Abstract
Background:
Determination of cell-associated antiretroviral drug concentrations is necessary for research into reservoirs of HIV. Variation exists in cell-associated drug concentrations among research groups. One cause for this may be washing cells during processing. We explored spinning cells through oil to minimize this variability.
Methods & results:
Raltegravir, atazanavir, darunavir, efavirenz, lopinavir and ritonavir concentrations were assessed in CEM.ss T cells washed with HBSS and oil-spun cells. Oil-spun cells had significantly higher concentrations for all drugs compared with samples washed with HBSS.
Conclusion:
The decline in cell-associated drug concentrations with saline washes compared with a single spin through oil shows the utility of a spin through oil. Oil centrifugation results in high cell-associated drug concentrations, and can be done in a fast, efficient manner.
Accurate determination of cell-associated drug concentrations is an important goal for research in sanctuary sites of HIV [1–5]. These are sites in the body where subtherapeutic concentrations of antiretroviral medications may result in reservoir sites for cryptic viral replication [5–7]. There is, however, the potential for considerable variation in reported cell-associated drug concentrations among laboratories [8–12]. These differences in reported drug concentrations among sites may be due to a variety of factors, including differences in sample processing methodologies. Some of these differences in processing methods include variations in the number and duration of cell washes, the cell processing buffer, the delay before cell processing and the temperature at which the samples are kept during and prior to processing. One proposed approach to minimize the variability associated with the number of washes and the type of wash buffer utilized is to centrifuge cells through an oil solution, rather than the buffered saline solutions that are traditionally used. While this spin through oil methodology has been described previously in the literature, it is not a standard in the processing of samples for cell-associated drug quantification [13]. Here we report our modification of this approach, as well as an investigation of cell-associated drug concentrations with traditional processing methods, consisting of multiple washes with an aqueous solution compared with a rapid spin through oil technique for a number of commonly utilized antiretroviral medications.
Methods
Cell culture
The immortalized T4 lymphoblastoid cell line CEM.ss (NIH AIDS Reagent program, Germantown, MD, USA) was utilized for all experiments [14]. This cell line is commonly utilized in experiments in HIV, and has been shown to be phenotypically very similar to primary CD4+ T cells. Low passage cells were cultured in RPMI + 10% fetal calf serum (Life Technologies, Grand Island, NY, USA) at 37°C with 5% CO2. CEM.ss cells were plated in T-75 flasks at an initial concentration of 4 × 105 cells/ml. Raltegravir (RAL), a gift from Merck & Co. Inc. (Rahway, NJ, USA) was added to the flasks with a final concentration of 3 μM to approximate average peak plasma concentrations [11]. In additional experiments cells were treated with a combination of 5 nM of the HIV-1 NNRTI efavirenz(EFV) as well as the HIV-1 protease inhibitors 0.5 nM atazanavir (ATV), and 1.25 nM darunavir (DRV), lopinavir (LPV) and ritonavir (RTV) simultaneously. Concentrations for this set of experiments were chosen to assess cell-associated drug concentrations in samples with low drug concentrations in order to assess the utility of the oil wash over a range of different drug concentrations. Efavirenz was purchased from the USA Pharmacopeia (Rockville, MD, USA). All other drugs were obtained from the NIH AIDS Research and Reference Program (Germantown, MD, USA). Cells were cultured with drugs for 24 h before harvesting.
Cell processing
After 24 h, cells were collected on ice, and enumerated with a Countess cell counter (Life Technologies, Grand Island, NY, USA). Cells were centrifuged at 400 rcf for 7 min. For the oil wash conditions, cell pellets were resuspended in 1 ml of supernatants, and the rest of the supernatants were discarded. The resuspended cells were then carefully layered over 150 μl of oil (Nyosil M25 oil, TAI Lubricants, Hockessin, DE, USA) in a 1.5 ml microcentrifuge tube. Samples were then centrifuged at 20,130 rcf for 1 min. After centrifugation, the cell pellet is at the bottom of the microcentrifuge tube, the oil solution is above that and the supernatant floats above the oil solution, ensuring that the cell pellet and extracellular drug are separate. The supernatant was aspirated, and the sides of the centrifuge tubes were washed twice with 1 ml of Hank's buffered saline solution (HBSS) (Fisher Scientific, Waltham, MA, USA) careful to not disturb the oil layer. This was done to ensure that no residual drug is left on the side of the microcentrifuge tube. After the second wash the oil was carefully removed without disturbing the cell pellet. The use of a single 1.5 ml microcentrifuge tube, followed by washing the sides of the tube offers a considerable time improvement over previously described approaches to an oil wash, which require multiple centrifuge tubes nested in each other [15]. Samples were lysed with 0.5 ml of ice-cold 70% methanol (Fisher Scientific), and stored at -80°C. For other conditions, cells were resuspended and washed with 5 ml of cold HBSS. After centrifugation at 400 rcf for 7 min, the wash buffer was discarded. Cells were washed between one- and three-times, depending on experimental conditions. Cells were lysed in 0.5 ml of ice-cold 70% methanol.
Sample quantification
Cell-associated RAL sample extracts were analyzed using a validated method published previously [11,12]. Briefly, methanol extracts were centrifuged to remove cellular debris. Samples were spiked with 13C6 RAL (a gift from Merck & Co Inc., Rahway, NJ, USA), and injected onto a Shimadzu Shim-pack XR-ODSII 2.0 mm x 75 mm column (Shimadzu Corp, Kyoto, Japan). RAL was separated using a gradient method and was detected using mass spectrometry on an API 5000 (AB SCIEX Corporation, Foster City, CA, USA). The assay standard curve has a range from 0.00562 fmol/μl to 22.5 fmol/μl and is linear over this range. HBSS wash samples were treated using a plasma sample protocol as published [11]. An aliquot of sample was spiked with 13C6 RAL, and extracted with methanol. The precipitate was centrifuged and the sample diluted and subjected to separation and detection using the API 5000 as described above. The assay has a range from 2.25 to 16,876 fmol/μl.
Cell-associated ATV, DRV, LPV, RTV and EFV were analyzed using a validated method previously published [16]. In brief, extracts were precipitated with a formic acid:acetonitrile mixture. Stable-isotope internal standards for ATV (used to track ATV/DRV), RTV (used to track LPV/RTV) and EFV (used to track EFV) were spiked into the precipitated samples. Cellular precipitants were removed by centrifugation and supernatant was diluted with water and injected onto an API 6500 (AB SCIEX Corporation, Foster City, CA, USA). Analyte separation was achieved with reversed-phase chromatography utilizing an ACE® 3 C18 column (3 mm × 100 mm). Detection was accomplished by multiple reaction monitoring mass spectrometry in both positive (ATV, DRV, LPV and RTV) and negative (EFV) ion mode. The analytical method was linear over the following ranges: 0.0200–10.0 fmol/μl for ATV, 0.0500–25.0 fmol/μl for DRV, LPV and RTV and 0.200–100 fmol/μl for EFV.
Calculations & statistics
To determine the cell-associated antiretroviral concentration, we first determined the amount of cells in each μl of cell lysate by dividing the cell count by the volume of the lysate. This calculation resulted in a determination of the amount of lysed cells in each μl of lysate. Statistical analysis was performed using SPSS (Armonk, NY USA). Results are reported as mean ±SD of three independent replications on multiple days. Comparisons were made via one-way ANOVA, with Tukey's post-test for individual comparisons. Differences were deemed statistically significant if p < 0.05.
Results
Differences in cell-associated concentrations with an oil versus an HBSS wash
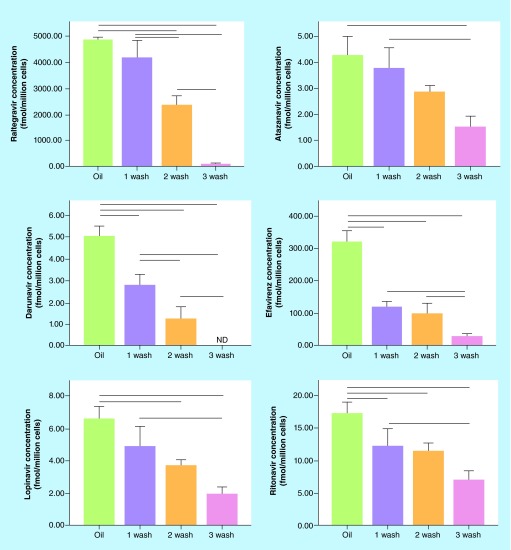
Figure 1 shows the differences in cell-associated drug concentrations between samples centrifuged through oil and those washed one-, two-, or three-times with HBSS. Oil washed cells had significantly higher cell-associated drug concentrations than cells washed three-times with HBSS for all drugs investigated, and higher drug concentrations than cells washed two-times for all drugs except for ATV (Figure 1). Significant differences were also observed between an oil wash and a single wash with HBSS for EFV and RAL, and nonsignificant trends with reduced concentrations were observed for all other drugs.
Figure 1. . Cell-associated concentrations with an oil versus a Hank's buffered saline solution wash.
Cells were treated with antiretrovirals for 24 h, harvested and centrifuged through oil or washed 1–3-times with HBSS. Lines between groups represent significant differences in cell-associated drug concentrations (p < 0.05 via one way ANOVA with Tukey's post-test).
HBSS: Hank's buffered saline solution.
Antiretroviral concentrations in wash buffer after one, two or three washes
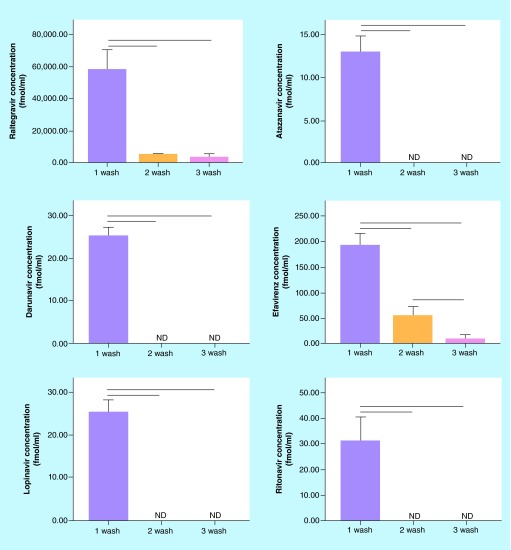
Figure 2 shows the concentrations of antiretrovirals present in the wash buffer of the samples. As we had observed large differences in the amount of cell-associated drug between washes, we also assessed the concentration of drugs in the HBSS wash buffer for the drugs, to attempt to determine the destination of lost cell-associated antiretrovirals between washes. We observed significant declines in extracellular drug concentrations between the first wash and both second and third washes. While second and third wash drug concentrations were undetectable for ATV, DRV, LPV and RTV, a significant decline was observed between second and third washes for EFV (Figure 2). While detectable concentrations of RAL were observed in second and third wash buffers, no significant differences were observed between the two washes.
Figure 2. . Antiretroviral concentrations in wash buffer.
Cells were treated with antiretrovirals for 24 h, harvested and washed 1–3-times with HBSS. Concentrations in HBSS washes were quantified. Lines between groups represent significant differences in drug concentrations (p < 0.05 via one way ANOVA with Tukey's post-test).
HBSS: Hank's buffered saline solution.
Differences in cell-associated concentrations between oil washed samples and samples washed with HBSS for variable amounts of time
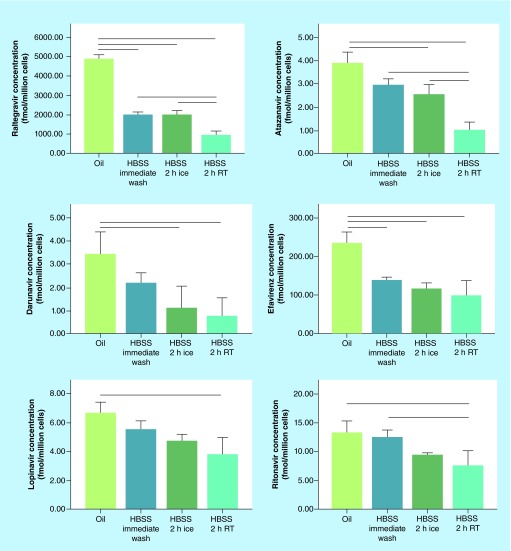
Figure 3 compares an immediate oil wash with samples left in HBSS for 2 h either on ice or at room temperature. It is common during cell processing for delays to occur preventing the timely processing of the samples. One set of samples were centrifuged through oil, while the samples left in HBSS were washed one time under the HBSS wash protocol. As anticipated, immediate processing with oil showed higher cell-associated concentrations than samples that were left in HBSS for 2 h for almost all drugs. Significant differences were observed between oil washed samples and both samples left at room temperature and on ice for RAL, ATV, DRV and EFV (Figure 3). Significant differences were observed between oil processing and samples left at room temperature for 2 h for LPV and RTV, with a nonsignificant trend observed between oil and samples processed on ice.
Figure 3. . Cell-associated concentrations in oil and Hank's buffered saline solution washed samples under different conditions.
Cells were treated with antiretrovirals for 24 h, harvested and centrifuged through oil, processed immediately with HBSS, or left for 2 h in HBSS either on ice or at room temperature. Lines between groups represent significant differences in cell-associated drug concentrations (p < 0.05 via one way ANOVA with Tukey's post-test).
HBSS: Hank's buffered saline solution.
Discussion
Protocols commonly utilized for quantification of cell-associated drug concentrations involve between two to three washes with either HBSS or phosphate buffered saline (PBS) [9,10,17–19]. Here, we report that processing samples through oil resulted in higher cell-associated drug concentrations compared with standard techniques utilizing multiple washes or even processing samples on ice [8,11,12]. We did observe similar amounts of antiretrovirals present in samples washed once with HBSS and samples centrifuged through oil. However, the large amount of drug present in the first wash buffer suggests that much of the quantified drug in these samples is due to incomplete washing of the sample as well as continued diffusion of cell-associated drug into the wash buffer, and thus may not provide a fully accurate, reproducible result. This process would continue in the repeated washes, and likely explains the continued decline in intracellular concentrations observed in cell-associated drug concentrations observed after washes two and three. Similarly, there is likely some loss of cells that occurs with repeated washes. While this may explain part of the decline in intracellular drug concentrations that we observed with repeated washes, the extreme decline in intracellular concentrations between one and three washes (50–90%) cannot be explained fully by a loss of cells.
When we assessed drug concentration in the wash buffer, large amounts of all drugs were found in the first wash buffer, with concentrations rapidly declining in subsequent washes. For EFV there was a detectable difference between concentrations in the second and third wash, but for the rest of the tested drugs there were no significant differences between wash buffer concentrations between the second and third wash, suggesting that there was little benefit in washing samples three instead of two-times. The large amounts of drug found in cell lysates that were washed once with HBSS is most likely due to incomplete removal of media from cells after one wash. This suggests a second wash is necessary to fully eliminate extracellular drug. However, with repeated washes with HBSS, there is an increased possibility for cell-associated drug to diffuse into the HBSS; this is a key concern that centrifuging through oil minimizes. The oil wash approach did show higher cell-associated concentrations of the drugs tested than the samples washed twice with HBSS, as well as requiring less time, demonstrating the advantage of this approach.
While we report here a method using immortalized cells in media, this approach can be easily modified for preparing peripheral blood mononuclear cells for cell-associated antiretroviral quantification. Cells suspended in buffer (either PBS or HBSS) can easily be layered over the oil layer, and cells otherwise be processed in a manner similar to that described above.
Tanaka et al. reported a similar method for processing samples for cell-associated quantification via centrifugation through an oil solution [15]. They similarly observed that the spin through oil technique displayed higher cell-associated concentrations than samples washed in PBS. They did this processing utilizing a dual-tube system, with an inner tube with the sample inside of an outer tube with the oil. By using this method, they observed significantly higher efavirenz concentrations by using an oil wash than by washing three-times with ice-cold PBS. Our methodology shows that high concentrations of cell-associated antiretrovirals can be collected utilizing only a single tube, rather than the double tube system, minimizing the time necessary to prepare for sample processing as well as the difficulty associated with processing the samples.
We assessed the role of immediate versus delayed processing of samples as well as the role of leaving processed samples on ice as compared with samples left at room temperature. Again, there was a nonsignificant trend for all drugs with samples processed through oil as compared with samples washed once immediately. As expected, oil processing resulted in significantly higher cell-associated concentrations compared with samples processed either on ice or at room temperature for all drugs except for LPV and RTV. Much of this difference is likely due to diffusion of the intracellular drug into the extracellular milieu. This process can be slowed, but not prevented by leaving the cells on ice. The oil wash procedure is considerably faster than traditional wash processes, and ideally should minimize the need to leave samples in wash buffers for extended amounts of time. By changing the extracellular environment during cell washes to an oil, rather than an aqueous solution, we can minimize the loss of intracellular drug due to passive diffusion to the extracellular environment. The log P value of the drugs that we assessed in these experiments were RAL: -0.39, ATV: 4.08, DRV:1.76, EFV: 3.89, LPV: 3.91 and RTV: 3.9, which represents a common range of partition coefficients [20]. When Tanaka et al. utilized their version of the oil spin on efavirenz, they observed similar advantages of the oil wash compared with washes with PBS [15].
There are some limitations associated with this research. First, we utilized cultured cells from an immortalized cell line, rather than cultured T cells or clinical samples from individuals receiving antiretroviral therapy. While utilizing primary preparing peripheral blood mononuclear cells would be more extrapolatable to clinical conditions, the CEM.ss cell line is commonly utilized in many in vitro experiments and has been shown to be phenotypically similar to primary T cells. [14,21]. In clinical practice using samples from individuals, collected mononuclear cells would usually be washed, and the cells in their wash buffer could then be processed according to the protocol outlined above. Second, in these experiments we washed cells with HBSS, rather than with PBS. While PBS is the wash buffer utilized for the vast majority of cell processing, HBSS is used in many circumstances [10]. Our laboratory previously assessed differences in RAL concentrations in clinical samples washed once with PBS compared with cells centrifuged through oil, showing similar results to what we reported here [12]. Here, we extended the previous data to five additional drugs, utilizing an extremely similar isotonic buffer. While these two buffers are not identical, they do function similarly for these purposes.
Conclusion
The development of accurate, reproducible processing methodologies is of key importance for studies of pharmacokinetics and concentration–response relationships. Once these accurate processing methodologies have been developed, standardization of these protocols is useful to allow fully accurate comparisons among laboratories. When multiple sites process samples differently, an additional source of variation is added, and it becomes increasingly difficult to make valid comparisons between samples. Standardizing processing methodology via separation through oil, rather than repeated washes with HBSS provides an inexpensive, fast and reliable method to minimize variability in the quantification of cell-associated antiretrovirals.
Future perspective
Determining ways to minimize the loss of cell-associated antiretrovirals during cell processing is a concern for the accurate quantification of drug concentrations in reservoir sites or target tissues. The spin through oil technique, coupled with other changes in the collection and processing of samples, may lead to less variation between samples. Differences in processing between laboratories may result in different determinations of cell-associated concentrations, making comparisons difficult to perform. The antiretroviral pharmacokinetic and exposure–response relationship field would benefit from standardization of processing methods among laboratories.
Key terms.
Spin through oil: Technique where cells are spun through an oil solution, rather than through a series of aqueous washes.
Raltegravir: Integrase inhibitor used in the treatment of HIV. The drug works by inhibiting the viral integrase enzyme.
Efavirenz: Non-nucleoside reverse transcriptase inhibitor used in the treatment of HIV. The drug works by inhibiting viral reverse transcriptase.
HIV-1 protease inhibitors: Class of antiretroviral drugs used in the treatment of HIV, which include atazanavir, darunavir, lopinavir and ritonavir that inhibit the activity of the viral protease enzyme.
Cell-associated quantification: Determination of drug concentration within the cell or bound to the cell surface.
Executive summary.
Traditional methods for quantifying cell-associated antiretrovirals can be time consuming, and result in the loss of cell-associated drug.
We centrifuged samples through an oil solution to minimize the loss of six antiretroviral medications, raltegravir, atazanavir, darunavir, efavirenz, lopinavir and ritonavir.
Samples centrifuged through oil had higher cell-associated concentrations than samples washed two- to three-times with Hank's buffered saline solution.
When we assessed drug remaining in the Hank's buffered saline solution wash solution, we observed large amounts of drug present in the first wash solution, with significantly less found in the second and third washes.
Processing samples immediately with oil resulted in higher cell-associated concentrations than samples left on ice or at room temperature during processing.
The spin through oil technique results in high cell-associated concentrations, in a fast, reliable and inexpensive method to process samples.
Footnotes
Financial & competing interests disclosure
This work was supported by a grants from the National Institute of Allergy and Infectious Diseases (P01 AI074340 and UM1AI06701, to CVF). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol. Pharm. 2012;10(2):459–466. doi: 10.1021/mp3002045. [DOI] [PubMed] [Google Scholar]
- 2.Bourry O, Mannioui A, Sellier P, et al. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology. 2010;7(1):78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr. Opin. HIV AIDS. 2013;8(3):190–195. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Mascio M, Srinivasula S, Bhattacharjee A, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob. Agents Chemother. 2009;53(10):4086–4095. doi: 10.1128/AAC.00419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solas C, Lafeuillade A, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2003;47(1):238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl Acad. Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zalar A, Figueroa MI, Ruibal-Ares B, et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res. 2010;87(2):269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Mello AF, Buclin T, Franc C, et al. Cell disposition of raltegravir and newer antiretrovirals in HIV-infected patients: high inter-individual variability in raltegravir cellular penetration. J. Antimicrob. Chemother. 2011;66(7):1573–1581. doi: 10.1093/jac/dkr151. [DOI] [PubMed] [Google Scholar]
- 9.Khoo SH, Hoggard PG, Williams I, et al. Intracellular accumulation of human immunodeficiency virus protease inhibitors. Antimicrob. Agents Chemother. 2002;46(10):3228–3235. doi: 10.1128/AAC.46.10.3228-3235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennessy M, Clarke S, Spiers JP, et al. Intracellular indinavir pharmacokinetics in HIV-infected patients: comparison with plasma pharmacokinetics. Antiviral Ther. 2003;8(3):191–198. [PubMed] [Google Scholar]
- 11.Sandkovsky U, Swindells S, Robbins BL, Nelson SR, Acosta EP, Fletcher CV. Measurement of plasma and intracellular concentrations of raltegravir in patients with HIV infection. AIDS. 2012;26(17):2257–2259. doi: 10.1097/QAD.0b013e328359a978. [DOI] [PubMed] [Google Scholar]
- 12.Robbins BL, Nelson SR, Fletcher CV. A novel ultrasensitive LC-MS/MS assay for quantification of intracellular raltegravir in human cell extracts. J. Pharm. Biomed. Anal. 2012;70:378–387. doi: 10.1016/j.jpba.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen PA, Schaumburg BP, Osterline K, Vinten J, Gammeltoft S, Gliemann J. A rapid technique for separation of thymocytes from suspensions by centrifugation through silicone oil. Anal. Biochem. 1974;59(2):610–616. doi: 10.1016/0003-2697(74)90314-5. [DOI] [PubMed] [Google Scholar]
- 14.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18(4):522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka R, Hanabusa H, Kinai E, Hasegawa N, Negishi M, Kato S. Intracellular efavirenz levels in peripheral blood mononuclear cells from human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 2008;52(2):782–785. doi: 10.1128/AAC.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes another method used to utilize an oil wash.
- 16.Podany AT, Winchester LC, Robbins BL, Fletcher CV. Quantification of cell associated atazanavir, darunavir, lopinavir, ritonavir and efavirenz in human mononuclear cell extracts. Antimicrob. Agents Chemother. 2014;58(5):2866–2870. doi: 10.1128/AAC.02551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford J, Boffito M, Maitland D, et al. Influence of atazanavir 200 mg on the intracellular and plasma pharmacokinetics of saquinavir and ritonavir 1600/100 mg administered once daily in HIV-infected patients. J. Antimicrob. Chemother. 2006;58(5):1009–1016. doi: 10.1093/jac/dkl379. [DOI] [PubMed] [Google Scholar]
- 18.Almond LM, Hoggard PG, Edirisinghe D, Khoo SH, Back DJ. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. J. Antimicrob. Chemother. 2005;56(4):738–744. doi: 10.1093/jac/dki308. [DOI] [PubMed] [Google Scholar]
- 19.Jackson A, Watson V, Back D, et al. Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 2011;58(5):450–457. doi: 10.1097/QAI.0b013e3182364c67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law VKC, Djoumbou Y, Jewison T, et al. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42(1):D1091–D1097. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]