Abstract
Delusions of schizophrenia have been found to be associated with alterations of some brain regions in structure and task-induced activation. However, the relationship between spontaneously occurring symptoms and spontaneous brain activity remains unclear. In the current study, 14 schizophrenic patients with delusions and 14 healthy controls underwent a resting-state functional magnetic resonance imaging (RS-fMRI) scan. Patients with delusions of schizophrenia patients were rated with Positive and Negative Syndrome Scale (PANSS) and Characteristics of Delusional Rating Scale (CDRS). Regional homogeneity (ReHo) was calculated to measure the local synchronization of the spontaneous activity in a voxel-wise way. A two-sample t-test showed that ReHo of the right anterior cingulate gyrus and left medial superior frontal gyrus were higher in patients, and ReHo of the left superior occipital gyrus was lower, compared to healthy controls. Further, among patients, correlation analysis showed a significant difference between delusion scores of CRDS and ReHo of brain regions. ReHo of the left medial superior frontal gyrus was negatively correlated with patients’ CDRS scores but not with delusional PANSS scores. These results suggested that altered local synchronization of spontaneous brain activity may be related to the pathophysiology of delusion in schizophrenia.
Introduction
“Delusion is a false belief based on incorrect inference about external reality that is firmly sustained despite what almost everyone else believes and despite what constitutes incontrovertible and obvious proof of evidence to the contrary” [American Psychiatric Association (APA), 1994]. Delusion is a core symptom for the diagnosis of schizophrenia [1], occurring in more than 70% of schizophrenia patients [2]. Identifying the neuroanatomical and functional underpinnings of specific symptoms offers significant insight into the etiology of schizophrenia [3].
Some evidence has been accumulated from structural magnetic resonance imaging (MRI) and task-related functional MRI (fMRI) studies. Structure alterations related to delusions in schizophrenia have been explored [4–12]. In previous studies, Whitford and his colleagues found that schizophrenic patients' delusion severity was positively correlated with the volume of the dorso-medial prefrontal cortex (DMPFC), centered on the medial frontal gyrus [13]. Although many structurally-altered brain areas were related to delusions of schizophrenia, structural brain changes usually occurred in relatively later stages of schizophrenia patients. Brain function research has been applied in the past to explore earlier brain changes, Many fMRI studies investigated brain activation in schizophrenic patients with delusions using cognitive tasks, such as a feedback task which was used to investigate the neural responses to feedback of (successful vs. unsuccessful) monetary gain or avoidance of loss [14]. A reference evoking task was also used in these studies, which was carried out by viewing video vignettes of referential conversations, non-referential conversations or no conversations between two people, filmed at varying distances of 1 m, 5 m or 10 m, while undergoing an fMRI scan [15]. A working memory task including auditory and visual memory was employed as well [16]. The severity of delusions in schizophrenic patients was negatively correlated with activation in the superior temporal sulcus in a reference-evoking task, which might be related to the formation of referential or persecutory delusions, and activation of the superior temporal sulcus might affect the severity of delusion [15]. However, the delusions in schizophrenic patients are spontaneous and it is difficult to design a specific task that can evoke delusions. Therefore, to explore the association of spontaneous brain activity and delusions may be helpful to reveal the underlying brain activity of delusions.
Positron emission tomography (PET) and resting-state fMRI (RS-fMRI) are important instruments to explore spontaneous brain activity. PET studies have shown the association between delusions and resting-state brain activity. For example, it was found that delusions showed a strong negative correlation with regional cerebral blood flow (rCBF) in the left frontal cortex [17] and left parahippocampal gyrus [18]. However, compared to PET, RS-fMRI does not require intravenous injection and has better temporal and spatial resolution. An increasing number of RS-fMRI studies have been carried out to investigate spontaneous activity since the first RS-fMRI report by Biswal and colleagues [19]. Most RS-fMRI studies have investigated the synchronization or functional connectivity of the time courses among “distinct” brain regions [20–26]; however regional homogeneity (ReHo) [27] has been used to analyze “local” synchronization. An abnormal functional connectivity between two distinct regions indicates an abnormal relationship between these two regions; however, the method does not confirm which specific region is abnormal. In contrast, an abnormal ReHo indicates abnormal brain activity in a specific region. For example, abnormally increased ReHo in the medial temporal lobe has been reported in medial temporal lobe epilepsy (mTLE) patients [28–30], suggesting increased neuronal local synchronization in the epileptic focus. ReHo assumes that, within a functional cluster, the hemodynamic feature of each voxel would be similar or synchronous with that of others, and that this similarity could be altered or modulated in different situations. Higher ReHo indicates more synchronization in the functional cluster, which may illustrate functional synchronization in certain brain regions [27]. This method has already been used for investigation of functional modulations in the resting state for patients with schizophrenia [31, 32], Alzheimer’s disease (AD) [33], Parkinson’s disease (PD) [34], and other pathological states (see review by [35, 36]).
In this study, we were interested in whether schizophrenic patients with delusions would show abnormal ReHo and, if so, whether the brain areas with abnormal ReHo were correlated with delusions.
Methods
2.1 Participants
Fourteen right-handed schizophrenia inpatients and outpatients of the Seventh People’s Hospital of Hangzhou were included in the study. All patients were diagnosed as paranoid schizophrenics according to DSM-IV (American Psychiatric Association, 1994) and the delusions were the predominant symptom of each patient; i.e., all patients were experiencing stable delusions during the experiment. Each patient had a single delusion. The most prevalent delusion was persecution delusion, which also included reference and guilt delusion. All the patients were assessed and diagnosed by experienced psychiatrists of the Seventh People’s Hospital of Hangzhou, using structured clinical interview for DSM-IV (SCID). The Positive and Negative Syndrome Scale (PANSS) [37] and Characteristics of Delusional Rating Scale (CDRS) [38] were used as instruments of clinical assessment. CDRS is a widely used multidimensional instrument for evaluating delusions [1] and is regarded as an expert rating scale of delusion [1]. It contains 11 dimensions: conviction, preoccupation, interference, resistance, dismissibility, absurdity, self-evidentness, reassurance, worry, unhappiness and pervasiveness [38]. All patients’ scales were rated by a trained and experienced psychiatrist. One patient missed one item (pervasiveness) in the CDRS. We used the mean CDRS score (total score divided by the number of items) to avoid the influence of the missing item. Fourteen right-handed healthy controls were recruited from Hangzhou by advertisements posted in the Hangzhou community. All healthy controls were also assessed by the psychiatrists of the Seventh People’s Hospital of Hangzhou, using the structured clinical interview according to the DSM-IV (SCID). None had psychosis. The two groups were matched for age, gender, and education level (Table 1).
Table 1. Demographic and clinical parameters of healthy control and schizophrenic patient groups (Mean ± SD).
| Group | ||
|---|---|---|
| Schizophrenia patients | Healthy controls | |
| Gender (M/F) | 9/5 | 9/5 |
| Age (years) | 33.2±10.7 | 34.9±13.6 |
| Education (years) | 11.7±2.7 | 11.3±2.3 |
| Illness duration (years) | 9.2±8.5 | N/A |
| Medication (average chlorpromazine equivalent, mg/day) | 586.5±327 | N/A |
| CDRS (average) | 6.2±1.6 | N/A |
| PANSS | N/A | |
| Total | 74.1±16.2 | |
| Positive | 16.4±5.3 | |
| Delusions(P1) | 3.9±1.2 | |
| Negative | 22.6±6.2 | |
| General | 30.8±8.7 | |
N/A: Not applicable. P1: The item of PANSS-Delusion subscale.
Exclusion criteria for participants were i) a history of head injury, neurological disease such as epilepsy, other serious illness, alcohol dependence, exposure to electroconvulsive therapy, and other psychiatric disorders (healthy controls with a history of schizophrenia and a family history of psychosis were also excluded), and ii) the intake of medication in 6 hours before the fMRI scan.
This study was approved by the ethics committee of the Seventh People’s Hospital of Hangzhou and Zhejiang University. We gave each participant or the legally authorized representative detailed information on the processes (including possible risks) of the study with commonly used words until that he/she understood completely. When he/she agreed, he/she would sign an informed consent. Written informed consent of the schizophrenic patient was obtained from his/her legally authorized representative and the control provided written informed consent himself/herself.
2.2 Image acquisition
Each participant was scanned with a 1.5 Tesla Siemens Sonata scanner. Foam pads were used to reduce head motion. The functional T2*-weighted resting-state images were acquired using an echo planar imaging (EPI) sequence (TR/TE 2000/40 ms, FA 90°, FOV 240 × 240 mm, matrix 64 × 64, slice thickness 5 mm with 1 a mm gap, 23 slices, scan time 8 min, 240 volumes). The participants were instructed to lie still with their eyes closed and not to think of anything in particular during RS-fMRI data acquisition [19, 27, 39].
2.3 Data analysis
2.3.1 ReHo calculation
The fMRI data preprocessing was performed with MATLAB (2008a, the MathWorks, Natick, MA, USA) and Data Processing Assistant for Resting-State fMRI (DPARSF, Basic edition) [40]. The first 10 volumes were discarded for scanner calibration and adaption of participants to the circumstances. Other preprocesses included slice-timing, realignment, resampling to a 3 mm isotropic voxel size and spatial normalization with an EPI template. No participant’s data was discarded due to excessive head motion (more than 2.5 mm in translation or 2.5 degree in rotation).
A temporal filter (0.01 Hz < f < 0.08 Hz) was used to reduce very low-frequency drifts and physiological high-frequency noise [19]. Linear trends were removed. By using DPARSF, ReHo was calculated using the Kendall coefficient of concordance to measure the similarity of time courses of 27 neighboring voxels in a voxel-wise way: where W is KCC among given voxels, ranging from 0 to 1. R i is the sum rank of the ith time point; where R = ((n+1)/K)/2 is the mean of the R i’s; K is the number of time series within a measured cluster (here, K = 27, respectively; one given voxel plus the number of its neighbors); n is the number of ranks (here n = 78) [27]. Thus, the individual ReHo map of each dataset was generated. Then the individual ReHo map was smoothed with a Gaussian filter of 6 mm full width at half-maximum (FWHM). Finally, each ReHo map was divided by the global mean ReHo of each participant for standardization purposes as done in PET studies [41]. Finally, we obtained the smReHo for statistical analysis.
2.3.2 Statistical analysis
A two-sample t-test was applied to compare the ReHo maps between schizophrenic patients with delusions and healthy controls. Multiple comparison correction was performed by using the AlphaSim program in REST 1.5 [42] (http://www.restfmri.net). The AlphaSim program of REST 1.5 was from AFNI [42]. A combination of individual voxel’s p < 0.05 and cluster size > 6156 mm3 (228 voxels) was used, which corresponds to a corrected p < 0.05 (rmm = 5, whole brain mask). Finally, a correlation analysis between the ReHo map and scores of CDRS and PANSS-Delusion subscale, respectively, were performed in the patient group in a voxel-wise way within the clusters, showing significant between-group differences, while the age, gender and medication dosage (chlorpromazine equivalents) were taken as covariates. To correct for the medication effect, all antipsychotic drugs the patients were taking were translated into the chlorpromazine equivalent [43]. The chlorpromazine equivalent of each patient was taken as a covariant during correlation analysis [13]. We also performed a correlation analysis between ReHo and CDRS while taking age, gender, medication dosage (chlorpromazine equivalents) and total score of PANSS, which reflected the severity of schizophrenia, as covariates, to reduce severity effect. Multiple comparison correction was performed by using the AlphaSim program in REST 1.5 [42] (http://www.restfmri.net). A combination of single voxel’s p < 0.05 and cluster size > 351 mm3 (13 voxels) was used, which corresponded to a corrected p < 0.05 (rmm = 5. Brain regions that survived the criteria for two sample t-tests were selected as a mask).
Results
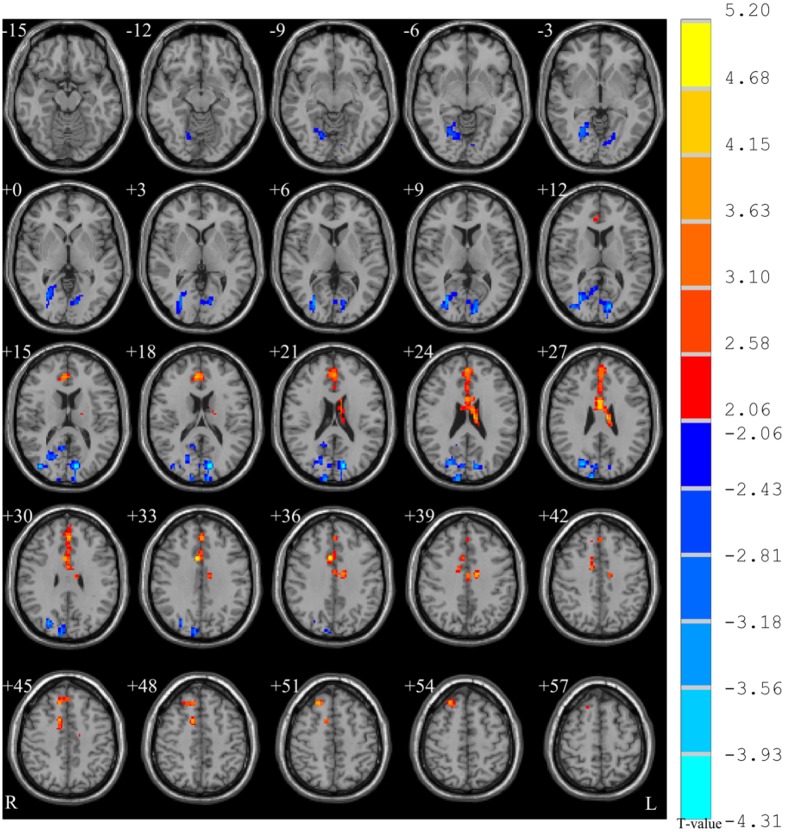
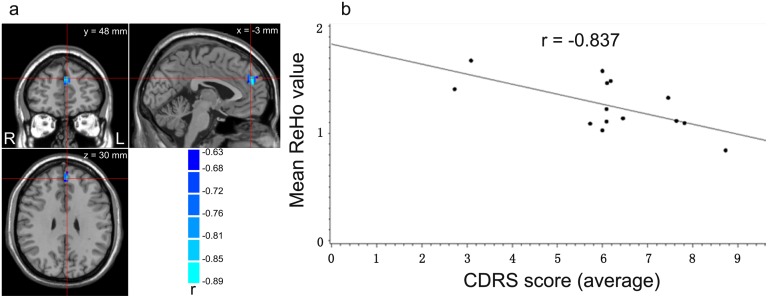
Two sample t-tests (p < 0.05, corrected; see Fig 1 and Table 2) showed that ReHo in the right anterior cingulate gyrus (ACC), extending to left medial superior frontal gyrus (SFG), was higher in the patients group than in controls, while ReHo in the left superior occipital gyrus was lower in the patient group. The medial SFG is also named the dorsal medial prefrontal cortex (dMPFC) [44, 45]. The correlation analysis (p < 0.05, AlphaSim corrected; see Fig 2a and 2b controlling for medication) showed that CDRS score was negatively correlated with the ReHo in the left medial SFG (BA 9). There was no difference between with and without controlling for medication in the correlation analysis results. The correlation between antipsychotic dose and severity of delusion symptoms was significant (r = 0.867, p < 0.001). No significant correlation was found between PANSS delusions subscale score and mean ReHo values within the mask, either with or without the total PANSS score as a covariate.
Fig 1. Two-sample t-test between groups (p < 0.05, corrected).
Warm color indicates that ReHo is higher in the schizophrenic patient group than in the healthy control group, and vice versa.
Table 2. Results of two-sample t-test between groups.
| Peak Coordinates (MNI) | |||||
|---|---|---|---|---|---|
| Brain region | x | y | z | t value | Volume (mm3) |
| R anterior cingulate gyrus, extending to left medial frontal gyrus (BA 24 and BA 9) | 6 | 12 | 33 | 3.53 | 12771 |
| L superior occipital gyrus (BA 18 and BA19) | -12 | -78 | 21 | -4.12 | 16362 |
R: Right. L: Left. BA: Brodmann area. MNI: Montreal Neurological Institute.
Fig 2. Results of correlation analysis (p < 0.05, corrected), controlling for medication.
(a) The left medial superior frontal gyrus (mSFG; 918 mm3, 34 voxels, with peak coordinates at [-3, 48, 30] in the Montreal Neurology Institute system). (b) The plot of negative correlation between scores of CDRS and mean ReHo values across the cluster shown in (a) in schizophrenic patients with delusions.
Discussion
To the best of our knowledge, this is the first study analyzing the ReHo of spontaneous brain activity and delusions of schizophrenic patients by RS-fMRI. We found that ReHo in the right ACC, extending to left dMPFC, was higher in schizophrenic patients compared to controls, while ReHo in the left superior occipital gyrus was lower in the schizophrenic patients group. The ReHo of the left dMPFC (BA 9) was negatively correlated with CDRS scores. No significant correlation of ReHo with PANSS delusions subscale score was found.
The alteration of ACC and dMPFC in schizophrenic patients has been found in many studies. Decreased volume of the right ACC [46, 47] and left dMPFC [13] has been reported in schizophrenic patients. The right ACC and left dMPFC were activated by a task designed to evoke sensations similar to delusions of reference in schizophrenia patients experiencing prominent referencing delusions [48]. In the current study, the increased ReHo in schizophrenic patients indicated that an increased local synchronization of spontaneous activity may be related to delusions. A previous resting-state fMRI study on schizophrenic patients found decreased ReHo in the left medial frontal gyrus, (BA 2, MNI coordinates: -12, -45, -18) [31], which was close to the left dMPFC in the current study. One possible explanation for the seemingly contradictory results is a difference in the schizophrenic patients. In our study, all schizophrenic patients had delusions while in that study the delusion symptoms were not mentioned [31]. As schizophrenia is a highly heterogeneous disorder, it will be important for future studies to use the same method and similar patients to replicate previous results.
The dMPFC was a highly active region. DMPFC was a hyper-perfusion area related to delusion in deluded patients with dementia with Lewy bodies [49]. Activation of dMPFC was elicited from both schizophrenia itself and endorsement of a psychosis state in schizophrenic patients experiencing referential delusions [48]. A delusion formation hypothesis suggests that the delusions arise from the brain’s attempts to integrate the disorganized neural processes experienced by patients with schizophrenia [13]. ReHo also indicated neural functional synchronization in certain brain regions [27]. Higher ReHo in DMPFC may indicate more integration of disorganized neural processes, which facilitates the formation of delusions.
In our study, the ReHo in dMPFC showed a negative correlation with delusion severity. This result is counterintuitive. Controlling for antipsychotic medicine has been considered as one reason. However, the correlated brain region between the ReHo map and delusion has not changed significantly based on whether or not medication was controlled for. Therefore, controlling medication does not appear to lead to the phenomenon. Head motion may influence RS-fMRI result [50, 51]. Yet head motion was also excluded. An explanation of the phenomenon may be the delusion formation hypothesis. They argued that the delusions arose from the brain’s attempts to integrate the disorganized neural processes experienced by patients with schizophrenia [13]. Compared to normal controls, schizophrenic patients needed to integrate disorganized neural processes for delusion. Therefore, patients showed higher ReHo than controls in the deluded brain region. However, among the patients themselves, the severe symptoms meant dysfunction of the brain. The severe patients did not have enough ability to integrate disorganized neural process. Therefore, the more severe delusional schizophrenic patients showed a decreased ReHo.
In this study, we found decreased ReHo in the left superior occipital gyrus of schizophrenic patients. However, in this area there was no significant correlation between ReHo values and CDRS scores. This was consistent with previous studies. Liu and colleagues also found decreased ReHo in the left inferior and middle occipital gyrus [31]. It seems that the decreased ReHo in the occipital area may not be closely related to delusions. Rather, an abnormal ReHo in the occipital area may be a more general abnormality in schizophrenic patients.
No significant correlation was found between ReHo maps and the PANSS delusions subscale, but a significant correlation with CDRS was found in schizophrenic patients with delusions. CDRS measures more dimensions of delusion than PANSS. Garety believed that delusion contained 11 dimensions, which were conviction, preoccupation, interference, resistance, dismissibility, absurdity, self-evidentness, reassurance, worry, unhappiness, and pervasiveness [38, 52]. However, the delusion subscale in PANSS only contains one item [37, 53]. This scale can only rate the severity of delusion in the most apparent aspects, such as conviction [54] or bizarreness [1]. It is quite plausible the CDRS provides more intrinsic information about delusions, which can be expressed in brain activity.
A few limitations in this study should be addressed. First, the sample is relatively small, especially considering that delusion is a very complex syndrome. If there is a source for an fMRI dataset, we may do more work similar to Turner’s [55]. Second, all patients were taking medicine. Results from drug naïve schizophrenia patients with delusions would be more helpful in understanding the brain mechanism of delusions. Third, although we have used the total score of PANSS as a covariate to exclude the confounding effects of other symptoms, it could not exclude the effects of other symptoms completely. If there is a direct comparison between delusion-dominated patients and non-deluded patients in the future, the results may be more significant. This should be studied further in the future.
In summary, the increased ReHo demonstrated by RS-fMRI in schizophrenic patients with delusions in the dMPFC may suggest that increased local synchronization of spontaneous brain activity may underlie the delusions.
Acknowledgments
We thank very much too all subjects taking part in this study. The authors further wish to thank prof. Xiaowei Tang and all members of Bio-x laboratory of Zhejiang University for their help in fMRI data processing.
Data Availability
This study contains private information of the subjects. Due to ethical and legal restrictions data are available upon request. Interested researchers may contact Yihong Zhu via email: zhyh@zju.edu.cn or Bin Gao via email: zjugaobin@163.com.
Funding Statement
This work was supported by the Key Specialty Project of Hangzhou Science and Technology Bureau (No. 20100733Q19, http://www.hzst.gov.cn/), and the Type A Project of Hangzhou Health Bureau (No. 2010A019, http://www.hzwsjsw.gov.cn/index/index.jhtml).
References
- 1. Kronmuller KT, von Bock A, Grupe S, Buche L, Gentner NC, Ruckl S, et al. Psychometric evaluation of the Psychotic Symptom Rating Scales. Compr Psychiatry. 2011;52(1):102–8. Epub 2011/01/12. doi: S0010-440X(10)00048-9 [pii] 10.1016/j.comppsych.2010.04.014 . [DOI] [PubMed] [Google Scholar]
- 2. Sartorius N, Jablensky A, Korten A, Ernberg G, Anker M, Cooper JE, et al. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO Collaborative Study on determinants of outcome of severe mental disorders. Psychol Med. 1986;16(4):909–28. Epub 1986/11/01. . [DOI] [PubMed] [Google Scholar]
- 3. Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8(9):725–32. Epub 2007/08/21. doi: nrn2218 [pii] 10.1038/nrn2218 . [DOI] [PubMed] [Google Scholar]
- 4. Yamasaki S, Yamasue H, Abe O, Yamada H, Iwanami A, Hirayasu Y, et al. Reduced planum temporale volume and delusional behaviour in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257(6):318–24. Epub 2007/05/01. 10.1007/s00406-007-0723-5 . [DOI] [PubMed] [Google Scholar]
- 5. Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161(9):1612–9. Epub 2004/09/01. 10.1176/appi.ajp.161.9.1612 161/9/1612 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6. Takahashi T, Zhou SY, Nakamura K, Tanino R, Furuichi A, Kido M, et al. Longitudinal volume changes of the pituitary gland in patients with schizotypal disorder and first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):177–83. Epub 2010/11/04. doi: S0278-5846(10)00409-4 [pii] 10.1016/j.pnpbp.2010.10.023 . [DOI] [PubMed] [Google Scholar]
- 7. Sumich A, Chitnis XA, Fannon DG, O'Ceallaigh S, Doku VC, Faldrowicz A, et al. Unreality symptoms and volumetric measures of Heschl's gyrus and planum temporal in first-episode psychosis. Biol Psychiatry. 2005;57(8):947–50. Epub 2005/04/12. doi: S0006-3223(05)00005-3 [pii] 10.1016/j.biopsych.2004.12.041 . [DOI] [PubMed] [Google Scholar]
- 8. Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66(4):366–76. Epub 2009/04/08. doi: 66/4/366 [pii] 10.1001/archgenpsychiatry.2009.12 . [DOI] [PubMed] [Google Scholar]
- 9. Spencer MD, Moorhead TW, McIntosh AM, Stanfield AC, Muir WJ, Hoare P, et al. Grey matter correlates of early psychotic symptoms in adolescents at enhanced risk of psychosis: a voxel-based study. Neuroimage. 2007;35(3):1181–91. Epub 2007/02/27. doi: S1053-8119(07)00039-0 [pii] 10.1016/j.neuroimage.2007.01.008 . [DOI] [PubMed] [Google Scholar]
- 10. Suga M, Yamasue H, Abe O, Yamasaki S, Yamada H, Inoue H, et al. Reduced gray matter volume of Brodmann's Area 45 is associated with severe psychotic symptoms in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(6):465–73. Epub 2009/12/19. 10.1007/s00406-009-0094-1 . [DOI] [PubMed] [Google Scholar]
- 11. Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007;89(1–3):59–71. Epub 2006/10/31. doi: S0920-9964(06)00386-0 [pii] 10.1016/j.schres.2006.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad KM, Rohm BR, Keshavan MS. Parahippocampal gyrus in first episode psychotic disorders: a structural magnetic resonance imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):651–8. Epub 2004/07/28. 10.1016/j.pnpbp.2004.01.017 S0278-5846(04)00036-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13. Whitford TJ, Farrow TF, Williams LM, Gomes L, Brennan J, Harris AW. Delusions and dorso-medial frontal cortex volume in first-episode schizophrenia: a voxel-based morphometry study. Psychiatry Res. 2009;172(3):175–9. Epub 2009/04/28. doi: S0925-4927(08)00098-X [pii] 10.1016/j.pscychresns.2008.07.011 . [DOI] [PubMed] [Google Scholar]
- 14. Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65(12):1032–9. Epub 2009/02/07. doi: S0006-3223(08)01598-9 [pii] 10.1016/j.biopsych.2008.12.016 . [DOI] [PubMed] [Google Scholar]
- 15. Park IH, Ku J, Lee H, Kim SY, Kim SI, Yoon KJ, et al. Disrupted theory of mind network processing in response to idea of reference evocation in schizophrenia. Acta Psychiatr Scand. 2011;123(1):43–54. Epub 2010/08/18. doi: ACP1597 [pii] 10.1111/j.1600-0447.2010.01597.x . [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto R, Lee K, Preus A, McCarley RW, Wible CG. An fMRI study of functional abnormalities in the verbal working memory system and the relationship to clinical symptoms in chronic schizophrenia. Cereb Cortex. 2010;20(1):46–60. Epub 2009/04/28. doi: bhp079 [pii] 10.1093/cercor/bhp079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349(9067):1735–9. Epub 1997/06/14. doi: S0140-6736(96)08380-8 [pii] 10.1016/S0140-6736(96)08380-8 . [DOI] [PubMed] [Google Scholar]
- 18. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–30. Epub 2005/08/27. doi: 1300837 [pii] 10.1038/sj.npp.1300837 . [DOI] [PubMed] [Google Scholar]
- 19. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. Epub 1995/10/01. . [DOI] [PubMed] [Google Scholar]
- 20. Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013;33(7):2889–99. Epub 2013/02/15. 10.1523/JNEUROSCI.3554-12.2013 33/7/2889 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alexander-Bloch AF, Vertes PE, Stidd R, Lalonde F, Clasen L, Rapoport J, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23(1):127–38. Epub 2012/01/26. 10.1093/cercor/bhr388 bhr388 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoptman MJ, Zuo XN, D'Angelo D, Mauro CJ, Butler PD, Milham MP, et al. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res. 2012;141(1):1–7. Epub 2012/08/23. 10.1016/j.schres.2012.07.027 S0920-9964(12)00421-5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38(2):285–94. Epub 2010/07/03. 10.1093/schbul/sbq074 sbq074 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H, Wei X, Tao H, Mwansisya TE, Pu W, He Z, et al. Opposite effective connectivity in the posterior cingulate and medial prefrontal cortex between first-episode schizophrenic patients with suicide risk and healthy controls. PLoS One. 2013;8(5):e63477 Epub 2013/05/25. 10.1371/journal.pone.0063477 PONE-D-13-03599 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barch DM. Cerebellar-Thalamic Connectivity in Schizophrenia. Schizophr Bull. 2014. Epub 2014/06/05. doi: sbu076 [pii] 10.1093/schbul/sbu076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen H, Wang L, Liu Y, Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage. 2010;49(4):3110–21. Epub 2009/11/26. 10.1016/j.neuroimage.2009.11.011 S1053-8119(09)01195-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. Epub 2004/04/28. 10.1016/j.neuroimage.2003.12.030 S1053811904000035 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28. Weaver KE, Chaovalitwongse WA, Novotny EJ, Poliakov A, Grabowski TG, Ojemann JG. Local functional connectivity as a pre-surgical tool for seizure focus identification in non-lesion, focal epilepsy. Front Neurol. 2013;4:43 Epub 2013/05/04. 10.3389/fneur.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng H, Pizarro R, Nair VA, La C, Prabhakaran V. Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia. 2013;54(4):658–66. Epub 2013/01/09. 10.1111/epi.12066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, et al. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res. 2011;1373:221–9. Epub 2010/12/15. 10.1016/j.brainres.2010.12.004 S0006-8993(10)02616-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17(1):19–22. Epub 2005/12/20. doi: 00001756-200601230-00005 [pii]. . [DOI] [PubMed] [Google Scholar]
- 32. Northoff G, Qin P. How can the brain's resting state activity generate hallucinations? A 'resting state hypothesis' of auditory verbal hallucinations. Schizophr Res. 2011;127(1–3):202–14. Epub 2010/12/15. doi: S0920-9964(10)01638-5 [pii] 10.1016/j.schres.2010.11.009 . [DOI] [PubMed] [Google Scholar]
- 33. He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35(2):488–500. Epub 2007/01/27. doi: S1053-8119(06)01163-3 [pii] 10.1016/j.neuroimage.2006.11.042 . [DOI] [PubMed] [Google Scholar]
- 34. Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp. 2009;30(5):1502–10. Epub 2008/07/24. 10.1002/hbm.20622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. Epub 2010/01/09. doi: nrneurol.2009.198 [pii] 10.1038/nrneurol.2009.198 . [DOI] [PubMed] [Google Scholar]
- 36. Margulies DS, Bottger J, Long X, Lv Y, Kelly C, Schafer A, et al. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23(5–6):289–307. Epub 2010/10/26. 10.1007/s10334-010-0228-5 . [DOI] [PubMed] [Google Scholar]
- 37. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 38. Garety PA, Hemsley DR. Characteristics of delusional experience. Eur Arch Psychiatry Neurol Sci. 1987;236(5):294–8. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 39. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. Epub 2002/12/31. 10.1073/pnas.0135058100 0135058100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan C-G, Zang Y-F. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13 Epub 2010/06/26. 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. Epub 2001/02/24. 10.1073/pnas.98.2.676 98/2/676 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031 Epub 2011/09/29. 10.1371/journal.pone.0025031 PONE-D-11-09456 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–7. Epub 2003/06/26. . [DOI] [PubMed] [Google Scholar]
- 44. Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421(1):16–21. Epub 2007/06/06. doi: S0304-3940(07)00451-X [pii] 10.1016/j.neulet.2007.04.074 . [DOI] [PubMed] [Google Scholar]
- 45. Xi Q, Zhao XH, Wang PJ, Guo QH, Yan CG, He Y. Functional MRI study of mild Alzheimer's disease using amplitude of low frequency fluctuation analysis. Chin Med J (Engl). 2012;125(5):858–62. Epub 2012/04/12. . [PubMed] [Google Scholar]
- 46. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41(8):1701–8. Epub 2010/12/15. doi: S0033291710002205 [pii] 10.1017/S0033291710002205 . [DOI] [PubMed] [Google Scholar]
- 47. Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, et al. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90(1):1–15. Epub 1999/05/13. . [DOI] [PubMed] [Google Scholar]
- 48. Menon M, Schmitz TW, Anderson AK, Graff A, Korostil M, Mamo D, et al. Exploring the Neural Correlates of Delusions of Reference. Biol Psychiatry. 2011. Epub 2011/08/13. doi: S0006-3223(11)00634-2 [pii] 10.1016/j.biopsych.2011.05.037 . [DOI] [PubMed] [Google Scholar]
- 49. Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133(Pt 2):557–67. Epub 2009/11/19. doi: awp295 [pii] 10.1093/brain/awp295 . [DOI] [PubMed] [Google Scholar]
- 50. Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. Epub 2013/03/19. 10.1016/j.neuroimage.2013.03.004 S1053-8119(13)00212-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014;111(16):6058–62. Epub 2014/04/09. 10.1073/pnas.1317424111 1317424111 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun YY. The chinese edition of Characteristics of delusional experience. Chinese Mental Health Journal. 2014. [Google Scholar]
- 53. Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 1989;(7):59–67. Epub 1989/11/01. . [PubMed] [Google Scholar]
- 54. Steel C, Garety PA, Freeman D, Craig E, Kuipers E, Bebbington P, et al. The multidimensional measurement of the positive symptoms of psychosis. Int J Methods Psychiatr Res. 2007;16(2):88–96. Epub 2007/07/12. 10.1002/mpr.203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turner JA, Calhoun VD, Michael A, van Erp TG, Ehrlich S, Segall JM, et al. Heritability of multivariate gray matter measures in schizophrenia. Twin Res Hum Genet. 2012;15(3):324–35. Epub 2012/08/04. 10.1017/thg.2012.1 S1832427412000011 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study contains private information of the subjects. Due to ethical and legal restrictions data are available upon request. Interested researchers may contact Yihong Zhu via email: zhyh@zju.edu.cn or Bin Gao via email: zjugaobin@163.com.