Abstract
Background
Few longitudinal studies have been conducted on occupational exposure and lung function. This study investigated occupational dust exposure effects on lung function and whether genetic variants influence such effects.
Methods
The study population (1,332 participants) was from the Framingham Heart Study, in which participant lung function measures were available from up to five examinations over nearly 17 years. Occupational dust exposures were classified into “more” and “less” likely dust exposure. We used linear mixed effects models for the analysis.
Results
Participants with more likely dust exposure had a mean 4.5 mL/year excess loss rate of FEV1 over time. However, occupational dust exposures alone or interactions with age or time had no significant effect on FEV1/FVC. No statistically significant effects of genetic modifications in the different subgroups were identified for FEV1 loss.
Conclusions
Occupational dust exposures may accelerate the rate of FEV1 loss but not FEV1/FVC loss.
Keywords: job exposure matrix, forced expiratory volume in one second, chronic obstructive pulmonary disease, environmental lung disease, environmental health, occupational health, occupational respiratory disease
Introduction
Chronic obstructive pulmonary disease (COPD) affects between 6% and 20% of people worldwide [Buist et al., 2008]. Diagnosis of COPD usually employs a measure of the forced expiratory volume in one second (FEV1) and ratio of FEV1 to forced vital capacity (FVC). FEV1 is a stable measure of lung function and has been shown to predict clinical outcome, as well to reflect the severity and natural history of obstructive lung disease. Smoking is a well-known important risk factor for COPD. However, in the United States, 15% to 20% of COPD cases are attributable to occupational exposure [Balmes et al., 2003].
Occupational exposure to dust has been associated with COPD or poor lung function in both industry-based [Higgins, 1973; Kauffmann et al., 1982; Oxman et al., 1993; Johnsen et al., 2008] and community-based [Korn et al., 1987; Krzyzanowski and Kauffmann, 1988; Bakke et al., 1991; Viegi et al., 1991; Heederik et al., 1992] cross-sectional studies, although some results are controversial [Petran et al., 2000]. Industry-based longitudinal studies have shown that occupational exposure is associated with accelerated lung function decline in the furniture industry [Jacobsen et al., 2008], in blue-collar workers [Thaon et al., 2012], in smelters [Johnsen et al., 2010], and in flavoring manufacturing workers [Kanwal et al., 2011; Kreiss et al., 2011]. Other longitudinal studies have shown that lung function declines in occupational settings [Wang and Petsonk, 2004; Wang et al., 2006; Hnizdo et al., 2010].
Few community-based studies have been published based on longitudinal pulmonary function data; importantly, the follow-up times were short (<10 years), and the numbers of repeated measurements were few (only two or three repeated measurements) for those studies. A recent review of occupational chronic obstructive pulmonary diseases summarized the results of the lung function/COPD studies using either industry-specific or population-based data [Omland et al., 2014].
A community-based longitudinal study offers several advantages. For example, studies in industry-based populations tend to focus on workers who are less susceptible participants, such as blue-collar workers who are usually healthier or stronger than the general population and thus more prone to healthy worker effect bias. Community-based studies, however, can provide more generalizable information since they include participants from many different industries. Additionally, in contrast to cross-sectional studies, longitudinal studies can help characterize aging and normal development as well as improvement or decline in lung function, to distinguish the effects of time. Longitudinal studies also allow for consideration of time-dependent variables such as change in smoking status between different examinations.
The pathophysiological mechanisms of all cause (e.g., aging and cigarette smoking) accelerated decline of FEV1 are thought to be multifactorial, involving genetic factors, cellular repair, and inflammatory response and resolution [MacNee and Tuder, 2009]. Genes and environmental interaction such as occupational exposure may thus interact with each other and affect the lung function. In a previous gene-environmental interaction study on cross-sectional lung function, we found a single nuclear polymorphism, rs9931086, in the gene SLC38A8 on chromosome 16, that significantly modified the association of occupational dust exposure with cross-sectional FEV1, and another single nuclear polymorphism, rs17051547, on chromosome 4, that modified the association of occupational dust exposure with cross-sectional FEV1/FVC (using the SNP with the smallest p value which did not, however, reach genome-wide significance) [Liao et al., 2013].
In this study, we stratified our participants into two genetic groups and assessed whether the single nucleotide polymorphisms rs9931086 or rs17051547 also modified the association of occupational dust exposure with longitudinal lung function. The purpose of the study was to determine how occupational dust exposures affect lung function change over time and whether genetic variants influence occupational dust exposure effects on lung function change over time in a longitudinal community-based study population with an average of 17 years of follow-up.
Materials and Methods
Study Population
Our study used the Framingham Heart Study (FHS) population, which includes mainly Whites who live in Framingham, Massachusetts, USA. Manufacturing, such as automobile production, had been a key economic feature of Framingham at the time of the first generation study, but in the past 3–4 decades, as in other United States industrial towns, manufacturing left and now Framingham is a retail center for the region. The FHS has recruited participants since 1948, and participants have returned approximately every two years for spirometry measurement, detailed medical history, physical examination, and laboratory tests. Three generations have participated in the FHS: the original cohort, their offspring, and the third generation. Here, we used the offspring cohort, which has available longitudinal lung function measurements. The study population comprises participants with at least one spirometry measurement, current occupation information, and covariates. A total of 1,332 participants (261 families and 352 individuals without relatives) with 4,734 observations were used for our longitudinal analysis.
Ethics Statement
Written informed consent was provided by all participants. Protocols were approved by local institutional review boards.
Spirometry Phenotypes, Covariates, and Genotypings
Spirometry data from participants having acceptable pulmonary function were used in our study. Accessible examinations were obtained from Offspring Exams 3, 5, 6, 7, and 8. FEV1 and FEV1/FVC values were used as continuous outcomes. Gender and baseline age were time-independent variables; height (inches), pack-years, years after baseline, and smoking status at the time of each examination were time-dependent variables and were used as covariates in our analysis. Smoking status (never, former, and current smokers) was coded as a dummy variable. The genotyping was conducted with Affymetrix 500 K mapping plus Affymetrix 50 K supplemental array. The method has been described in detail in our previous study [Liao et al., 2013].
Occupational Dust Exposure
Although the lung function was measured longitudinally (exams 3, 5, 6, 7, 8), the occupation information was only measured at exam 8. We did not have information on how long participants remained on the job or whether their occupations changed during the follow-up period. We excluded participants who retired or reported their jobs as unknown or other (retired, other, and unknown are three separate categories). For occupational dust exposure classification, we used a mini population-specific job exposure matrix (JEM) (Table I) [Liao et al., 2013] modified from the UCSF COPD Job Exposure Matrix (January, 2009 revision) [Blanc et al., 2009] for occupational exposure (with job category codes rather than specific occupational codes in the UCSF COPD JEM). Occupational dust exposure was classified as “more likely dust exposure” for job categories (factory/assembly/mechanic, skilled labor, general labor, and heavy labor) that were classified as high exposure in the UCSF COPD Job Exposure Matrix. Occupational dust exposure was classified as “less likely dust exposure” for homemakers and the remaining job categories. There were 29 job categories in the FHS occupations classification, which were less detailed than the UCSF COPD Job Exposure Matrix. For example, in the FHS occupational classification, the category of skilled labor (classified as more likely dust exposure) included some jobs (e.g., plumber, carpenter, and painter) classified as job with dust exposure and some jobs (e.g., hairdresser) classified as no dust exposure in the UCSF COPD Job Exposure Matrix.
Table I. Job Categories for Dust Exposure Classification.
| Dust exposure groups | Job categories |
|---|---|
| More likely dust exposure | Factory/assembly/mechanic |
| Skilled labor (e.g., plumber, carpenter, painter, hairdresser) | |
| General labor (e.g., custodian, delivery, mailman, truck driver) | |
| Heavy labor (e.g., construction, landscaping) | |
| Less likely dust exposure | Nurse/medical personnel/laboratory technician |
| Physical/occupational/speech therapist | |
| Homemaker | |
| Self-employed business owner | |
| Physician/dentist/scientist/research | |
| Lawyer/judge | |
| Psychologist/social worker/mental health counselor | |
| Engineer/computer science | |
| banker/accountant | |
| Manager/consultant (e.g., production manager) | |
| Administrative (e.g., personnel) | |
| Educator | |
| Secretary/clerk/data entry | |
| Retail/cashier | |
| Sales/marketing/insurance | |
| Realtor | |
| Police/fire/security/military | |
| Restaurant/food worker | |
| Writer/editor | |
| Artist/graphic Designer/craftsperson | |
| Musician | |
| Clergy (minister, priest, rabbi) | |
| Sports pro/coach/exercise instructor/other | |
| Statistician | |
| Student |
Statistical Analysis
For longitudinal analysis we used a linear mixed effects model for the association between FEV1 or FEV1/FVC and dust exposure groups, adjustment for relevant covariates. Random effects were used to account for the correlation between repeated measures within each subject and the correlation among observations from multiple individuals within a family. Specifically, we used a family random intercept to account for the correlation of the measures of different individuals within the same family, and individual random intercept/random age slope to account for correlation of repeated measures over time within the same individual. In addition to main effects, we also included two interaction terms, dust exposure (E) and baseline age (centered by mean baseline age for whole the population, Age-Agemean) and years after baseline in which the lung function was measured (T). These two interaction terms are presented as Eij* (Age-Agemean) ij and Eij*Tijt below. Yijt denotes the outcome (FEV1 or FEV1/FVC) for subject j of family i measured at year t. The mixed effects model can be written as below:
where the Xijt are other covariates at year t, the bi are family random effects, the aij and cij are subject-specific random intercept and slopes and eijk are residuals. Analyses were performed using SAS PROC MIXED (version 9.2; SAS Institute Inc., Cary, NC).
We stratified our participants into subgroups based on the genotype of the single nucleotide polymorphisms rs9931086 and rs17051547 that we previously identified as potential interacting variants for occupational exposure and lung function [Liao et al., 2013]. For analysis of the genetic modification of rs9931086, one subgroup comprised participants with one or two C alleles on rs9931086 (AC/CC), and the other subgroup comprised participants without a C allele (AA). For analysis of the genetic modification of rs17051547, one subgroup comprised participants with one or two A alleles on rs17051547 (AC/AA), and the other subgroup comprised participants without an A allele (CC). We analyzed the data separately and compared the effect of occupational dust exposures in these subgroups. We also tested the difference of the effects in these subgroups using three way interaction analyses with the model written as below:
where Ggroup are coded as 1 and 0 to represent the two subgroups and tested for β9.
Results
There were 1,332 participants with an average of 3.55 (median = 4) repeated lung function measurements/per person. Among them, 1,188 participants were in the less likely dust exposure group, with an average of 3.58 (median = 4) repeated measurements/per person, and 144 participants were in the more likely dust exposure group with an average of 3.38 (median = 4) repeated measurements/per person.
Table II summarizes the baseline characteristics for groups with different dust exposure likelihood at final follow-up (Exam 8). The mean follow-up time was about 17 years in both groups. At baseline, FEV1 was higher in the group with more likely dust exposure, but the FEV1/FVC ratio was the same for both groups. Additionally, the prevalence of current and former smokers at baseline was higher in the group with more likely dust exposure.
Table II. Characteristics of Participants at the Baseline Examination, Stratified by Dust Exposure Groups Classified at the Final Examination.
| Total (n =1332) | More likely dust exposure (n =144) | Less likely dust exposure (n =11 8 8) | |
|---|---|---|---|
| Male, n (%) | 570 (42.79) | 118 (81.94) | 452 (38.05) |
| FEV1, mL (SD) | 3130 (820) | 3520 (820) | 3080 (800) |
| FEV1/FVC, (SD) | 0.77 (0.07) | 0.76 (0.08) | 0.77 (0.07) |
| Follow-up time, years (SD) | 16.88 (5.44) | 17.25 (4.36) | 16.96 (4.53) |
| Age, years (SD) | 47.44 (10.55) | 45.89 (11.15) | 47.63 (10.47) |
| Height, inches (SD) | 66.30 (3.72) | 68.10 (3.32) | 66.08 (3.71) |
| Pack-years*, (SD) | 21.35 (19.80) | 27.80 (21.24) | 20.30 (19.38) |
| Smoking status, n (%) | |||
| Never smokers | 583 (43.77) | 40 (27.78) | 543 (45.71) |
| Former smokers | 429 (32.21) | 52 (36.11) | 377 (31.73) |
| Current smokers | 320 (24.02) | 52 (36.11) | 268 (22.56) |
| Genotyping, n (%) | |||
| rs 9931086** | |||
| AC/CC genotypes | 370 (27.78) | 32 (22.22) | 338 (28.45) |
| AA genotype | 733 (55.03) | 80 (55.56) | 653 (54.97) |
| rs17051547** | |||
| AC/AA genotypes | 239 (17.94) | 20 (13.89) | 219 (18.43) |
| CC genotypes | 990 (74.32) | 111 (84.09) | 879 (73.99) |
FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; SD: Standard deviation; rs: Reference single nucleotide polymorphism number.
Values reported as means unless otherwise noted.
Pack-years mean and standard deviation calculated among current and former smokers.
Not all participants had genotyping.
Table III shows the results of the linear mixed model fit of FEV1 and FEV1/FVC on centered baseline age, years after baseline, centered baseline age by dust exposure interaction, and years after baseline by dust interaction, adjusting for covariates. The association of dust exposure as a main effect on FEV1 was not significant at baseline for subjects with a mean age of 47.44 years old. The decrement in FEV1 associated with a one-year increase in the baseline age (cross-sectional age effect) for the less likely dust exposure group was − 30.6 ± 1.3 mL/year, adjusted by gender, height, smoking, and pack-years at baseline (P-value < 0.0001). Some evidence indicated different cross-sectional age effects between the two dust exposure groups (P-value = 0.05), with a bigger mean difference between the two dust exposure groups for older subjects.
Table III. Characteristics Affecting FEV1 and FEV1/FVC by Linear Mixed Model.
| Covariate | FEV1 (mL) | FEV1/FVC | ||
|---|---|---|---|---|
|
|
|
|||
| Estimate (SE) | P value | Estimate (SE) | P value | |
| Time-independent | ||||
| More likely vs. less likely dust exposure | −15.1 (41.6) | 0.7173 | −0.0039 (0.0058) | 0.5030 |
| Male vs. female | 712.6 (34.1) | <0.0001 | −0.0128 (0.0048) | 0.0083 |
| Baseline Age* | −30.6 (1.3) | <0.0001 | −0.0027 (0.0002) | <0.0001 |
| Time-dependent | ||||
| Years after Baseline | −25.8 (0.6) | <0.0001 | −0.0029 (0.0001) | <0.0001 |
| Current vs. never smoker | −135.9 (22.4) | <0.0001 | −0.0175 (0.0036) | <0.0001 |
| Former vs. never smoker | −92.8 (19.6) | <0.0001 | −0.0125 (0.0031) | <0.0001 |
| Pack-years | −2.8 (0.4) | <0.0001 | −0.0004 (0.0000) | <0.0001 |
| Height | 61.5 (4.2) | <0.0001 | −0.0036 (0.0006) | <0.0001 |
| Interaction terms | ||||
| Baseline age*×More likely dust exposure | −7.1 (3.7) | 0.0573 | 0.0001 (0.0005) | 0.8907 |
| Years after baseline age×More likely dust exposure | −4.5 (1.7) | 0.0074 | −0.0001 (0.0003) | 0.6643 |
FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; SD: Standard deviation.
Baseline age was centered on mean baseline age (47.44 years old).
The FEV1 annual loss rate (longitudinal time effect) for the less likely dust exposure group was −25.8 ± 0.6 mL/year adjusted by other covariates (P-value < 0.0001). Subjects with more likely dust exposure had a statistically significant 4.5 ± 1.7 mL/year mean excess loss of FEV1 over time compared to those with less likely dust exposure (P-value = 0.0074). No significant effect of occupational dust exposure was observed on the FEV1/FVC ratio.
As expected, males had a significantly higher (712.6 ± 34.1 mL) mean FEV1 than females. Current smokers (−135.9 ± 22.4 mL) and former smokers (−92.8 ± 19.6 mL) had a significantly lower mean FEV1 than never smokers. Each increase in pack-years significantly decreased the mean FEV1 by 2.8 ± 0.4 mL. For each one-inch increase in height, the FEV1 significantly increased by 61.5 ± 4.2 mL.
Comparing the two subgroups stratified by genotype of rs9931086, there was a mean 6.5 ±3.4 mL/year mean excess loss of FEV1 over time (P = 0.05) among those with more likely dust exposure compared to those with less likely dust exposure in the AC/CC group (at least one C allele in rs9931086) and a 3.8 ±2.1 mL/year mean excess loss of FEV1 over time (P = 0.07) in the AA group (no C allele in rs9931086). However, the difference in FEV1loss across dust exposure strata was not significant across the two genotype subgroups (P = 0.53 in the three way interaction analysis). No significant effect of occupational dust exposure was observed on the FEV1/FVC ratio loss in either the CC (P = 0.19) or the AA/AC group (P = 0.42) stratified by genotype of rs17051547 although the difference in FEV1/FVC loss across dust exposure strata was significant across the two genotype subgroups (P = 0.03 in the three way interaction analysis).
Figure 1 demonstrates different annual FEV1 loss for a male who was 67 inches tall across the different dust exposure groups, baseline age, and pack-years. The more likely dust exposure group had a larger FEV1 annual loss. Compared to former smokers with 20 pack-years smoking history but no dust exposure, former smokers with dust exposure had greater loss in FEV1 after 8 to 9 years, even with 10 pack-years less smoking history. The effect of occupational dust exposure on the FEV1 annual loss (4.5 mL/year) was greater than the effect of each pack-year (2.8 mL). For older participants (e.g., baseline age of 60 years old), the mean difference between different dust exposure groups was larger than for younger participants.
Figure 1.
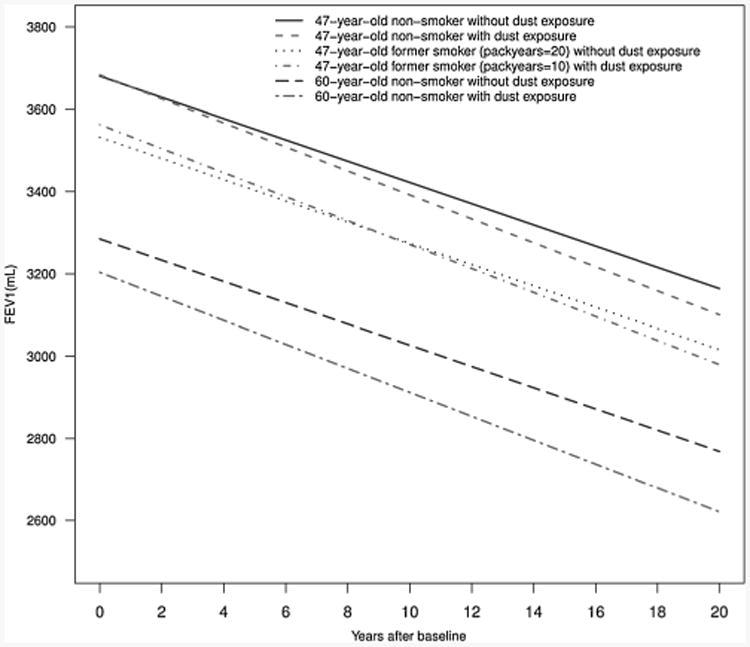
Annual FEV1 loss for a male of 67-inch height in different dust exposure groups, different smoking status, baseline age, and pack-years based on the linear mixed model.
Discussion
We found that occupational dust exposure was not associated with cross-sectional FEV1 cross-sectional FEV1/FVC, or longitudinal FEV1/FVC annual loss in a general population study. However, we did find dust associated accelerated longitudinal FEV1 annual loss, and this effect was greater than the effect of each pack-year increase. Dust exposure also modified the effect of age at baseline on cross-sectional FEV1.
We distinguished the age effect as two components: cross-sectional age effect and longitudinal time effect. In our model, the coefficient of baseline age represents the cross-sectional age effect, and the coefficient of years after baseline represents the longitudinal time effect. For the cross-sectional age effect, we found that, for participants at baseline, each year of age decreased the FEV1 significantly when other covariates remained constant. This effect was modified by dust exposure. For example, at baseline, the difference between each male non-smoker of 67-inch height and a matched male one year older was greater in the group with dust exposure. The difference between different exposure groups was higher in older subjects.
For the longitudinal time effect, we found that participants having the same baseline age had significant annual FEV1 loss, but the annual loss were higher in participants in the more likely dust exposure group. This result implies that, in populations similar to the Framingham Offspring Cohort, occupational dust exposure may not affect FEV1 (occupational dust exposure is not significantly associated with lower average FEV1 in this population), but may accelerate FEV1 loss. The effect of dust exposure (4.5 mL/year excess loss) is greater than the loss caused by each pack-year increase in smoking (2.8 mL). This accelerated FEV1 annual loss rate is consistent with a previous study [Johnsen et al., 2010]. Estimates of moderate to heavy smoking in a review of FEV1 decline by age and smoking show a mean excess loss of 15 mL/year compared to non-smokers [Kerstjens et al., 1997]. The mean excess loss of FEV1 attributable to occupational dust exposure in our study is about 1/3 of that due to smoking and therefore can be clinically significant over a lifetime. The absence of an overall effect of dust exposure on FEV1 is also consistent with some previous studies [Petran et al., 2000; Zock et al., 2001].
The non-significant occupational dust exposure main effect may result from population selection. For our studies, occupational information was only available at exam 8 (the latest exam). Therefore, our participants were assumed to be active workers at exam 8. Participants without occupational information included retired individuals. This could skew selection toward healthier workers, especially in the job categories that require heavy labor (e.g., workers with respiratory diseases might not enter high-exposure jobs or might leave these jobs before the end of the study), causing a healthy worker effect confounding bias [Monson, 1990; Eisen et al., 1995; Li and Sung, 1999]. Since heavy labor job categories were classified as more likely for dust exposure, and if we assume that participants in the group were those in better health, then the magnitude of dust exposure's effect on FEV1 is an underestimate. In addition, a healthy smoker effect confounding bias [Becklake and Lalloo, 1990] may explain why mean FEV1 was higher in the more likely exposure group despite a higher prevalence of smoking.
However, even though these participants might be healthier, their FEV1 loss rates were a significant 4.5 mL/year higher than participants in the less likely dust exposure group. Our observed accelerated loss is lower than a previous study that reported an excess loss in FEV1 of 5.7 to 6.4 mL/year, although that study was industry-based [Johnsen et al., 2010]. One study of blue-collar workers exposed to welding fumes concluded that the absence of a significant effect of occupational dust exposure on lung function in smokers was because of a healthy worker effect [Thaon et al., 2012], but we did not find a significant joint effect (interaction) of tobacco use and occupational dust exposure (data not shown). Genetic factors may also play an important role in the lung function through interactions with occupational dust exposure, but we did not find a significant effect of genetic modifications on FEV1or FEV1/FVC loss.
Even though our results have merit in offering insights into the effects of occupational dust exposures on lung function loss in a community-based study, we acknowledge some limitations. One limitation concerns the assessment of the occupational dust exposure. We constructed a population-specific JEM for dust exposure based on 29 job categories that were less detailed than the UCSF COPD Job Exposure Matrix. This may cause exposure misclassification. This bias can be viewed as non-differential misclassification since the exposure assessment was not related to outcome [Blair et al., 2007]. This misclassification might reduce statistical power and may bias the estimate toward the null, but would not affect the direction of the results (i.e., relation to outcome in the same direction) [Goldberg et al., 1993]. This may explain why we did not find a significant association between dust exposure and cross-sectional FEV1.
In addition, the actual dust exposure level may vary among people who have the same job categories but have different job tasks. We included “students” and “homemaker” in our analysis, i.e., viewed them as having an occupation; although they are not currently employed for salary they can be categorized as low exposure. Another limitation is that we did not exclude participants with pre-existing conditions such as COPD or asthma, due to a lack of such information. Future research that aims at finding the effect of dust exposure on lung function loss in a population-based study will be improved by more detailed job classifications and by direct personal measurements on at least a representative sample of individual job categories.
Acknowledgments
The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195). This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or NHLBI. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. Funding support for the Framingham Social Network datasets was provided by NIA grant P01 AG 031093. This study was funded by NIH (NIEHS) ES00002. The findings and conclusions in this report are those of the authors. We would like to acknowledge the comments of Edwin K. Silverman, MD, PhD from Harvard Medical School and research assistance of Zhaoxi Wang, PhD from Harvard School of Public Health.
Footnotes
Disclosure Statement: The authors report no conflicts of interests.
References
- Bakke PS, Baste V, Hanoa R, Gulsvik A. Prevalence of obstructive lung disease in a general population: Relation to occupational title and exposure to some airborne agents. Thorax. 1991;46:863–870. doi: 10.1136/thx.46.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, Milton D, Schwartz D, Toren K, Viegi G. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–797. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- Becklake MR, Lalloo U. The ‘healthy smoker’: A phenomenon of health selection? Respiration. 1990;57:137–144. doi: 10.1159/000195837. [DOI] [PubMed] [Google Scholar]
- Blair A, Stewart P, Lubin JH, Forastiere F. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50:199–207. doi: 10.1002/ajim.20281. [DOI] [PubMed] [Google Scholar]
- Blanc PD, Iribarren C, Trupin L, Earnest G, Katz PP, Balmes J, Sidney S, Eisner MD. Occupational exposures and the risk of COPD: Dusty trades revisited. Thorax. 2009;64:6–612. doi: 10.1136/thx.2008.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12:703–708. [PubMed] [Google Scholar]
- Eisen EA, Wegman DH, Louis TA, Smith TJ, Peters JM. Healthy worker effect in a longitudinal study of one-second forced expiratory volume (FEV1) and chronic exposure to granite dust. Int J Epidemiol. 1995;24:1154–1161. doi: 10.1093/ije/24.6.1154. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Kromhout H, Guenel P, Fletcher AC, Gerin M, Glass DC, Heederik D, Kauppinen T, Ponti A. Job exposure matrices in industry. Int J Epidemiol. 1993;22(Suppl 2):S10–15. doi: 10.1093/ije/22.supplement_2.s10. [DOI] [PubMed] [Google Scholar]
- Heederik D, Kromhout H, Kromhout D, Burema J, Biersteker K. Relations between occupation, smoking, lung function, and incidence and mortality of chronic non-specific lung disease: The Zutphen study. Br J Ind Med. 1992;49:299–308. doi: 10.1136/oem.49.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins IT. The epidemiology of chronic respiratory disease. Prev Med. 1973;2:14–33. doi: 10.1016/0091-7435(73)90005-4. [DOI] [PubMed] [Google Scholar]
- Hnizdo E, Yan T, Hakobyan A, Enright P, Beeckman-Wagner LA, Hankinson J, Fleming J, Lee Petsonk E. Spirometry longitudinal data analysis software (SPIROLA) for analysis of spirometry data in workplace prevention or COPD treatment. Open Med Inform J. 2010;4:94–102. doi: 10.2174/1874431101004010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen G, Schlunssen V, Schaumburg I, Taudorf E, Sigsgaard T. Longitudinal lung function decline and wood dust exposure in the furniture industry. Eur Respir J. 2008;31:334–342. doi: 10.1183/09031936.00146806. [DOI] [PubMed] [Google Scholar]
- Johnsen HL, Hetland SM, Benth JS, Kongerud J, Soyseth V. Dust exposure assessed by a job exposure matrix is associated with increased annual decline in FE V1: A 5-year prospective study of employees in Norwegian smelters. Am J Respir Crit Care Med. 2010;181:1234–1240. doi: 10.1164/rccm.200809-1381OC. [DOI] [PubMed] [Google Scholar]
- Johnsen HL, Kongerud J, Hetland SM, Benth JS, Soyseth V. Decreased lung function among employees at Norwegian smelters. Am J Ind Med. 2008;51:296–306. doi: 10.1002/ajim.20557. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Fedan KB, Kreiss K. Occupational lung disease risk and exposure to butter-flavoring chemicals after implementation of controls at a microwave popcorn plant. Public Health Rep. 2011;126:480–494. doi: 10.1177/003335491112600405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann F, Drouet D, Lellouch J, Brille D. Occupational exposure and 12-year spirometric changes among Paris area workers. Br J Ind Med. 1982;39:221–232. doi: 10.1136/oem.39.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: Facts, figures, and fallacies. Thorax. 1997;52:820–827. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn RJ, Dockery DW, Speizer FE, Ware JH, Ferris BG., Jr Occupational exposures and chronic respiratory symptoms. A population-based study. Am Rev Respir Dis. 1987;136:298–304. doi: 10.1164/ajrccm/136.2.298. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Fedan KB, Nasrullah M, Kim TJ, Materna BL, Prudhomme JC, Enright PL. Longitudinal lung function declines among California flavoring manufacturing workers. Am J Ind Med. 2011;55:657–668. doi: 10.1002/ajim.21013. [DOI] [PubMed] [Google Scholar]
- Krzyzanowski M, Kauffmann F. The relation of respiratory symptoms and ventilatory function to moderate occupational exposure in a general population. Results from the French PAARC study of 16,000 adults. Int J Epidemiol. 1988;17:397–406. doi: 10.1093/ije/17.2.397. [DOI] [PubMed] [Google Scholar]
- Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med (Lond) 1999;49:225–229. doi: 10.1093/occmed/49.4.225. [DOI] [PubMed] [Google Scholar]
- Liao SY, Lin X, Christiani DC. Gene-environment interaction effects on lung function- a genome-wide association study within the Framingham heart study. Environ Health. 2013;12:101. doi: 10.1186/1476-069X-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009;6:527–531. doi: 10.1513/pats.200905-027DS. [DOI] [PubMed] [Google Scholar]
- Monson R, editor. Occupational Epidemiology. Boca Raton: CRC Press; 1990. [Google Scholar]
- Omland O, Wurtz ET, Aasen TB, Blanc P, Brisman JB, Miller MR, Pedersen OF, Schlunssen V, Sigsgaard T, Ulrik CS, Viskum S. Occupational chronic obstructive pulmonary disease: A systematic literature review. Scand J Work Environ Health. 2014;40:19–35. doi: 10.5271/sjweh.3400. [DOI] [PubMed] [Google Scholar]
- Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, Lange HJ. Occupational dust exposure and chronic obstructive pulmonary disease. A systematic overview of the evidence. Am Rev Respir Dis. 1993;148:38–48. doi: 10.1164/ajrccm/148.1.38. [DOI] [PubMed] [Google Scholar]
- Petran M, Cocarla A, Baiescu M. Association between bronchial hyper-reactivity and exposure to silicon carbide. Occup Med (Lond) 2000;50:103–106. doi: 10.1093/occmed/50.2.103. [DOI] [PubMed] [Google Scholar]
- Thaon I, Demange V, Herin F, Touranchet A, Paris C. Increased lung function decline in blue-collar workers exposed to welding fumes. Chest. 2012 doi: 10.1378/chest.11-0647. [DOI] [PubMed] [Google Scholar]
- Viegi G, Prediletto R, Paoletti P, Carrozzi L, Di Pede F, Vellutini M, Di Pede C, Giuntini C, Lebowitz MD. Respiratory effects of occupational exposure in a general population sample in north Italy. Am Rev Respir Dis. 1991;143:510–515. doi: 10.1164/ajrccm/143.3.510. [DOI] [PubMed] [Google Scholar]
- Wang ML, Avashia BH, Petsonk EL. Interpreting periodic lung function tests in individuals: the relationship between 1- to 5-year and long-term FEV1 changes. Chest. 2006;130:493–499. doi: 10.1378/chest.130.2.493. [DOI] [PubMed] [Google Scholar]
- Wang ML, Petsonk EL. Repeated measures of FEV1 over six to twelve months: What change is abnormal? J Occup Environ Med. 2004;46:591–595. doi: 10.1097/01.jom.0000128159.09520.2a. [DOI] [PubMed] [Google Scholar]
- Zock JP, Sunyer J, Kogevinas M, Kromhout H, Burney P, Anto JM. Occupation, chronic bronchitis, and lung function in young adults. An international study. Am J Respir Crit Care Med. 2001;163:1572–1577. doi: 10.1164/ajrccm.163.7.2004195. [DOI] [PubMed] [Google Scholar]


