-term tamoxifen treatment resulted in efficient Cre recombination in cardiomyocytes. This new Myh6 MerCreMer/+ animal is a useful tool for deletion of genes of interest in myocardium with Cre-LoxP technology at desirable stages.
A few mouse lines were created previously for inducible genetic deletion in myocardium with tetracycline and MerCreMer systems [11, 12]. The tetracycline-inducible system requires two transgenes: a reverse tetracycline-controlled transactivator (rtTA) directed by a rat cTnT promoter and a Cre recombinase driven by a tetracycline responsive promoter (TetO), thereby making breeding scenarios complicated [11]. Another limitation of the tetracycline-inducible system is potential leakiness [38]. The α-MHC-MerCreMer transgenic mouse line was generated and had been used widely for gene inactivation in the myocardium [12, 39–42]. However, a few studies showed that the α-MHC-MerCreMer mouse line displayed myocardial fibrosis and cardiac dysfunction due to Cre-induced DNA damage and myocardial apoptosis after tamoxifen induction [15–21]. In this new Myh6 MerCreMer/+ mouse, Cre recombination is strongly activated within cardiomyocytes following tamoxifen induction. This animal exhibited relatively good tolerance to tamoxifen (no myocardial fibrosis or apoptosis) and displayed normal cardiac structure and function after appropriate induction. Moreover, a low dosage of tamoxifen (0.05 mg/g body weight for 3 days) also introduces robust and specific recombination in the cardiomyocytes at adult stage.
Myh6 MerCreMer/+ is a heterozygous null for Myh6 (Myh6 +/−). Myh6 +/− animals were shown to have cardiac functional defects with sarcomeric structural alterations and fibrosis [43]. However, by performing trichrome staining and echocardiography, we did not detect effects on Myh6 MerCreMer/+ hearts (2–3 months old, n = 10, Fig 5, Table 1, and S1 and S2 Videos). The discrepancy could be due to the difference in the gene targeting strategy and/or a genetic divergence between these animals: in Myh6 MerCreMer/+ mice, the MerCreMer-FRT-Neo-FRT cassette was inserted into the ATG locus and the Neomycin sequence was removed by Flippase deleter mice. In the Myh6 +/− mice examined by Jones et al [43], the pgk-Neo-polyA cassette was targeted into the Myh6 locus with a deletion of an approximately 2-kb fragment of the Myh6 gene. The deleted sequence includes the first three exons, the 5' untranslated region, and the initiating methionine codon [43]. It is uncertain whether the existing Neomycin cassette in this strong myocardial locus has any negative effects on cardiac function. Moreover, it is important to note that the physiologic and pathologic phenotypes in Myh6 +/− mice are not completely penetrant [43]. Myh6 MerCreMer/+ animals in this study are in hybrid background (Black Swiss), and genetic and epigenetic variations could potentially be important factors for Myh6 +/− heart function [43]. In the future, it will be of interest to determine whether the inbred background of Myh6 MerCreMer/+ mice has any impact on their cardiac performance.
As mentioned before, tamoxifen injection into α-MHC-MerCreMer transgenic line could lead to severe toxicity to the heart [15–21]. Mice with three doses of tamoxifen at 0.03–0.09 mg/g body weight/day displayed cardiac fibrosis and dysfunction, with 10–50% mortality within one week [20, 21]. In this study, we found that with three doses of tamoxifen at 0.1 mg/g body weight/day, the Myh6 MerCreMer/+ mice appeared normal in cardiac function and structure and no lethality was observed. No myocardial fibrosis or apoptosis was found in Myh6 MerCreMer/+ mice after one and five weeks of administration (Figs 4 and 5). This may be explained by the genetic difference between α-MHC-MerCreMer and Myh6 MerCreMer/+ mice. α-MHC-MerCreMer is a transgenic line and each myocardial cell may have multiple copies of MerCreMer (note each MerCreMer cassette has its own α-MHC promoter) [44]. In contrast, Myh6 MerCreMer/+ knock-in animals only have one copy of MerCreMer in their genome. Therefore, MerCreMer expression in α-MHC-MerCreMer myocardial cells might be much higher than that in Myh6 MerCreMer/+ myocardial cells. Under certain dosage of tamoxifen induction (e.g., 0.1 mg/g body weight/day for three days), the high level MerCreMer expression may lead to excessive amount of Cre translocation to nuclei which in turn may induce DNA damage and cell death in the cardiomyocytes. Myh6 MerCreMer/+ cardiomyocytes have lower MerCreMer expression and do not have a large amount of Cre translocation under this dosage.
The major application of this new Myh6 MerCreMer/+ mouse model will be the temporal disruption of genes of interest in cardiomyocytes in vivo. Given that almost all the myocardial cells robustly express Cre after tamoxifen induction, this inducible Cre mouse line can also be applied to determine myocardial lineage during development and after cardiac injury.
Supporting Information
(AVI)
(AVI)
(AVI)
(AVI)
Acknowledgments
The authors thank Dr. Kevin Kelly in the Transgenic Core at Mount Sinai in generating the Myh6 MerCreMer/+ knock-in mouse.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by grants to C.L.C. from the NHLBI (1R01HL095810 and 1K02HL094688), NYSTEM (C026426), the American Heart Association (15GRNT25710153) and the March of Dimes Foundation (5-FY07-642), and to A.S. from NIH T32 GM066704 (Bach). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gelb BD, Chung WK. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb Perspect Med. 2014;4(7):a013953 10.1101/cshperspect.a013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf M, Basson CT. The molecular genetics of congenital heart disease: a review of recent developments. Curr Opin Cardiol. 2010;25(3):192–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruneau BG, Srivastava D. Congenital heart disease: entering a new era of human genetics. Circ Res. 2014;114(4):598–9. 10.1161/CIRCRESAHA.113.303060 [DOI] [PubMed] [Google Scholar]
- 4. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. . [DOI] [PubMed] [Google Scholar]
- 5. Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117(3):373–86. Epub 2004/04/28. S0092867404004052 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6. Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, et al. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 351(1):62–9. Epub 2010/12/28. S0012-1606(10)01264-9 [pii] 10.1016/j.ydbio.2010.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–18. Epub 2006/08/18. dev.02519 [pii] 10.1242/dev.02519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, et al. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114(4):298–308. Epub 2006/07/19. CIRCULATIONAHA.105.608968 [pii] 10.1161/CIRCULATIONAHA.105.608968 . [DOI] [PubMed] [Google Scholar]
- 9. Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 23(2):280–91. Epub 2012/08/18. S1534-5807(12)00280-8 [pii] 10.1016/j.devcel.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai X, Zhang W, Hu J, Zhang L, Sultana N, Wu B, et al. Tbx20 acts upstream of Wnt signaling to regulate endocardial cushion formation and valve remodeling during mouse cardiogenesis. Development. 140(15):3176–87. Epub 2013/07/05. dev.092502 [pii] 10.1242/dev.092502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu B, Zhou B, Wang Y, Cheng HL, Hang CT, Pu WT, et al. Inducible cardiomyocyte-specific gene disruption directed by the rat Tnnt2 promoter in the mouse. Genesis. 2010;48(1):63–72. 10.1002/dvg.20573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89(1):20–5. . [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Riesterer C, Ayrall AM, Sablitzky F, Littlewood TD, Reth M. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 1996;24(4):543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robbins J. Genetic modification of the heart: exploring necessity and sufficiency in the past 10 years. J Mol Cell Cardiol. 2004;36(5):643–52. . [DOI] [PubMed] [Google Scholar]
- 15. Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, et al. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail. 2006;12(5):392–8. Epub 2006/06/10. S1071-9164(06)00136-9 [pii] 10.1016/j.cardfail.2006.03.002 . [DOI] [PubMed] [Google Scholar]
- 16. Hall ME, Smith G, Hall JE, Stec DE. Systolic dysfunction in cardiac-specific ligand-inducible MerCreMer transgenic mice. Am J Physiol Heart Circ Physiol. 2011;301(1):H253–60. 10.1152/ajpheart.00786.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, et al. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105(1):12–5. Epub 2009/06/13. CIRCRESAHA.109.198416 [pii] 10.1161/CIRCRESAHA.109.198416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hougen K, Aronsen JM, Stokke MK, Enger U, Nygard S, Andersson KB, et al. Cre-loxP DNA recombination is possible with only minimal unspecific transcriptional changes and without cardiomyopathy in Tg(alphaMHC-MerCreMer) mice. Am J Physiol Heart Circ Physiol. 2010;299(5):H1671–8. Epub 2010/08/31. ajpheart.01155.2009 [pii] 10.1152/ajpheart.01155.2009 . [DOI] [PubMed] [Google Scholar]
- 19. Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 2009;46(2):130–6. Epub 2008/10/11. S0022-2828(08)00584-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lexow J, Poggioli T, Sarathchandra P, Santini MP, Rosenthal N. Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis Model Mech. 2013;6(6):1470–6. 10.1242/dmm.010470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, et al. Moderate and high amounts of tamoxifen in alphaMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis Model Mech. 2013;6(6):1459–69. 10.1242/dmm.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, et al. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112(1):54–9. . [DOI] [PubMed] [Google Scholar]
- 23. Ching YH, Ghosh TK, Cross SJ, Packham EA, Honeyman L, Loughna S, et al. Mutation in myosin heavy chain 6 causes atrial septal defect. Nat Genet. 2005;37(4):423–8. . [DOI] [PubMed] [Google Scholar]
- 24. Posch MG, Waldmuller S, Muller M, Scheffold T, Fournier D, Andrade-Navarro MA, et al. Cardiac alpha-myosin (MYH6) is the predominant sarcomeric disease gene for familial atrial septal defects. PLoS One. 2011;6(12):e28872 10.1371/journal.pone.0028872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng WA, Grupp IL, Subramaniam A, Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991;68(6):1742–50. . [DOI] [PubMed] [Google Scholar]
- 26. Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28(3–4):106–10. . [PubMed] [Google Scholar]
- 27. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–1. . [DOI] [PubMed] [Google Scholar]
- 28. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park EJ, Sun X, Nichol P, Saijoh Y, Martin JF, Moon AM. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev Dyn. 2008;237(2):447–53. Epub 2007/12/28. 10.1002/dvdy.21415 . [DOI] [PubMed] [Google Scholar]
- 30. Yan J, Zhang L, Xu J, Sultana N, Hu J, Cai X, et al. Smad4 Regulates Ureteral Smooth Muscle Cell Differentiation during Mouse Embryogenesis. PLoS One. 2014;9(8):e104503 10.1371/journal.pone.0104503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan J, Sultana N, Zhang L, Park DS, Shekhar A, Hu J, et al. Generation of a tamoxifen inducible Tnnt2 knock-in mouse model for cardiac studies. Genesis. 2015. 10.1002/dvg.22861 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301(5):H1765–80. Epub 2011/08/30. ajpheart.00559.2011 [pii] 10.1152/ajpheart.00559.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyons GE, Schiaffino S, Sassoon D, Barton P, Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990;111(6 Pt 1):2427–36. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol. 1996;178(2):375–92. . [DOI] [PubMed] [Google Scholar]
- 35. Mack CP, Owens GK. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5' and first intron promoter regions. Circ Res. 1999;84(7):852–61. . [DOI] [PubMed] [Google Scholar]
- 36. Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101(11):1139–45. . [DOI] [PubMed] [Google Scholar]
- 37. Walsh S, Ponten A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010;86(3):365–73. 10.1093/cvr/cvq005 [DOI] [PubMed] [Google Scholar]
- 38. Jaisser F. Inducible gene expression and gene modification in transgenic mice. J Am Soc Nephrol. 2000;11 Suppl 16:S95–S100. . [PubMed] [Google Scholar]
- 39. Nemir M, Metrich M, Plaisance I, Lepore M, Cruchet S, Berthonneche C, et al. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur Heart J. 2014;35(32):2174–85. 10.1093/eurheartj/ehs269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111(24):8850–5. 10.1073/pnas.1408233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27(12):1365–77. 10.1101/gad.215871.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123(10):1073–82. 10.1161/CIRCULATIONAHA.110.977066 [DOI] [PubMed] [Google Scholar]
- 43. Jones WK, Grupp IL, Doetschman T, Grupp G, Osinska H, Hewett TE, et al. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest. 1996;98(8):1906–17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pinkert CA. Transgenic animal technology: a laboratory handbook. 2nd ed Amsterdam; Boston: Academic Press; 2002. xv, 618 p. p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(AVI)
(AVI)
(AVI)
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.