Abstract
Stimulation of the spinal cord has been shown to have great potential for improving function after motor deficits caused by injury or pathological conditions. Using a wide range of animal models, many studies have shown that stimulation applied to the neural networks intrinsic to the spinal cord can result in a dramatic improvement of motor ability, even allowing an animal to step and stand after a complete spinal cord transection. Clinical use of this technology, however, has been slow to develop due to the invasive nature of the implantation procedures and the difficulty of ascertaining specific sites of stimulation that would provide optimal amelioration of the motor deficits. Moreover, the development of tools available to control precise stimulation chronically via biocompatible electrodes has been limited. In this paper, we outline the use of a multisite electrode array in the spinal rat model to identify and stimulate specific sites of the spinal cord to produce discrete motor behaviors in spinal rats. The results demonstrate that spinal rats can stand and step when the spinal cord is stimulated tonically via electrodes located at specific sites on the spinal cord. The quality of stepping and standing was dependent on the location of the electrodes on the spinal cord, the specific stimulation parameters, and the orientation of the cathode and anode. The spinal motor evoked potentials (sMEP) in selected muscles during standing and stepping are shown to be critical tools to study selective activation of interneuronal circuits via responses of varying latencies. The present results provide further evidence that the assessment of functional networks in the background of behaviorally relevant functional states is likely to be a physiological tool of considerable importance in developing strategies to facilitate recovery of motor function after a number of neuromotor disorders.
Keywords: Spinal cord epidural stimulation, spinal motor evoked potentials, electrode array, electric enabling motor control (eEmc), locomotion, neurorehabilitation
Introduction
The combination of spinal cord epidural stimulation (electrical enabling motor control, eEmc) and proprioceptive input from the hindlimbs while stepping on a moving treadmill belt has been successful in restoring some weight-bearing standing ability in rats (Gad et al., 2013a) and humans (Harkema et al., 2011; Angeli et al., 2014) and stepping ability in rats (Ichiyama et al., 2008, Courtine et al., 2009; Musienko et al., 2011) and cats (Gerasimenko et al., 2003, Musienko et al., 2012; Rossignol et al., 1999, Barbeau et al., 1999, Brustein et al., 1999, Barthelemy et al., 2007) after a spinal cord injury (SCI). To begin to better understand the mechanisms underlying the of regulation complex motor tasks, we characterize how sMEPs vary as a function of the physiological state of the spinal networks. In a previous study, we compared the sMEPs as a function of the phases of the step cycle in spinal rats while stepping bipedally on a treadmill at different speeds and weight-bearing conditions under the influence of eEmc with and without quipazine (a serotoninergic agonist) or strychnine (a glycinergic antagonist) (Gad et al., 2013c). The evoked potentials (middle responses, MRs and late responses, LRs) were modulated during different stepping speeds and body weight support conditions, suggesting a correlation between the physiological state of the spinal networks responsible for generation of these responses and the functional state of the hindlimbs (Lavrov et at., 2006, 2008a, 2008b). These observations provide the groundwork for understanding how the spinal cord circuitry can respond to a range of stimulation parameters using a chronically implanted epidural electrode array.
We hypothesize that chronically implanted electrode arrays placed over the lumbosacral spinal cord in rats with complete paralysis of the lower limbs can be used to differentially activate spinal networks projecting to specific flexor and extensor motor pools that are constantly changing their physiological states under non-anesthetized in vivo conditions. We examined the modulation of sMEPs to different stimulation parameters, i.e., location and orientation of the anode and cathode, frequency of stimulation, etc. Specifically, we asked the following questions, 1) to what degree does the variability in electrode design (wire vs. microelectrode array) affect the evoked potentials, 2) are the modulatory features of sMEPs spatially unique at different anatomical points along the lumbosacral spinal cord, 3) to what degree can such spatially unique sensorimotor networks be selectively activated by different stimulation configurations, and 4) how is the composition of sMEPs affected by location, frequency, and intensity of the spinalcord stimulation?
Methods
Data were obtained from 4 adult female Sprague Dawley rats (270-300 g body weight) at 10-12 days complete spinalization post-injury. Pre- and post-surgical animal care procedures have been described in detail previously (Roy et al., 1992). The rats were housed individually with food and water provided ad libitum. All survival surgical procedures were conducted under aseptic conditions and with the rats deeply anesthetized (isoflurane gas administered via facemask as needed). All procedures described below are in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Committee at UCLA. Details of the implant and electrode array fabrication and multiplexor techniques, have been described previously (Nandra et al., 2011; Gad et al., 2013a)
Implant fabrication
The electrode array is fabricated with a sandwich structure of parylene-metal-parylene. Parylene-C is a USP class VI biocompatible material and its mechanical properties provide the necessary flexibility to make good epidural contact with the spinal cord. The micro-fabrication process begins with an optional layer of sacrificial photoresist being spun onto a wafer followed by a deposition of a layer of 10-μm thick parylene-C. This layer is patterned to form a structural frame around the outside of the electrode array and is followed by another layer of 5-μm thick parylene-C. The metal layer, patterned using liftoff, was deposited using e-beam evaporation and was composed of a titanium adhesion layer of 100 Å followed by 2000 Å of platinum. The top layer of parylene-C is also 5-μm thick. Openings to expose the metal, formation of the frame, and overall device outline were achieved with oxygen plasma etching. The completed devices were released from the wafer using acetone or water and annealed in a vacuum oven at 200°C for 48 h. The full micro-fabricated device is 59 mm × 3 mm and has a 9 × 3 array of electrodes which are 200 × 500 μm with a parylene grid structure to help prevent delamination (Gad et al., 2013). The complete implant consists of this electrode array, a multiplexer circuit, various wires, and a head connector. The multiplexer circuit routes connections and performs pre-amplification to reduce the total number of head connector wires needed from 37 (passive implant, Nandra et al., 2011) to just 12 (active implant, Gad et al., 2013a). This design reduces surgery complications and also serves as a stepping-stone for a fully wireless design. The electrode array is interfaced to the multiplexer board with conductive epoxy. The implant then is sealed with 20 μm of parylene, biocompatible silicone (MDX 4–4210), biocompatible epoxy (Loctite M-121HP), and another 20-μm layer of parylene.
Control box and multiplexer circuit board description
The stimulation host computer has a software interface to choose the electrodes to be stimulated and the stimulation intensity (specified by pulse voltage or current), pulse duration, and pulse frequency. The software generates a 5 MHz signal stream to be output by an ADC/DIO card (National Instruments PXI-6123) and fed to the control box. This signal stream consists of the EN, Clock, and Data signals to control the multiplexer circuit in the implant, PWM (pulse-width modulation) and Mode signals for stimulation, and a Sync signal to synchronize EMG recordings. The control box has an op-amp circuit to generate the stimulation signal. The PWM signal is passed through an RC filter and creates any required analog waveform at Vin (0–2.5 V, ∼5 μs effective pulse rise time). (Gad et al., 2013a)
Head connector and intramuscular EMG electrode implantation
A small incision was made at the midline of the skull. The muscles and fascia were retracted laterally, small grooves were made in the skull with a scalpel, and the skull was dried thoroughly. Amphenol head connectors with Teflon-coated stainless steel wires (AS632, Cooner Wire, Chatsworth CA) were securely attached to the skull with screws and dental cement as described previously (Roy et al., 1991, Gerasimenko et al., 2006, Courtine et al., 2009). The tibialis anterior (TA), medial gastrocnemius (MG), and soleus muscles were implanted bilaterally with EMG recording electrodes as described previously (Roy et al., 1991). Skin and fascial incisions were made to expose the belly of each muscle. Two wires extending from the multiplexer circuit were routed subcutaneously to each muscle (Gad et al., 2013a). The wires were inserted into the muscle belly using a 23-gauge needle and a small notch (∼0.5-1.0 mm) was removed from the insulation of each wire to expose the conductor and form the electrodes. The wires were secured in the belly of the muscle via a suture on the wire at its entrance into and exit from the muscle belly. The wires were looped at the entrance site and in the mid-back region to provide stress relief. The proper placement of the electrodes was verified 1) during the surgery by stimulating through the stimulator in the control box and by selecting the correct channels on the multiplexer circuit board and 2) post-surgery via dissection.
Spinal cord transection and array implantation
A partial laminectomy was performed at the T8-T9 vertebral level and a complete spinal cord transection to include the dura was performed at ∼T8 spinal level using microscissors. Two surgeons verified the completeness of the transection by lifting the cut ends of the spinal cord and passing a glass probe through the lesion site. Gel foam was inserted into the gap created by the transection as a coagulant and to separate the cut ends of the spinal cord.
To implant the array, the spinous processes and portions of the dorsal and lateral aspects of the T11 vertebrae and the rostral portions the T12 and L4 vertebrae were removed. A suture (4.0 Ethilon) was inserted through the opening at T11 and passed down to the opening at L4. This suture then was threaded into holes at the most rostral end of the electrode array and used to gently pull the array rostrally between the dura and the vertebral column. The most rostral row of electrodes was placed at the middle of the T12 vertebra. Once the array was positioned satisfactorily over the dorsal surface of the spinal cord, the rostral end of the array was sutured (8.0 Ethilon) to the dura to secure it in position. The spinous process of the L3 vertebra was removed to form a flat surface. A multiplexer circuit board was placed on the vertebral column over the L3 vertebra. A “U” notch on the ventral surface of the implant was secured into the L2 spinous process via a suture (4.0 Ethilon) threaded through a hole on the circuit board and then tied around the L2 spinous process.
All incision areas were irrigated liberally with warm, sterile saline throughout the surgery. All surgical sites were closed in layers, i.e., muscle and connective tissue layers with 5.0 Vicryl (Ethicon, New Brunswick, NJ) and the skin incisions on the back and the limbs with 5.0 Ethilon. All closed incision sites were cleansed thoroughly with warm saline solution. Analgesia was provided by buprenex (0.5–1.0 mg/kg, 3 times/day s.c.). The analgesics were initiated before the completion of the surgery and continued for a minimum of 2 days post-surgery. The rats were allowed to fully recover from anesthesia in an incubator. The spinal rats were housed individually in cages that had ample CareFresh bedding and their bladders were expressed manually 3 times/day for the first 2 weeks after surgery and 2 times per day thereafter. The hindlimbs of the spinal rats were moved passively through a full range of motion once per day to maintain joint mobility.
Stimulation and testing procedures
Two bipolar stimulation protocols were used for testing. Firstly, on the testing day, the cathode and anode combinations were selected sequentially among all electrodes on the array to systematically cover the entire surface of the array. Evoked potentials were recorded from the TA and soleus muscles bilaterally for each electrode combination. The evoked potentials were produced by stimulating the spinal cord at a low frequency (1 Hz) and voltage sweep from 1-8 V (1 V increments) with the rat suspended in a jacket with its hindpaws in contact with a stationary treadmill. Secondly, a bipolar configuration where both the cathode and anode were selected from the set of 27 electrodes on the array was used to facilitate the standing and stepping ability of the spinal rats. Sub-sets of bipolar configurations were tested on different test days. The stimulation frequency was based on previously reported values (Ichiyama et al., 2005, Ichiyama et al., 2008, Gad et al., 2013a) and the stimulation intensity was varied (range from 1–8 V) to optimize the standing and stepping ability of the spinal rats. EMG was recorded from the MG, TA, and soleus bilaterally while the rats stepped bipedally on a specially designed motor-driven rodent treadmill at 13.5 cm/s (de Leon et al., 2002). The treadmill belt had an anti-slip material that minimized slipping while stepping. The rats were placed in a body weight support system that allowed the rat to support the maximum amount of its body weight while stepping with plantar placement.
Data collection and analysis
EMG recordings from the hindlimb muscles were pre-amplified by the multiplexer circuit board and an external control box before being sent to a band-pass filter (1 Hz to 5 KHz), externally amplified (A-M Systems Model 1700 differential AC amplifier: A-M Systems, Carlsborg, WA), and sampled at a frequency of 10 KHz using a custom data acquisition program written in the LabView development environment (National Instruments, Austin, TX) as described previously (Courtine et al., 2009). Evoked potentials during suspension, standing, and stepping were analyzed as described previously (Lavrov et al., 2006, 2008a, Gad et al., 2013a, 2013b, 2013c). These evoked potentials then were divided into early (ER, 1-4 ms latency), middle (MR, 4-8 ms latency), and late (LR > 8 ms) responses.
Results
When we previously used individual wire electrodes for spinal cord stimulation in normal and spinal cord transected rats, we recorded three motor evoked responses when the rats were suspended (Gerasimenko et al., 2006, Gad et al., 2013c), weight-bearing standing (Gad et al., 2013a), or stepping bipedally (Gad et al., 2013b, 2013c) under the influence of eEmc. We observed an ER (latency 1-3 ms), MR (4-6 ms), and LR (7-9 ms). Using the multielectrode array in the present study, we observed responses similar in pattern, but with slightly longer latencies, i.e., ER, (1-4 ms), MR (4-8 ms), and LR (>8 ms).
All stimulation combinations did not generate all three responses (ER, MR, and LRs). Stimulation at the rostral end of the lumbar spinal cord (∼L2-L3 spinal segments) resulted in prominent ERs in the TA but not in the soleus. Stimulation at the middle of the spinal cord (∼L4-L5 spinal segments) resulted in lower amplitude ERs and MRs in both the TA and soleus than the rostral electrodes. Stimulation at the caudal end of the spinal cord (∼L6-S1 spinal segments) resulted in large ERs in the TA. The largest MR amplitudes were observed in the soleus muscle when stimulating the caudal end of the spinal cord. Very few LRs were observed, most likely due to the time point after injury (12 days post-injury, Lavrov et al., 2006).
All stepping experiments were performed while maintaining the frequency of eEmc at 40 Hz but changing the sites and orientation of the anode and cathode (Fig. 2). Stepping performance varied considerably across the monopolar/bipolar (Gad et al., 2013a) stimulation combinations. The stepping patterns varied from robust bilateral weight-bearing stepping, to partial weight-bearing inconsistent stepping, to unilateral non-weight-bearing stepping, to hindlimb dragging. The most stable stepping was observed with diagonal pairs of electrodes covering multiple levels of the spinal cord either at the rostral (rows 1 to 3) or caudal pairs of electrodes (rows 7 to 9).
Figure 2.
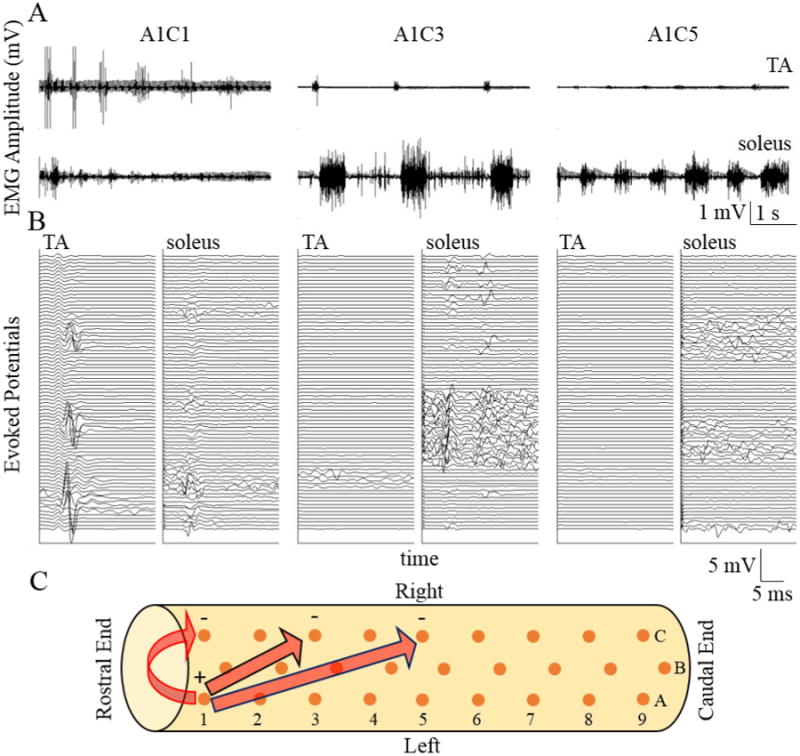
(A) Raw EMG from a flexor (tibialis anterior, TA) and an extensor (soleus) muscle from a spinal rat while stepping on a treadmill at 13.5cm/s under the influence of eEmc (40 Hz) using different combinations of anodes and cathodes. Identification of the electrode pairs is shown in (C). (B) Evoked potentials from the first 2 sec of data recorded in each muscle shown in A. Each trace is triggered of the stimulation pulse with the first trace being the lowest and the topmost being the last pulse. Data presentation is similar to that in previous publications (Gad et al., 2013a). (C) Schematic of the electrode array (orange dots) implanted epidurally on the spinal cord between L2 and S2 spinal levels. The arrows indicate the electrode combinations shown in (A).
Figure 2 demonstrates three cases of consistent bilateral stepping with varying weight-bearing capabilities as the combination of electrodes was changed. Keeping the anode consistent at A1 and moving the cathode from electrode C1 to C3 to C5, the TA EMG amplitudes were reduced, whereas the soleus EMG amplitudes were increased. Evoked potentials during stepping revealed some interesting aspects of evoked responses. Moving the cathode from electrode C1 to C5 lowered the amplitude of the MRs, while increasing the number of LRs. The evoked potentials produced when stimulating with A1C5 as compared to A1C1 and A1C3 were associated with a more normal EMG bursting pattern (similar to those observed in control rats).
While maintaining the sites of stimulation constant at A1C7 and varying the frequency, the tuning of the spinal cord varied widely during quiet standing (Fig. 3). This frequency effect was observed in both the EMG responses (Fig. 3A) and the evoked potentials (Fig. 3B). Stimulation at 1 Hz resulted in a flexion motion at the ankle with MRs in the TA, soleus, and MG and ERs only in the soleus and MG. Stimulation at 10 Hz produced twitches in the hindlimbs, whereas 40-Hz stimulation resulted in partial weight-bearing standing. There was an over-excitation of the neural networks at the highest frequency of stimulation (100 Hz) causing unorganized activation of the flexors and extensors. Increasing the stimulation frequency between 1 and 100 Hz reduced the MRs in the TA, whereas increasing the frequency between 1 to 40 Hz increased the amplitudes of the MRs in the soleus. Note the presence of an ER at 1 and 10 Hz but not at 40 Hz and an MR at 1 and 40 Hz but not at 10 Hz. Due to the unorganized bursting pattern in the TA and soleus, no evoked potentials could be identified at 100 Hz.
Figure 3.
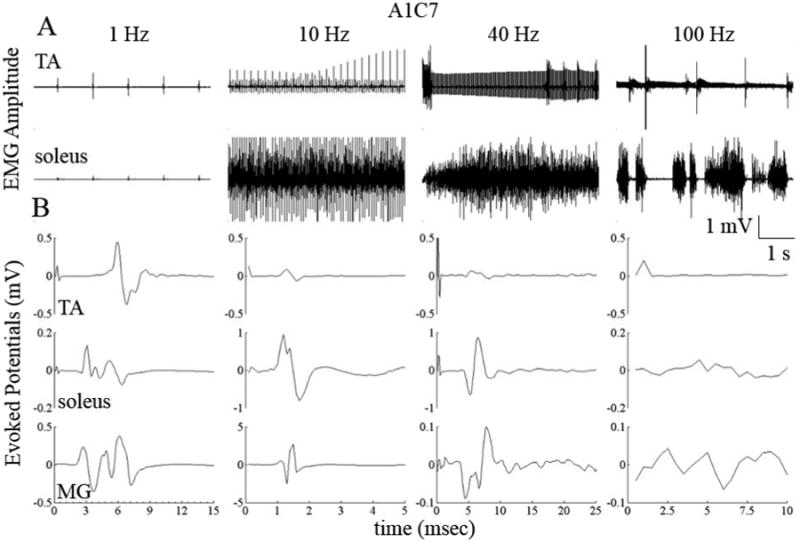
(A) Raw EMG from the TA and soleus muscles from a spinal rat while standing bilaterally under the influence of eEmc at different frequencies using electrodes A1 and C7 on the array (see Fig. 2(C)) as the anode and cathode, respectively. (B) Average evoked potentials from the data shown in (A). Note the differences in the time and amplitude scales.
Discussion
We have begun to characterize the properties of sMEPs evoked in selected hindlimb muscles when using a novel high-density parylene-based multi-electrode platinum array to stimulate the lumbosacral spinal cord. These data are critical for determining the degree to which selective activation of spinal circuits can be used to facilitate standing and stepping in rats after a complete spinal cord transection at a low-thoracic level. The results suggest that spinal rats can stand and step more quickly when the spinal cord is stimulated tonically at 40 Hz by microelectrodes located at specific sites on the spinal cord compared to wire electrodes. The quality of stepping and standing was dependent on the location of the electrodes on the spinal cord, the specific stimulation parameters, and the orientation of the cathode and anode. In addition, the amplitudes and latencies of evoked potentials were determined in non-anesthetized spinal rats during standing and stepping to assess the efficacy of selected spinal circuits. The evoked potentials during standing and stepping are critical tools for studying selective activation of interneuronal circuits via responses of varying latencies.
Incongruity of clinical and physiological assessments of completeness of paralysis: Need for the ability to record evoked potentials from the spinal cord
Recently we reported (Harkema et al., 2011, Angeli et al., 2014) changes in the physiological state of the spinal cord in 4 out of 4 clinically motor complete subjects (2 AIS A and 2 AIS B) implanted with a 16-electrode epidural array over the L1-S1 spinal levels within weeks of implantation. The results show recovery and progressive improvement in volitional motor control in the presence of epidural stimulation as a result of daily motor training. The increased excitability using eEmc was sufficiently close to the motor threshold so that the newly evolved supraspinal descending input to the lumbosacral spinal cord was sufficient to reach motor threshold. Kakulas (1998) reported a remarkable finding in the study of 564 human cadavers with SCI. He studied variables such as axonal lesions, traumatic demyelination-remyelination, and quantification of white matter tracts. Surprisingly, many of the cadavers had a proportion of their spinal cord white matter remaining across the level of lesion even though they were completely paralyzed as assessed clinically. Thus, there appears to be residual connectivity that is dormant and could be accessed via spinal cord stimulation paradigms. The full potential for the use of high-density epidural electrode arrays as a diagnostic tool in clinical and basic scientific studies cannot yet be realized due to limitations in currently available implantable stimulating electronics. The stimulators currently FDA-approved for human studies are too limited in the types of stimulation needed and have no capability to record electrical potentials. For this reason, we are unable to detect dynamic changes in identified intra-spinal cord network interactions during stimulation. Furthermore, we have little to no information about the ascending signals that can provide significant input to both the spinal and supraspinal networks. Adding the ability to record from intrinsic networks of the spinal cord could reveal novel insight in the feedback mechanisms that form the basis for locomotor pattern generation with and without supraspinal input. This will require that the technology for the electrodes and stimulating and recording devices provide optimal characteristics for both stimulation and recording.
Comparison between traditional wired electrodes and multi-electrode arrays
Several studies have shown that epidural stimulation at L2 and/or S1 using wire electrodes in combination with motor training can facilitate stepping within 3–4 weeks after complete paralysis in rats (Lavrov et al., 2006, Gerasimenko et al., 2008, Courtine et al., 2009, Musienko et al., 2011, van den Brand et al., 2012, Wenger et al., 2014). Using the parylene-based platinum electrode arrays described herein we have been successful in facilitating weight-bearing standing and stepping within 8–10 days post-transection (Present data, Gad et al., 2013a). Thus use of the electrode array allows more effective selectivity in activating spinal networks to enable stepping sooner after injury as compared to using conventional wire electrodes. This could be due to the presence of the parylene substrate directing the electric field microelectrodes in a more focused manner as compared to the wired electrodes. Further studies involving both mathematical modeling (Capogrosso et al., 2013, Danner et al., 2011) and immunohistochemical analyses (Edgerton et al., 2004) to decode the activation patterns of the electric fields are needed to maximize the clinical and scientific impact of the multi-electrode arrays.
Neurophysiological mechanisms and specific sensorimotor integration impacting motor function via the electrode array after SCI
There is a range of motor behaviors that can be generated with modest levels of stimulation, i.e., primarily sub-motor threshold levels, using different combinations of electrodes and at different frequencies. The results indicate that it is evident that second to second modulation of interneuronal network excitability toward the threshold for excitation of selected motor pools is an important strategy in controlling movement. Conceptually our strategy for facilitating these motor behaviors is to achieve a physiological state that enables the proprioceptive input derived from stepping and standing to serve as the source of control. That is, the ‘sub-threshold’ intensity of stimulation that modulates the spinal circuitry associated with stepping and standing may not, and actually preferably does not, induce action potentials of motoneurons, but excite interneuronal networks extending from sensory afferents to all of the motor pools. Rather than imposing a specific motor response by stimulating at high intensities, and thus precluding proprioceptive modulation, the activated pathways are determined by the ensemble of normally occurring weight-bearing sensory information being projected in real time to the spinal circuitry. Regarding the degree of selectivity of specific pathways that could be modulated, the extensive divergence of a single Ia afferent fiber from each muscle spindle has extensive synaptic connectivity to not only the homonymous motor pools, but also to a lower percentage of its synergists and indirectly to antagonistic motor pools through Ia inhibitory interneurons. In addition, robust intersegmental connectivity among the lumbar segments via ascending projections from the sacral segments has recently been reported. Combined, these observations are consistent with the interpretation that epidural stimulation can impact many different combinations of spinal networks simultaneously but in different degrees and proportions based on the multiple stimulation parameters described in the present paper.
In summary results from earlier studies demonstrated that epidural stimulation can be used to facilitate recovery of stepping and standing in rats after a complete spinal cord transection (Iwahara et al., 1991, Ichiyama et al., 2005, Courtine et al., 2009, Gad et al., 2013a). We have extended several details that provide strategies for further success in recovery of these tasks even with a complete absence of supraspinal input. More specifically the present results demonstrate that microelectrode arrays provide a means for fine-tuning multiple networks within the spinal cord. Relatively small changes in the site of stimulation can have marked effects on the motor output. The responses to these positionally sensitive sites are highly interactive with simultaneous modulation of stimulation intensity. The present results do not provide other stimulation-sensitive parameters that also have facilitory effects on postural and locomotor tasks. These results do, however, provide very strong evidence that recording the dynamic modulation of multiple sMEPs among multiple muscles of interest under in vivo non-anesthetized conditions represents a source of much more rich data that can be obtained from anesthetized preparations in which massive sources of synaptic interactions within these networks are eliminated. The present results provide evidence that the assessment of functional networks in the background of behaviorally relevant physiological states is likely to be a physiological tool of considerable importance in developing strategies to facilitate recovery of motor function after a number of neuromotor disorders.
Figure 1.
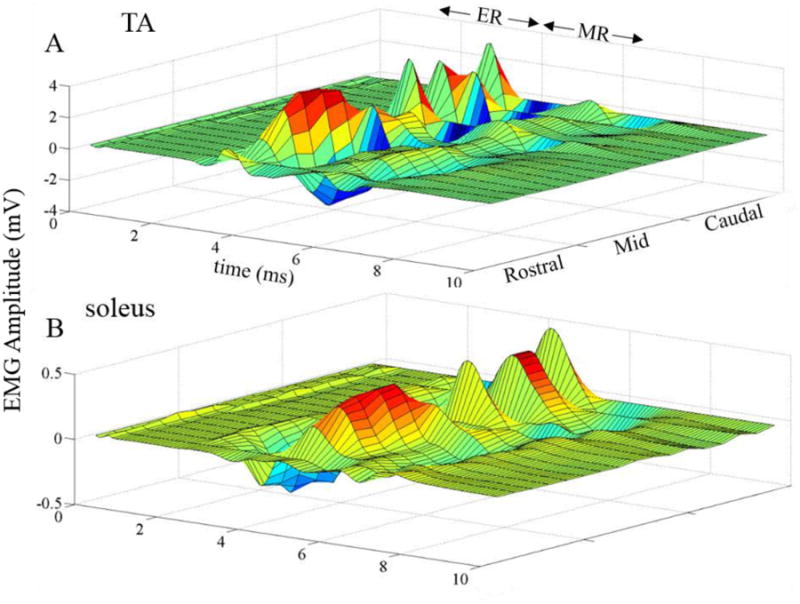
Average evoked potentials (n = 3 rats) from the TA (A) and soleus (B) muscles using different combinations of electrodes on the array (rostral: rows 1, 2, and 3; mid: rows 4, 5, and 6; and caudal: rows 7, 8, and 9) with eEmc at 1 Hz. Note the variations in the early responses (ER, latency 1-4 ms) and middle responses (MR, latency 4-8 ms) for the different electrode combinations used for the stimulation.
Acknowledgments
This research was funded in part by NIH U01EB15521, R01EB007615 through NIBIB, NINDS, and NICD, the Christopher and Dana Reeve Foundation, the Hemsley Foundation, the Broccoli Foundation, the Walk about Foundation, and the F. M. Kirby Foundation. Y.G. is supported by RFBR №13-04-12030 ofi-m, and by Russian Scientific Fund project № 14-45-00024.
Footnotes
PG, RRR, YG and VRE designed the experiments. MN and YT designed and fabricated the implant. RRR and HZ performed all the surgeries. PG and JC performed the experiments. PG analyzed the data. PG, YG, RRR and VRE wrote the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest: VRE (along with RRR and YG) researchers on the study team hold shareholder interest in NeuroRecovery Technologies. VRE (RRR and YG) hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and it's subsidiaries.
References
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain : a journal of neurology. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, McCrea DA, O'Donovan MJ, Rossignol S, Grill WM, Lemay MA. Tapping into spinal circuits to restore motor function. Brain research Brain research reviews. 1999;30:27–51. doi: 10.1016/s0165-0173(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Leblond H, Rossignol S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. Journal of neurophysiology. 2007;97:1986–2000. doi: 10.1152/jn.00818.2006. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. Journal of neurophysiology. 1999;81:1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19326–19340. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nature neuroscience. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artificial organs. 2011;35:257–262. doi: 10.1111/j.1525-1594.2011.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Progress in brain research. 2002;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annual review of neuroscience. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Gad P, Choe J, Nandra MS, Zhong H, Roy RR, Tai YC, Edgerton VR. Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. Journal of neuroengineering and rehabilitation. 2013a;10:2. doi: 10.1186/1743-0003-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. Journal of neuroengineering and rehabilitation. 2013b;10:108. doi: 10.1186/1743-0003-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Lavrov I, Shah P, Zhong H, Roy RR, Edgerton VR, Gerasimenko Y. Neuromodulation of motor-evoked potentials during stepping in spinal rats. Journal of neurophysiology. 2013c;110:1311–1322. doi: 10.1152/jn.00169.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Experimental neurology. 2008;209:417–425. doi: 10.1016/j.expneurol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neuroscience and behavioral physiology. 2003;33:247–254. doi: 10.1023/a:1022199214515. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. Journal of neuroscience methods. 2006;157:253–263. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neuroscience letters. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Locomotion induced by spinal cord stimulation in the neonate rat in vitro. Somatosensory & motor research. 1991;8:281–287. doi: 10.3109/08990229109144751. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. The journal of spinal cord medicine. 1998;22(2):119–124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008a;28:7774–7780. doi: 10.1523/JNEUROSCI.1069-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Dy CJ, Fong AJ, Gerasimenko Y, Courtine G, Zhong H, Roy RR, Edgerton VR. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:6022–6029. doi: 10.1523/JNEUROSCI.0080-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. Journal of neurophysiology. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, Roy RR, Edgerton VR, Gerasimenko Y. Somatosensory control of balance during locomotion in decerebrated cat. Journal of neurophysiology. 2012;107:2072–2082. doi: 10.1152/jn.00730.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9264–9278. doi: 10.1523/JNEUROSCI.5796-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandra M, Lavrov I, Edgerton VR, Tai YC. Micro Electro Mechanical Systems (MEMS), 2011 IEEE 24th International Conference on. IEEE; 2011. A Parylene-based microelectrode array implant for spinal cord stimulation in rats; pp. 1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Progress in brain research. 1999;123:349–365. doi: 10.1016/s0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hodgson JA, Lauretz SD, Pierotti DJ, Gayek RJ, Edgerton VR. Chronic spinal cord-injured cats: surgical procedures and management. Laboratory animal science. 1992;42:335–343. [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. Journal of applied physiology. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, Morari M, Micera S, Courtine G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Science translational medicine. 2014;6:255ra133. doi: 10.1126/scitranslmed.3008325. [DOI] [PubMed] [Google Scholar]


