Abstract
Prospective validation of the hematopoietic cell transplantation-comorbidity index (HCT-CI) using contemporary patients treated with HCT across the Unites States is necessary to confirm its widespread applicability. We performed a prospective observational study including all patients (8115 recipients of allogeneic and 11,652 recipients of autologous HCT) who underwent first HCT that was reported to the CIBMTR between 2007 and 2009. In proportional hazards models, increased HCT-CI scores were independently associated with increases in hazard ratios for NRM (p<0.0001) and overall mortality (p<0.0001) among recipients of allogeneic HCT. HCT-CI Scores of ≥3 were uniformly associated with higher risks for outcomes in both allogeneic and autologous HCT, and all subgroups regardless of diagnoses, age, and conditioning intensity. Recipients of allogeneic HCT with scores of 1–2 who were aged <18 or were treated with lower intensity conditioning regimens had similar outcomes compared to those with score 0. Higher risks for overall mortality, but not for NRM, were observed among recipients of autologous HCT with scores of 1–2 versus 0. Our results confirm the validity the HCT-CI in both allogeneic and autologous HCT. The index should be used as a valid standard-of-care health measure in counseling patients for HCT, in clinical trial design, and in adjusting outcome analyses.
Keywords: Bone marrow transplantation, hematopoietic cell transplantation, autologous, allogeneic, HCT-CI, comorbidities, validity, inter-rater reliability
Introduction
Organ dysfunctions (comorbidities) impact both treatment decisions and treatment outcomes in oncology, and is particular salient in hematopoietic cell transplantation (HCT), where the morbidity associated with the procedure is high.[1] Until 2004, age alone had been widely used as the primary measure of a patient’s ability to tolerate the conditioning regimens for allogeneic HCT.[2] Recently, the HCT-CI was designed as a health measure suited for capturing the burden and complexity of organ dysfunctions among recipients of allogeneic HCT. The index was modeled to predict non-relapse mortality (NRM) and initial analysis validated its ability to discriminate risks for NRM as well as overall mortality in an independent randomly selected cohort from the same institution.[3] Subsequently, comorbidity evaluation integrated in transplant-related analyses have demonstrated the importance of risk assessment prior to HCT[4–7] or even conventional therapies[8–11], and to better select patients for different regimen intensities.[12,13] Additional studies suggested that comorbidities may have more important role than calendar age in determining HCT eligibility.[14]
While some investigators have confirmed the prognostic significance of the HCT-CI in their respective patients,[15–18] others did not confirm the importance of the HCT-CI.[19,20] Therefore, it became important to study the prognostic significance of the HCT-CI in a prospective, well designed, multi-center setting to confirm its utility as a prognostic health-status measure of HCT outcomes. Further, there has been only limited number of studies that assessed the performance of the HCT-CI in the autologous HCT settings.[21,22] If the utility of the HCT-CI is confirmed in adequately designed large validation studies, this index would allow for consistent integration of comorbidities into the design of randomized clinical trials in HCT, adjustment of clinical trial results across transplant institutions, and better understanding of the biological causes of post-HCT morbidities.
We hereby summarize the results from a large multi-institutional prospective study gathering information from all United States transplant centers that report to the Center of International Blood and Marrow Transplantation Research (CIBMTR). The study was aimed to determine the discriminative capacity of the HCT-CI among recipients of allogeneic and autologous HCT, and the effectiveness of the HCT-CI in stratifying outcomes among HCT patients with different diagnoses, age groups, and conditioning intensities.
Patient and Methods
Data Source
The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a research affiliate of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) established in 2004. It comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute data on consecutive allogeneic and autologous HCT procedures to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, Minnesota. Participating centers report longitudinal data on all transplants and compliance is monitored by on-site audits. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
Study Design and Patients
In 2007, a new prospective multi-institutional observational study was initiated at the CIBMTR to collect comorbidities from all transplant centers by their respective evaluators and to validate the predictive power of the HCT-CI in a large sample of patients. The HCT-CI was adapted into the Pre-Transplant Essential Data (pre-TED) collection form #2400. Data managers from all institutions attended an education session on comorbidity coding per the HCT-CI at the 2007 Tandem BMT Meeting in Keystone, Colorado. This session was then made public to all data managers at the CIBMTR website “www.cibmtr.org/Meetings/Materials/CRPDMC/Pages/feb2007sorror.aspx”.
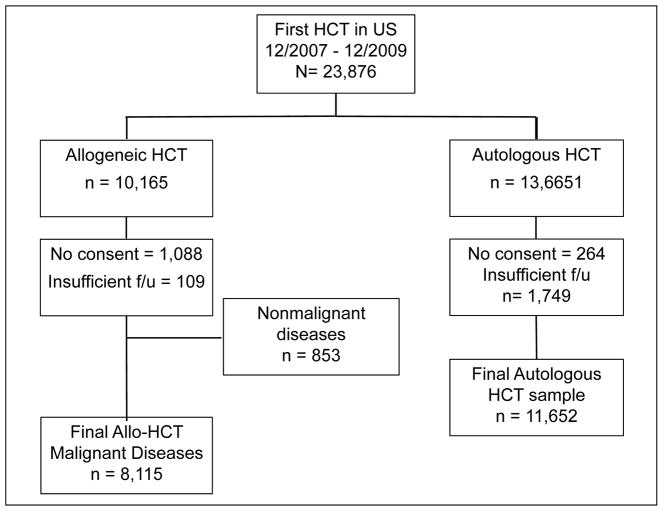
The study was designed to score comorbidities prospectively for all patients meeting the following criteria: 1) diagnoses of hematological malignant diseases, 2) treatment with autologous or allogeneic HCT between December 1st 2007 and December 31st 2009, 3) receiving conditioning regimens of any intensity or composition, 4) receiving grafts from HLA-matched related or unrelated donors, and 5) given marrow or granulocyte-colony stimulating factor-mobilized peripheral blood mononuclear cells (G-PBMC) grafts. No upper limit was stated for the number of patients to be enrolled into the study. Figure 1 is an organization chart depicting patient eligibility and enrollment into the study. Among 23,876 recipients (Figure 1) of first HCT in the United States between 2007 and 2009 who were reported to CIBMTR, final samples of 8,115 recipients of allogeneic HCT and 11,652 recipients of autologous HCT contributed to this analysis.
Figure 1. Organization chart of patient eligibility and enrollment into the prospective observational study.
Among a total sample of 23,876 patients who received hematopoietic cell transplantation in United States between 12/2007 and 12/2009, 8,115 recipients of allogeneic and 11,652 recipients of autologous HCT contributed to the study analyses.
Study end points and definitions
The primary outcomes studied were non-relapse (NRM) and overall mortality. NRM was defined as post-transplantation death that was not preceded by disease progression or relapse. Progression was defined as >50% increase in the burden of primary disease compared to pre-transplant disease status and/or development of disease at new sites. Relapse was defined as reappearance of primary disease following achievement of post-HCT complete remission. For survival, patients were considered to have an event at time of death from any cause; survivors were censored at last contact. Conditioning regimens were classified into high-dose, reduced-intensity (RIC), or nonmyeloablative (NMA) intensity based on the previously published criteria.[23] Comorbidities were evaluated by respective staff at each site, while total scores were assigned by the CIBMTR statistical team following previously published guidelines.[3] The HCT CI score was derived directly from the presence of a comorbidity per the HCT-CI as collected in the TED forms. Additional comorbidities that were not part of the HCT-CI, but collected in free text fields under the “other” category, were not considered for the validation of this score. We analyzed a subset of these “other comorbidity” fields to assess discrepancies between what was documented in the free text field and the HCT CI components. We found that the content in this free text field could potentially change the overall HCT CI score in less than 5% of cases. Consequently, the “other comorbidity” field was not used to modify the score reported in the HCT-CI specific fields. To further rule out the contribution of these write-in entries, patients with a HCT CI score of 0 but with any “other comorbidity” reported in the free text field were analyzed as a separate risk group in the statistical models.
Statistical methods
Cumulative incidence and Kaplan Meier estimates were calculated to evaluate the unadjusted associations between the HCT-CI scores and NRM and survival, respectively. Relapse or progression of the primary disease was treated as a competing risk for NRM and vice versa. Because this study investigates the impact of the HCT-CI on outcomes following the first transplant, all outcomes were censored at the second transplant.
Proportional hazards models were used to estimate the hazard ratio (HR) for NRM and survival associated with HCT-CI scores among the whole patient population as well as among adults versus children, high-dose versus RIC/NMA regimens, and among patients with different diagnoses. The models were adjusted for patient-related risk factors: age, Karnofsky performance status (KPS) score, race, and cytomegalovirus (CMV) serology results; disease-related risk factors: diagnosis category, sensitivity to last chemotherapy among patients with lymphoma, and disease status among patients with acute leukemia, and interval between diagnosis and HCT; and transplant-related risk factors: donor type/HLA typing, stem cell source, conditioning regimen, and GVHD prophylaxis regimen. Multivariate p-values for a variable were based on adjustment for all other variables in the model. All p-values were derived from likelihood ratio statistics and were two-sided. In these multivariate analyses, the HCT-CI was primarily modeled as a categorical variable with group stratifications of 0, 1–2, and ≥3 similar to the initially recommended model to allow for almost uniform distribution of patient samples per risk group. A subset analysis using categorization of 0, 1, 2, 3, 4, and ≥5 was also performed with nested comparisons of both stratification models.
Three samples of 58, 80, and 77 patients were selected randomly from patients reported from Dana Farber Cancer Institute, Roswell Park Cancer Institute, and Fred Hutchinson Cancer Research Center to assess the magnitude of inter-rater reliability. These samples of patients were re-evaluated for comorbidity coding and score assignment by the study co-investigators V.T.H., P.L.M, and M.L.S, respectively. Score assignments were compared between two raters from each institution; one that collected original data reported to the CIBMTR and another that assigned scores independently.
Kappa statistic is a measure used to analyze inter-rater agreement,[24,25] and it adjusts for the degree of agreement that would be expected to occur by chance, and is therefore more appropriate than Pearson’s product moment, Spearman’s correlation, or percent agreement.[26] It is reported from 0.0 to 1.0. Weighted Kappa statistic (Kw),[27] which assigns less weight to agreement as risk categories are further apart, was computed with Fleiss-Cohen weights[28] to analyze the magnitude of inter-rater agreement between two raters on assignment of patients to the HCT-CI risk-categories of 0–1, 2, 3, and ≥4. Standard errors (S.E.) for kappa and Kw statistics were calculated as previously described.[29] Weighted Kw has been used to compare agreement between two raters.[27] The Landis scale was used for interpretation of the magnitude of Kw statistics where values <0 indicate no agreement; 0.0–0.20, slight; 0.21–0.40, fair; 0.40–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect agreement, respectively.[30]
Results
Patient Characteristics
Patient-, disease-, and transplant-related characteristics are described in Table 1 for recipients of high-dose allogeneic (n=5,460), RIC/NMA allogeneic (n=2,655), and autologous HCT (n=11,652). Median (range) ages were 45 (<1–74), 59 (1–78), and 56 (<1–83) years, respectively. Median (range) intervals between diagnoses and HCT were 7(<1–377), 13 (1–347), and 11 (<1–389) months, respectively. Cyclophosphamide (Cy) combined with high-dose total body irradiation (TBI, 33%) and busulfan (Bu) combined with Cy (26%) or fludarabine (20%) were the most frequently used regimens among recipients of high dose conditioning, while fludarabine (Flu) combined with Bu (31%), melphalan (20%), or low-dose TBI (23%) were the most frequent regimens among recipients of RIC/NMA conditioning. Methotrexate combined with tacrolimus was the most frequently used graft-versus-host disease (GVHD) prophylaxis regimen among recipients of both high-dose (53%) and RIC/NMA regimens (41%). Other frequent GVHD prophylaxis regimens included MTX/cyclosporine (CSP, 15%) among high dose regimens, and mycophenolate mofetil combined with CSP (16%) or tacrolimus (15%) among the RIC/NMA regimens. Among recipients of autologous HCT, the most common conditioning regimens were melphalan-based (50%) and carmustine, etoposide, cytarabine, and melphalan combination (BEAM, 25%).
Table 1.
Characteristics of US patients who received an allogeneic or autologous HCT for malignant diseases between 2007 and 2009, registered with the CIBMTR
| Characteristics of patients | High-dose n=5460 (%) |
RIC/NMA n=2655 (%) |
Autologous n=11,652 (%) |
|---|---|---|---|
| Age of patients | |||
| >0–19 | 894 (16) | 50 (2) | 811 (7) |
| 20–39 | 1315 (24) | 191 (8) | 1423 (13) |
| 40–49 | 1179 (22) | 327 (12) | 1695 (15) |
| 50–59 | 1438 (26) | 853 (32) | 3420 (29) |
| ≥60 | 634 (12) | 1224 (46) | 4303 (37) |
| Race of patients | |||
| Caucasian | 4233 (78) | 2274 (86) | 9022 (77) |
| African-American | 324 (6) | 120 (5) | 1301 (11) |
| Asian/Pacific Islander | 181 (3) | 76 (3) | 289 (2) |
| Hispanic | 636 (12) | 148 (6) | 920 (8) |
| Others | 86 (2) | 37 (1) | 120 (1) |
| Karnofsky score, % | |||
| < 90 | 1615 (30) | 937 (35) | 3752 (32) |
| ≥ 90 | 3566 (65) | 1578 (59) | 7201 (62) |
| Missing | 279 (5) | 140 (5) | 699 (6) |
| Disease | |||
| Acute myelogenous leukemia | 2391 (44) | 926 (35) | 268 (2) |
| Acute lymphoblastic leukemia | 1228 (22) | 134 (5) | 21 (<1) |
| Other leukemia | 148 (3) | 364 (14) | 8 (<1) |
| Chronic myelogenous leukemia | 298 (5) | 59 (2) | 0 |
| Myelodysplastic | 600 (11) | 344 (13) | 0 |
| Myeloprolifterative disorders | 161 (3) | 131 (5) | 0 |
| Lymphomas | 481 (9) | 652 (25) | 4763 (41) |
| Myelomas | 73 (1) | 21 (1) | 5717 (49) |
| Other Malignancies | 80 (1) | 24 (1) | 875 (8) |
| AML/ALL disease status at transplant | |||
| Never treated | 29 (<1) | 15 (1) | 0 |
| Primary Induction Failure | 406 (11) | 101 (10) | 292 (99) |
| Complete Remission | 2756 (76) | 857 (81) | 0 |
| Relapse | 426 (12) | 85 (8) | 3 (1) |
| Missing | 2 (<1) | 2 (<1) | 0 |
| Lymphoma disease status prior to HCT | |||
| Sensitive | 352 (73) | 513 (79) | 4335 (91) |
| Resistant | 120 (25) | 129 (20) | 377 (8) |
| Unknown/untreated | 9 (2) | 10 (2) | 51 (1) |
| Donor/recipient CMV status | |||
| −/− | 1548 (28) | 675 (25) | - |
| +/+ | 1637 (30) | 823 (31) | - |
| +/− | 596 (11) | 327 (12) | - |
| −/+ | 1568 (29) | 746 (28) | - |
| unknown | 111 (2) | 84 (3) | - |
| Graft type | |||
| Marrow | 1343 (25) | 271 (10) | 72 (<1) |
| G-PBMC | 4117 (75) | 2384 (90) | 11580 (99) |
| Donor type | |||
| HLA-identical sibling | 2266 (42) | 1012 (38) | - |
| Other related | 257 (5) | 225 (8) | - |
| Unrelated donor | 2882 (53) | 1399 (53) | - |
| Twins | 51 (1) | 19 (<1) | - |
| URD HLA match status | |||
| 8/8 | 1853 (64) | 976 (70) | - |
| 7/8 | 483 (17) | 198 (14) | - |
| 6/8 | 66 (2) | 15 (1) | - |
| 5/8 | 10 (<1) | 1 (<1) | - |
| Missing | 470 (16) | 209 (15) | - |
| HCT-CI score | |||
| 0 | 2825 (52) | 1088 (41) | 5851 (50) |
| 1 | 767 (14) | 436 (16) | 1714 (15) |
| 2 | 610 (11) | 320 (12) | 1375 (12) |
| 3 | 611 (11) | 345 (13) | 1306 (11) |
| 4 | 341 (6) | 217 (8) | 673 (6) |
| ≥5 | 296 (5) | 246 (9) | 667 (6) |
| Missing | 10 (<1) | 3 (<1) | 66 (<1) |
RIC indicates reduced-intensity conditioning; NMA, nonmyeloablative; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HCT, hematopoietic cell transplantation; CMV, cytomegalo-virus; G-PBMC, granulocyte colony stimulating factor-mobilized peripheral blood mononuclear cells; HLA, human leukocyte antigen; URD, unrelated donor; CI, comorbidity index.
Prevalence of Comorbidities and Distribution of Comorbidity Scores
Overall, comorbidities were common among recipients of RIC/NMA allogeneic HCT when compared with those of high-dose allogeneic and autologous HCT. Patients with scores 0 were found in 41% compared to 52% and 51%, respectively, while those with scores of 1–2 and ≥3 were found in 28% and 31% compared to 25% and 23%, and 27% and 22%, respectively. Overall, pulmonary comorbidities were the most prevalent among the three groups of patients (26% compared to 22% and 21%, respectively) followed by psychiatric (14% compared to 13% and 11%, respectively) and combined cardiac comorbidities (18% compared to 9% and 12%, respectively) that included arrhythmia, heart failure, low ejection fraction, and heart valve disease (Figure 2). Overall, 11% samples within each of the three groups were reported as having other comorbidities that did not acquire a score per the HCT-CI.
Figure 2. Prevalence of comorbidities as captured by the HCT-CI among recipients of high-dose allogeneic, reduced-intensity/nonmyeloablative allogeneic, and autologous HCT.
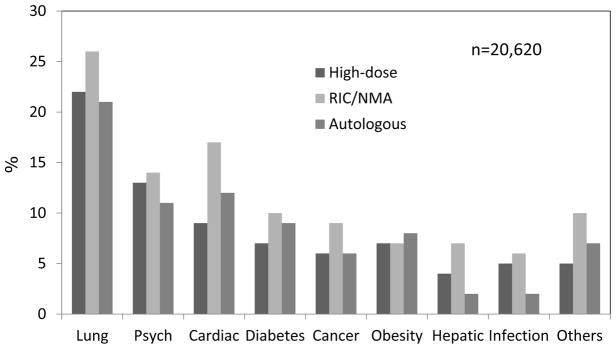
Pulmonary, psychiatric and heart comorbidities were the most prevalent among all three groups of patients
Validation of the HCT-CI among recipients of allogeneic HCT
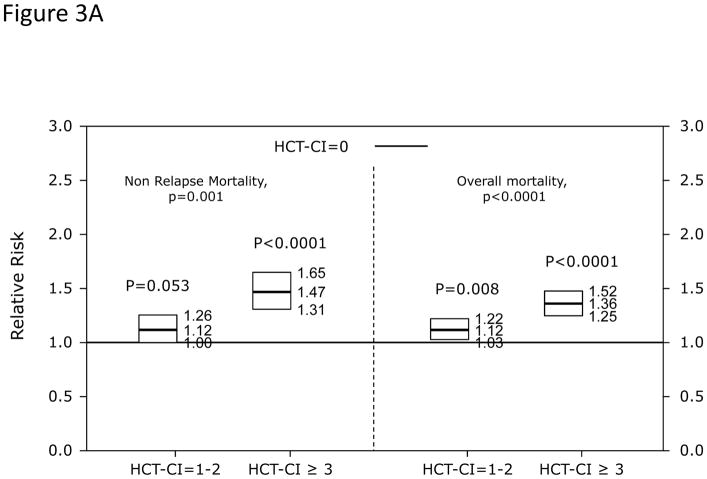
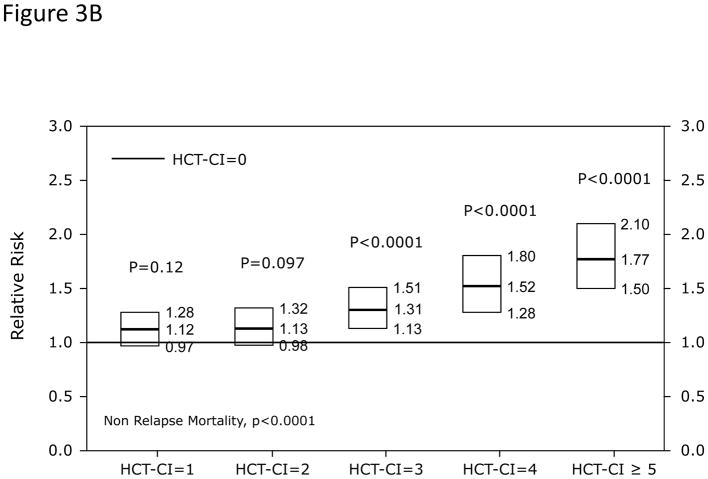
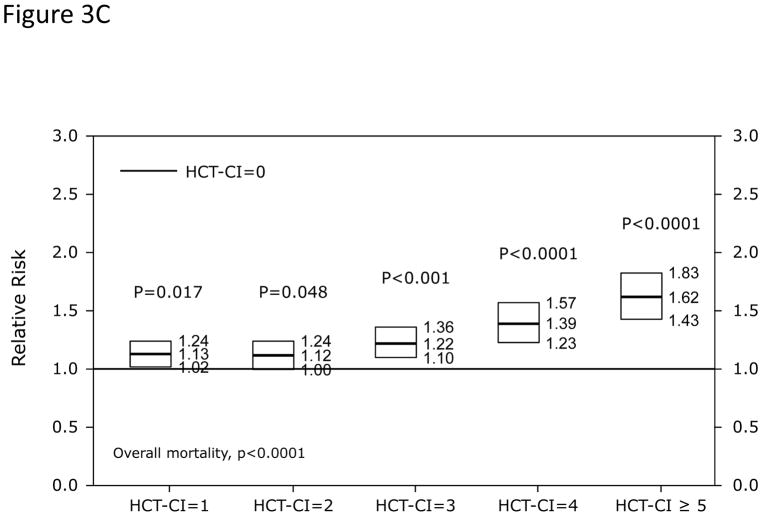
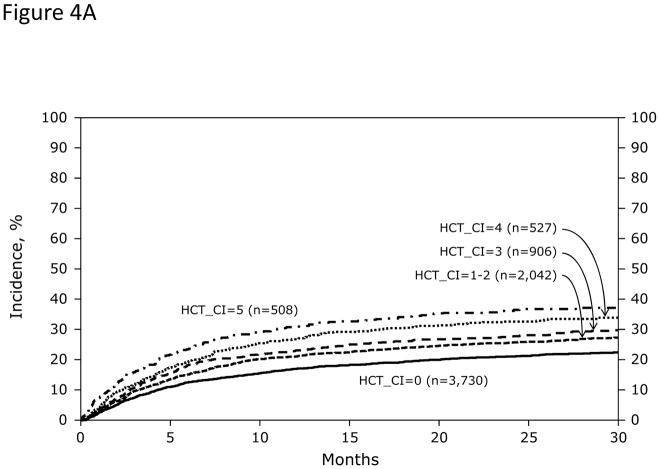
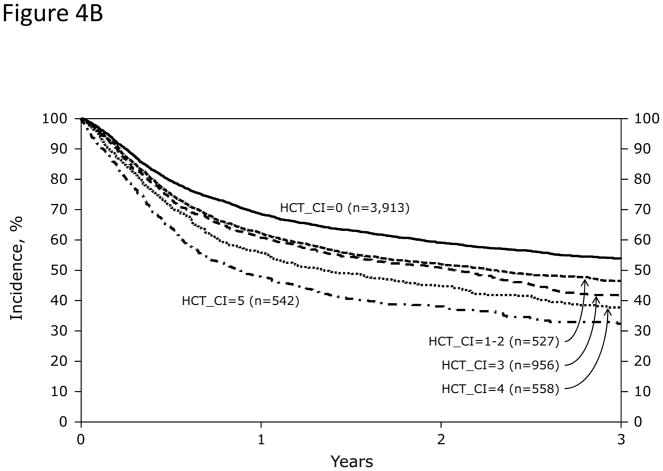
In Cox regression models adjusted for other co-variables, HCT-CI scores of 1–2 and ≥3 were statistically significantly associated with increased risks for NRM and overall mortality compared to score 0 (Table 2 and Figure 3A). Patients with scores of 0, 1–2, and ≥3 had 1-year probabilities of NRM of 17%, 21%, and 26% (p<0.001) and overall survival of 69%, 62%, and 56%, respectively, (p<0.001). The corresponding figures for 3-year NRM were 24%, 28%, and 35% (p<0.001) and for 3-year overall survival were 54%, 47%, and 38%, respectively (p<0.001). When the HCT-CI was categorized into 0, 1, 2, 3, 4, ≥5, each increased score was statistically significantly associated with higher risks for NRM and overall mortality with the exception of the associations between scores 1 and 2 with NRM that did not reach statistical significance (Table 2 and Figure 3B and C). Figure 4A and B demonstrated increasing probabilities of NRM and decreasing overall mortality, respectively, with each increment in HCT-CI scores. A nested comparison between the two categorization models suggested that the later had statistically significant better stratification power for both NRM (p=0.005) and overall mortality (p<0.001).
Table 2.
Cox regression models for associations of HCT-CI scores with risks of non-relapse (NRM) and overall mortality among recipients of allogeneic and autologous HCT. Three group stratification of HCT-CI scores predicted outcomes well among both cohorts of patients but the six group stratification model performed better for the former patients
| NRM | Overall mortality | ||||||
|---|---|---|---|---|---|---|---|
| HCT-CI scores | n | HR* (95% CI) | P | n | HR* (95% CI) | P | |
| Recipients of allogeneic HCT | * overall | <0.0001 | <0.0001 | ||||
| 0 | 2887 | 1.00 | 3026 | 1.00 | |||
| 0, but other comorbidity reported | 826 | 0.93 (0.79–1.10) | 0.385 | 866 | 0.96 (0.85–1.08) | 0.474 | |
| 1–2 | 2036 | 1.12 (1.00–1.26) | 0.053 | 2126 | 1.12 (1.03–1.22) | 0.008 | |
| 3+ | 1936 | 1.47 (1.31–1.65) | <0.0001 | 2051 | 1.36 (1.25–1.48) | <0.0001 | |
| * overall | <0.0001 | <0.0001 | |||||
| 0 | 2887 | 1.00 | 3026 | 1.00 | |||
| 0, but other comorbidity reported | 826 | 0.93 (0.79–1.10) | 0.396 | 866 | 0.96 (0.85–1.08) | 0.483 | |
| 1 | 1149 | 1.12 (0.97–1.28) | 0.122 | 1199 | 1.13 (1.02–1.24) | 0.017 | |
| 2 | 887 | 1.13 (0.98–1.32) | 0.097 | 927 | 1.12 (1.00–1.24) | 0.048 | |
| 3 | 903 | 1.31 (1.13–1.51) | <0.0001 | 953 | 1.22 (1.10–1.36) | <0.0001 | |
| 4 | 527 | 1.52 (1.28–1.80) | <0.0001 | 558 | 1.39 (1.23–1.57) | <0.0001 | |
| 5+ | 506 | 1.77 (1.50–2.10) | <0.0001 | 540 | 1.62 (1.43–1.83) | <0.0001 | |
| Recipients of autologous HCT | * overall | 0.001 | <0.0001 | ||||
| 0 | 4090 | 1.00 | 4621 | 1.00 | |||
| 0, but other comorbidity reported | 1086 | 0.93 (0.67–1.28) | 0.641 | 1229 | 0.85 (0.73–1.00) | 0.047 | |
| 1–2 | 2811 | 1.16 (0.93–1.44) | 0.200 | 3089 | 1.23 (1.11–1.37) | <0.0001 | |
| 3+ | 2385 | 1.49 (1.20–1.85) | 0.000 | 2645 | 1.37 (1.23–1.52) | <0.0001 | |
The Cox regression models were adjusted for diagnosis category, disease status for acute leukemia, chemo-sensitivity for lymphoma, donor type/HLA matching, stem cell source, KPS percentage, CMV serology status, conditioning regimen, GVHD prophylaxis regimen, recipient age, recipient race, and interval between diagnosis and HCT.
Figure 3. Illustration of the independent associations between HCT-CI score groups and risks of NRM and overall mortality among recipients of HCT using Cox regression models.
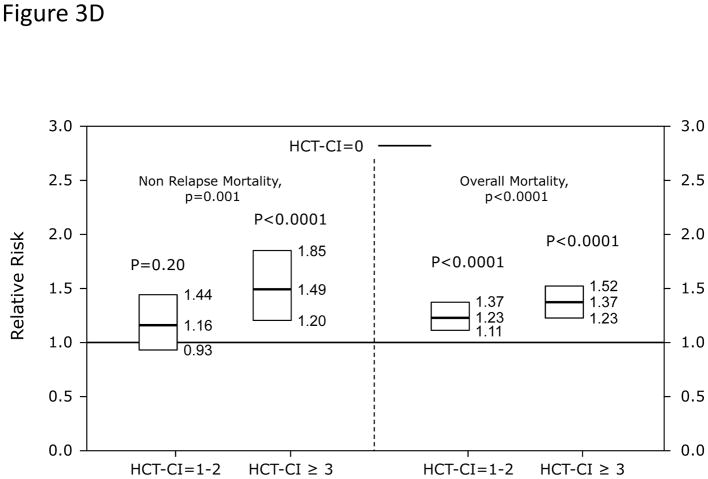
Among recipients of allogeneic HCT, (Figure 3A) HCT-CI scores of 1–2 and ≥3 were statistically significantly associated with increased risks for NRM and reduced overall mortality compared to score 0. In When the HCT-CI was categorized into 0, 1, 2, 3, 4, ≥5, for prediction of (Figure 3B) NRM and (Figure 3C) overall mortality among recipient of allogeneic HCT, each increased score was statistically significantly associated with higher risks for these outcomes with the exception of the associations between scores 1 and 2 with NRM that did not reach statistical significance. Among recipients of autologous HCT, HCT-CI score stratification of 1–2 and ≥3 was used for prediction of risks for NRM and overall mortality in (Figure 3D).
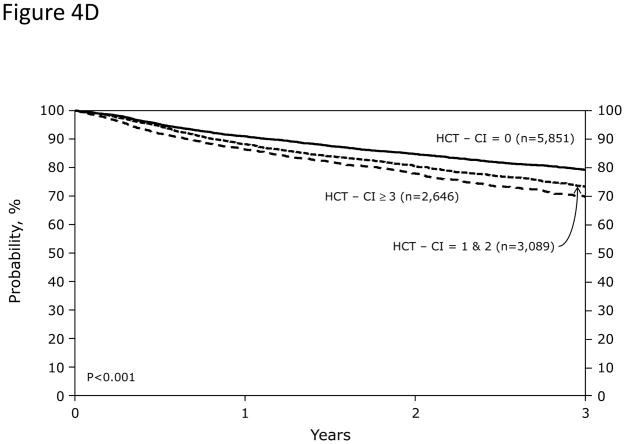
Figure 4. Stratification of probabilities of outcomes by the HCT-CI scores among recipients of allogeneic or autologous HCT.
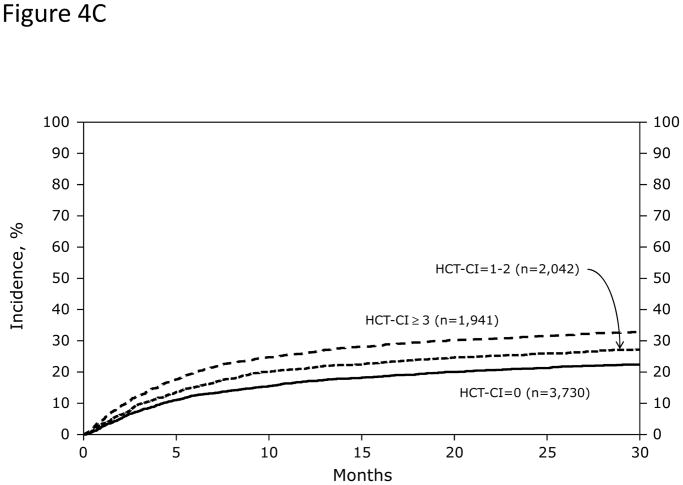
Among recipients of allogeneic HCT (Figures 4A and 4B), HCT-CI scores of 0, 1–2, 3, 4, and ≥5 stratified well probabilities of (Figure 4A) NRM and (Figure 4B) survival. Among recipients of autologous HCT, HCT-CI scores of 0, 1–2, ≥3 stratified well probabilities of (Figure 4C) NRM and (Figure 4D) survival.
Overall, patients who were assigned score 0 with versus without “other comorbidities that are not included within the HCT-CI” reported similar risks for NRM and overall mortality.
Performance of the HCT-CI among Subgroups of Recipients of Allogeneic HCT
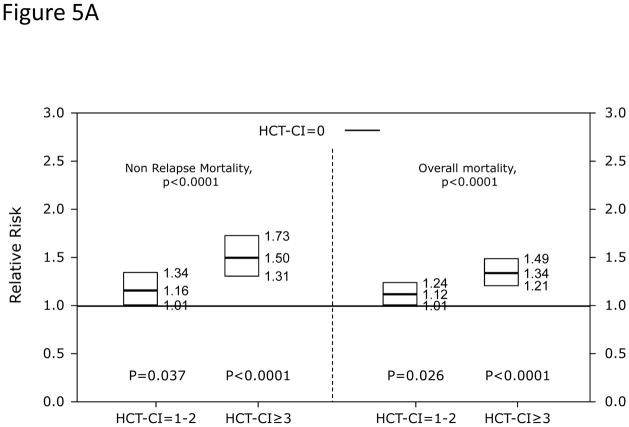
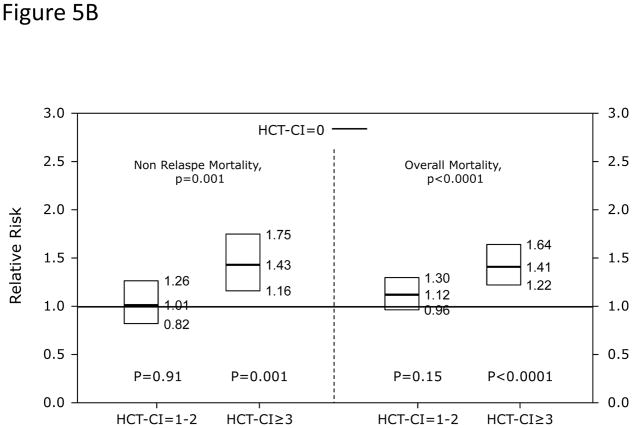
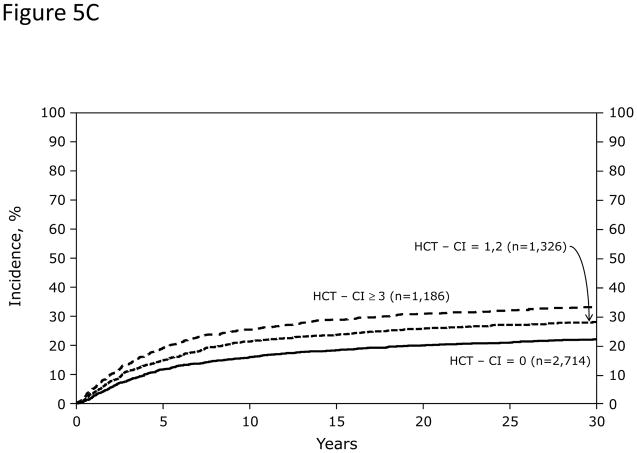
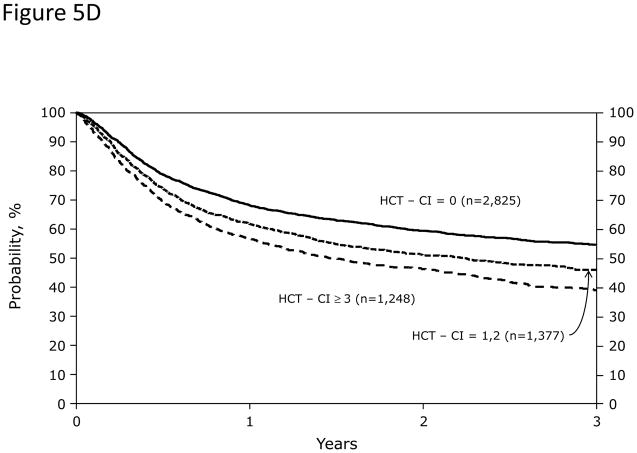
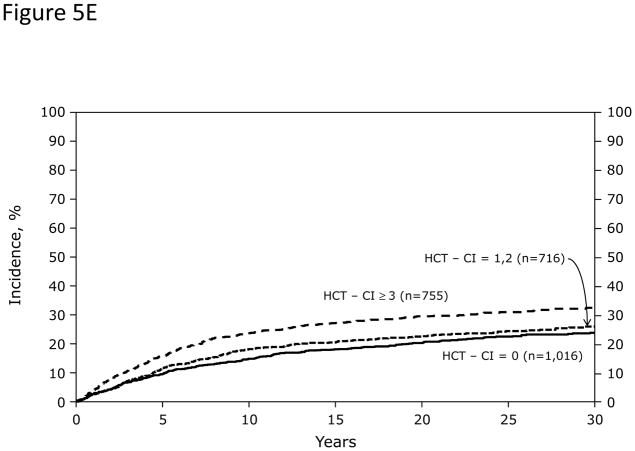
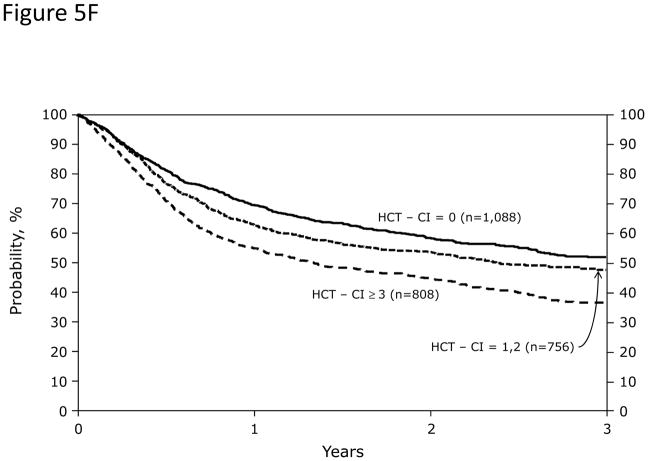
In Cox regression models adjusted for other co-variables, recipients of high-dose conditioning and allogeneic HCT with HCT-CI scores of 1–2 and ≥3 had statistically significantly higher risks for NRM and overall mortality compared to those with score 0 (Figure 5A). Likewise, patients with scores of 1–2 and ≥3 experienced respectively higher probabilities of NRM and overall survival when conditioned with high-dose regimens (Figures 5C and D). By contrast, patients with scores of 1–2 tolerated RIC/NMA regimens equally well compared to those with score 0, but those with scores of ≥3 experienced higher risks and probabilities for NRM and overall mortality compared to score 0 (Figures 5B, E, and F).
Figure 5. Subgroup validation of the predictive power of the HCT-CI among recipients of allogeneic HCT following high-dose versus reduced-intensity/nonmyeloablative conditioning regimens.
Figures 5A and 5B demonstrate results from Cox regression models focusing on NRM and overall mortality. HCT-CI scores of 1–2 and ≥3 were statistically significantly associated with increased risks for NRM and overall mortality among recipients of (Figure 5A) high-dose conditioning regimens, while only scores of ≥3 were associated with the same outcomes among recipients of (Figure 5B) reduced-intensity/nonmyeloablative conditioning regimens compared to those with score of 0.
Figures 5C, 5D, 5E, and 5F demonstrate probabilities of TRM and survival. HCT-CI scores of 1–2 and ≥3 stratified well probabilities of (Figure 5C) NRM and (Figure 5D) overall survival among recipients of high-dose conditioning.
Only patients with HCT-CI scores of ≥3 experienced (Figure 5E) increased probabilities of NRM and (Figure 5F) decreased probabilities of survival among recipients of reduced-intensity/nonmyeloablative conditioning regimens compared to those with score of 0.
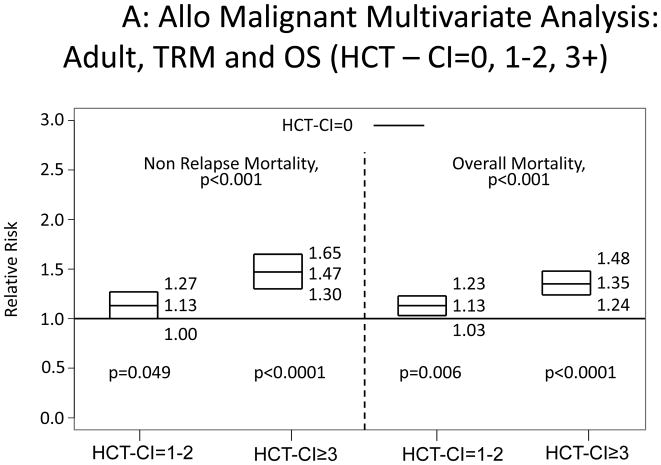
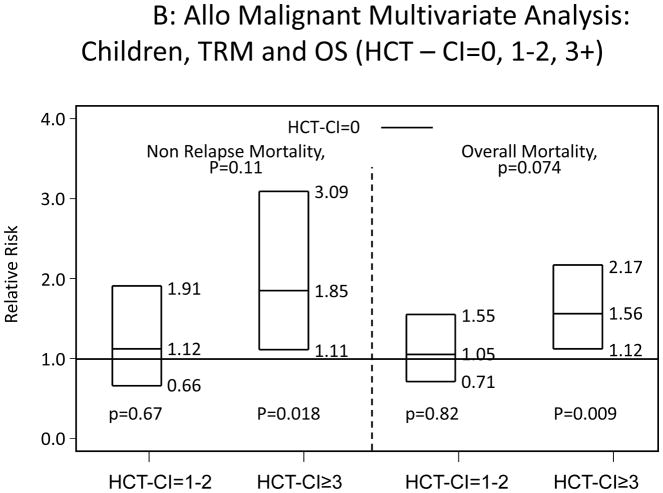
All recipients of allogeneic HCT were categorized into children and adults. Among adults, scores of 1–2 and ≥3 were associated with statistically significantly increased risks for NRM and overall mortality compared to score 0 (Appendix 2A). Children with HCT-CI scores of ≥3 were associated with statistically significant higher risks for NRM and overall mortality compared to score 0, while the higher hazard ratios (HRs) associated with scores of 1–2 did not reach statistical significance (Appendix 2B).
Appendix 2.
Illustration of the independent associations between HCT-CI scores of 0, 1–2, and ≥3 and risks of NRM and overall mortality among recipients of allogeneic HCT, who were A) adults or B) children using Cox regression models.
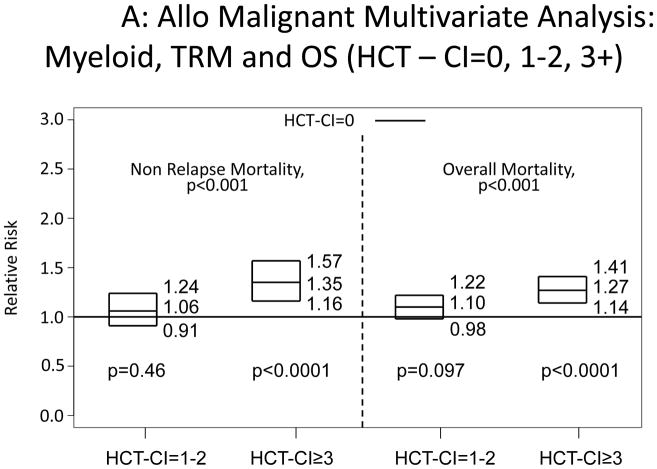
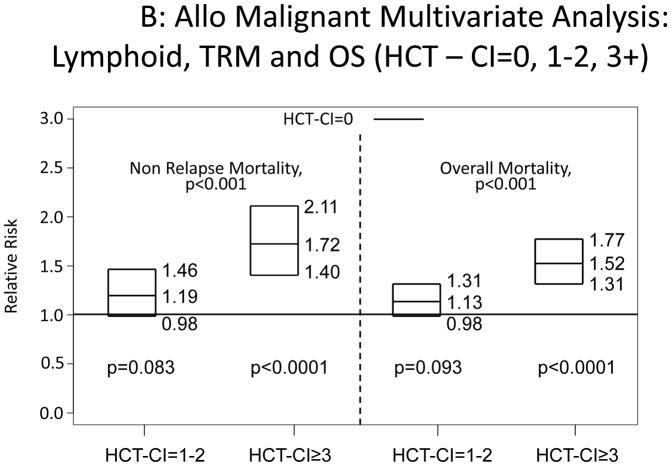
Increasing HCT-CI scores were also associated with increased risks for NRM and overall mortality among patients diagnosed with myeloid or lymphoid malignancies (Appendix 3).
Appendix 3.
Illustration of the independent associations between HCT-CI scores of 0, 1–2, and ≥3 and risks of NRM and overall mortality among recipients of allogeneic HCT, who were diagnosed with A) myeloid or B) lymphoid malignancies using Cox regression models.
Validation of the HCT-CI among recipients of autologous HCT
Recipients of autologous HCT with scores of 1–2 had statistically non-significant higher HRs for NRM but statistically significant higher HRs for overall mortality compared to those with score 0 (Table 2). Patients with scores of ≥3 experienced statistically significantly higher HRs for both outcomes compared to those with score of 0. Nested comparison between this stratification system and a more detailed one (0, 1, 2, 3, 4, and ≥5) did not show additional benefit in regards to prediction of NRM (p=0.297) or overall mortality (p=0.433).
Probabilities of NRM at 1-year were 3%, 3%, and 5% (p<0.001) for those with scores of 0, 1–2, and ≥3; while the figures for 3-year probabilities were 5%, 6%, and 9%, respectively (p<0.001). One-year and 3-year rates of survival were 91%, 88%, and 86% (p<0.001), respectively and 79%, 73%, and 70% (p<0.001), respectively (Figures 4C and D).
Performance of the HCT-CI among recipients of autologous HCT Diagnosed with Lymphoma versus Multiple Myeloma
Similar to the general population of recipients of autologous HCT, the associations between scores 1–2 among those with lymphoma or multiple myeloma only reached statistical significance for overall mortality but not for NRM (Appendix 1). Alternatively, scores of ≥3 were uniformly associated with higher HRs for both outcomes in both groups of patients. Day 100 NRM for the 3 HCT-CI score groups were 1%, 2%, and 3% (p<0.001), respectively among patients with multiple myeloma and 3%, 4%, and 6% (p<0.001), respectively, among those with lymphoma. At 3-years, NRM probabilities were 4%, 6%, and 7% (p=0.007), respectively, for myeloma patients, while survival rates were 84%, 76%, and 74%, respectively (p<0.001). Among lymphoma patients, the 3-year NRM probabilities were 5%, 7%, and 10% (p<0.001), respectively, while survival rates were 77%, 72%, and 67% (p<0.001), respectively.
Appendix 1.
Cox regression models for associations of HCT-CI scores with risks of non-relapse (NRM) and overall mortality among recipients of autologous HCT and within subgroups of lymphoma versus multiple myeloma.
| NRM | Overall mortality | ||||||
|---|---|---|---|---|---|---|---|
| HCT-CI scores | n | HR (95% CI) | P | n | HR (95% CI) | P | |
| Lymphoma subgroup | * overall | 0.043 | 0.001 | ||||
| 0 | 1243 | 1.00 | 1264 | 1.00 | |||
| 0, but other comorbidity reported | 311 | 0.96 (0.57–1.61) | 0.863 | 318 | 0.99 (0.77–1.28) | 0.959 | |
| 1–2 | 977 | 1.24 (0.88–1.73) | 0.218 | 988 | 1.23 (1.05–1.46) | 0.013 | |
| 3+ | 832 | 1.56 (1.12–2.17) | 0.009 | 838 | 1.37 (1.15–1.62) | <0.0001 | |
| Myeloma subgroup | * overall | 0.048 | <0.0001 | ||||
| 0 | 1657 | 1.00 | 1949 | 1.00 | |||
| 0, but other comorbidity reported | 586 | 0.95 (0.59–1.55) | 0.844 | 682 | 0.76 (0.59–0.98) | 0.032 | |
| 1–2 | 1382 | 1.32 (0.93–1.87) | 0.119 | 1620 | 1.39 (1.18–1.64) | <0.0001 | |
| 3+ | 1168 | 1.55 (1.09–2.20) | 0.015 | 1386 | 1.43 (1.21–1.69) | <0.0001 | |
The Cox regression models were adjusted for diagnosis category, chemo-sensitivity for lymphoma, stem cell source, KPS percentage, conditioning regimen, recipient age, recipient race, and interval between diagnosis and HCT.
Reliability of the HCT-CI
Weighted kappa statistic estimates were 0.54 in DFCI, 0.81 in RPCI, and 0.47 in FHCRC data samples, suggesting a fair-moderate agreement rate among evaluators.
Discussion
This prospective, multi-center study has generated several key findings about the performance of the HCT-CI as a prognostic comorbidity model for HCT outcomes. First, The HCT-CI stood the test of time as it was shown here to predict outcomes among a group of patients treated about almost a decade after those who originally contributed to its design. Second, it was valid in predicting both NRM and survival among recipients of both allogeneic and autologous HCT. The ability of the HCT-CI to predict NRM after autologous HCT is an important finding, especially considering that the idnex was originally designed based on data from a cohort of allogeneic HCT recipients, and considering the substantial differences between both transplant strategies. Other comorbidity indices have also been shown to be useful in settings beyond those from which they were developed.[2,31,32] Third, we found that patients with score 0 plus additional comorbidities coded under “other”, in aggregate, did not influence outcomes compared to patients with scores of 0 alone. These results confirm the original design of the index that dropped most of these comorbidities from consideration due to lack of statistical association. Fourth, the index in this prospective contemporary patient cohort demonstrated sensitive stratification of outcomes that varied based on criteria of age or conditioning intensity. Specifically, patients undergoing RIC/NMA allogeneic HCT or children tolerated allogeneic HCT equally well when they had scores of 1–2 versus 0. This confirms the benefit of the style that was used to build the HCT-CI, where its association with increased risks of NRM was meant to be a range of values that would vary based on other variables such as conditioning intensity, age, or disease-risk. Finally, the index performed well among subgroups of diagnoses, age categories, and conditioning intensities. The findings of this large study, in conglomerate, have affirmed the adaptability and integrity of the HCT-CI in the real world HCT setting.
Since its development, the HCT-CI has been tested in a number of retrospective analyses with conflicting results. Many of these retrospective analyses suffered from limited sample size, lack of complete comorbidity data, and apparent inaccurate coding of comorbidities. [19,20,33–35] There has been an unmet need for prospective evaluation of the comorbidity impact in a well-designed and appropriately powered study. A recent prospective study from Italy confirmed validity of the index.[36] Our study is the first prospective study to evaluate the performance of the HCT-CI among a large number of patients treated at transplant centers across the US. The proven value of the HCT-CI in the current study should encourage investigators and community physicians to incorporate comorbidity assessment in their daily practice.
The prospective nature of this study, together with the inclusion of large groups of patients from various transplant centers, would ensure generalizability of the study findings. In 3 randomly selected samples, we have found that the rate of agreement between evaluators ranged from fair to moderate. Variable IRR is a common problem among comorbidity indices[37–39] and it was recently underscored for the HCT-CI promoting the production of comprehensive guidelines for comorbidity coding.[40] The guidelines were summarized in a web-based application (www.hctci.org ), and has been validated to improve the IRR among novice evaluators to an excellent magnitude.[40] It is interesting that the fair-moderate degree of IRR in the current study did not obscure the validity of the index in predicting outcomes. Still, it would be important to use the new guidelines consistently in order to standardize comorbidity coding across institutions, which is critical when using the index to adjust comparisons of center performances or to test new associations with outcomes. Nested comparison analyses showed that each digit increase in the score of the HCT-CI between 0–≥5 was associated with statistically significant increases in risks for NRM and survival among overall recipients of allogeneic HCT. Nevertheless, the stratification of 0, 1–2, ≥3 is preferable for patient counseling and assessment of outcomes at relatively smaller studies given the ease of use and given the limitations in sample size outside of registry studies.
The prospective validation of the HCT-CI as achieved in this study should promote consistent use of this index in the stratification of patients in future randomized HCT studies. The index could also be incorporated as a variable to adjust comparisons of outcomes and performances across transplant centers. This strategy is already being used by the Center of International Blood and Marrow Transplantation Research (CIBMTR) in the determination of the center specific outcomes comparisons, and should prove valuable to patients, insurers, and investigators. Finally, the HCT-CI would be helpful to clinicians in counseling potential HCT recipients about their risks of NRM, and choosing the appropriate conditioning regimen.
Patients with score 1–2 has similar NRM risks to those with score 0 when patients with children or when they received either autologous HCT or allogeneic HCT after reduced-intensity/nonmyeloablative regimens. These results validate findings from previous studies about the performance of the HCT-CI in these specific cohorts of patients.[41,42] These results also highlight the sensitivity of the index in differentiation between adults versus children and recipients of high-intensity versus lower intensity regimens in regards to tolerability of HCT. This differentiation is important in the clinic to help transplant physicians to decide between the variable options of transplant strategies. Patients with HCT-CI scores of ≥3 consistently have higher risks for NRM and overall mortality, compared to those with scores of 0, regardless of transplant strategy, conditioning intensity, diagnoses, or age groups.
In the future, we could achieve finer discrimination of outcomes by combining the HCT-CI with other relevant metrics. This could be specifically important for vulnerable patients such as those of age 60 years or older. For example, one could potentially further stratify risks for mortality by combining the HCT-CI scores with biomarkers,[6,33,43] performance status,[44] and/or some components of a geriatric assessment (GA) for older patients.[45] The recent Bone Marrow Transplant-Clinical Trial Network (BMT-CTN)-State of the Science Symposium (SOSS) has suggested a prospective study to develop a novel risk assessment tool comprising of the HCT-CI, performance status model, biomarkers and GA to improve risk-assessment prior to HCT.[46] In summary, our study confirms that the HCT-CI is a powerful tool for predicting NRM and survival after HCT. Co-morbidity assessments should be applied in future research and clinical care of HCT recipients. This is of particular importance considering the increasing age and vulnerability of the population eligible for HCT in the United States.
Here are the Highlights for this manuscript as requested.
High HCT CI is linked to mortality after allogeneic and autologous transplants
HCT CI as a prognostic tool is validated for all transplants for malignancies
Only HCT CI ≥3 were prognostic for mortality in children and in RIC HCT
Acknowledgments
We would like to acknowledge Dr. Theresa Hahn for her contributions to portions of the analysis.
M.L.S. is supported by grant HL088021 from the National Institutes of Health, #RSG-13-084-01-CPHPS Research Scholar Grant from the American Cancer Society, and a Patient-Centered Outcome Research Institute contract, #CE-1304-7451.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from * Actinium Pharmaceuticals; Allos Therapeutics, Inc.; * Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; * Blue Cross and Blue Shield Association; * Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; * Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;* Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; * Milliman USA, Inc.; * Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; * Remedy Informatics; * Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; * Tarix Pharmaceuticals; * TerumoBCT; * Teva Neuroscience, Inc.; * THERAKOS, Inc.; University of Minnesota; University of Utah; and * Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
Financial Disclosure Statement: The authors do not have any relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chron Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 2.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplant comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlu J, Kew AK, Taylor-Roberts B, et al. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010;115:4018–4020. doi: 10.1182/blood-2010-01-263624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–350. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 9.Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica. 2009;94:729–732. doi: 10.3324/haematol.2008.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breccia M, Frustaci AM, Cannella L, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukaemia patients. Hematol Oncol. 2009;27:148–153. doi: 10.1002/hon.889. [DOI] [PubMed] [Google Scholar]
- 11.Sperr WR, Wimazal F, Kundi M, et al. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann Oncol. 2010;21:114–119. doi: 10.1093/annonc/mdp258. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama D, Fukuda T, Kato R, et al. Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant. 2007;13:932–941. doi: 10.1016/j.bbmt.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status-based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 14.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23:1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 16.Barba P, Piñana JL, Martino R, et al. Comparison of two pretransplant predictive models and a flexible HCT-CI using different cut points to determine low-, intermediate-, and high-risk groups: the flexible HCT-CI is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biol Blood Marrow Transplant. 2010;16:413–420. doi: 10.1016/j.bbmt.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Lim ZY, Ingram W, Brand R, et al. Impact of pretransplant comorbidities on alemtuzumab-based reduced-intensity conditioning allogeneic hematopoietic SCT for patients with high-risk myelodysplastic syndrome and AML. Bone Marrow Transplant. 2010;45:633–639. doi: 10.1038/bmt.2009.236. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka K, Nannya Y, Ueda K, Kumano K, Takahashi T, Kurokawa M. Differential prognostic impact of pretransplant comorbidity on transplant outcomes by disease status and time from transplant: a single Japanese transplant centre study. Bone Marrow Transplant. 2010;45:513–520. doi: 10.1038/bmt.2009.194. [DOI] [PubMed] [Google Scholar]
- 19.Guilfoyle R, Demers A, Bredeson C, et al. Performance status, but not the hematopoietic cell transplantation comorbidity index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplant. 2009;43:133–139. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- 20.Birninger N, Bornhäuser M, Schaich M, Ehninger G, Schetelig J. The hematopoietic cell transplantation-specific comorbidity index fails to predict outcomes in high-risk AML patients undergoing allogeneic transplantation--investigation of potential limitations of the index. Biol Blood Marrow Transplant. 2011;17:1822–1832. doi: 10.1016/j.bbmt.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Sorror M, Storer B, Gopal A, et al. Comorbidity, lactate dehydrogenase (LDH), and chemosensitivity are independent predictors of mortality after autologous hematopoietic cell transplantation (HCT) for patients (pts) with lymphoma. Blood. 2007;110 (Part 1):190a. [abstr.] [Google Scholar]
- 22.Labonté L, Iqbal T, Zaidi MA, et al. Utility of comorbidity assessment in predicting transplantation-related toxicity following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14:1039–1044. doi: 10.1016/j.bbmt.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 25.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 26.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 27.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educational and Psychological Measurement. 1973;33:613–619. [Google Scholar]
- 29.Fleiss JL, Cohen J, Everitt BS. Large sample standard errors of kappa and weighted kappa. Psychol Bull. 1969;72:323–327. [Google Scholar]
- 30.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 31.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared to myeloablative conditioning before hematopoietic cell transplantation from HLA matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 32.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terwey TH, Hemmati PG, Martus P, et al. A modifed EBMT risk score and the hematopoietic cell transplantation-specific comorbidity index for pre-transplant risk assessment in adult acute lymphoblastic leukemia. Haematologica. 2010;95:810–818. doi: 10.3324/haematol.2009.011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xhaard A, Porcher R, Chien JW, et al. Impact of comorbidity indexes on non-relapse mortality. Leukemia. 2008;22:2062–2069. doi: 10.1038/leu.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raimondi R, Tosetto A, Oneto R, et al. Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood. 2012;120:1327–1333. doi: 10.1182/blood-2012-03-414573. [DOI] [PubMed] [Google Scholar]
- 37.Imamura K, McKinnon M, Middleton R, Black N. Reliability of a comorbidity measure: the Index of Co-Existent Disease (ICED) J Clin Epidemiol. 1997;50:1011–1016. doi: 10.1016/s0895-4356(97)00128-5. [DOI] [PubMed] [Google Scholar]
- 38.Liu M, Domen K, Chino N. Comorbidity measures for stroke outcome research: a preliminary study. Archives of Physical Medicine & Rehabilitation. 1997;78:166–172. doi: 10.1016/s0003-9993(97)90259-8. [DOI] [PubMed] [Google Scholar]
- 39.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 40.Sorror M. How I assess comorbidities prior to hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsawy M, Storer BE, Pulsipher MA, et al. Multi-center validation of the prognostic value of the hematopoieitc cell transplantation-specific comorbidity index among recipients of allogeneic hematopoietic cell transplantation. Br J Haematol. 9999 doi: 10.1111/bjh.13476. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughn JE, Storer BE, Chauncey T, et al. Comorbidity, history of alcohol disorders, and LDH predict non-relapse mortality (NRM) among recipients of autologous hematopoietic cell transplantation (HCT) for lymphoma. Biol Blood Marrow Transplant. 2014;20:S66. [abstr.] [Google Scholar]
- 43.Sorror ML, Storer BE, Schoch G, et al. Low albumin, high ferritin, and thrombocytopenia before transplant predict non-relapse mortality (NRM) independent of the hematopoeitic cell transplantation comorbidity index (HCT-CI) Blood. 2009;114:271. [abstr.] [Google Scholar]
- 44.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 45.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appelbaum FR, Anasetti C, Antin JH, et al. Blood and marrow transplant clinical trials network state of the science symposium 2014. Biol Blood Marrow Transplant. 2015;21:202–224. doi: 10.1016/j.bbmt.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]