Abstract
Purpose/Introduction
Clinical data have documented a clear increase in fracture risk associated with chronic kidney disease (CKD). Preclinical studies have shown reductions in bone mechanical properties although the tissue-level mechanisms for these differences remain unclear. The goal of this study was to assess collagen cross-links and matrix hydration, two variables known to affect mechanical properties, in animals with either high or low turnover CKD.
Methods
At 35 weeks of age (>75% reduction in kidney function), the femoral diaphysis of male Cy/+ rats with high or low bone turnover rates, along with normal littermate (NL) controls, were assessed for collagen cross-links (pyridinoline (PYD), deoxypyridinoline (DPD), and pentosidine (PE)) using a high performance liquid chromatography (HPLC) assay as well as pore and bound water per volume (pw and bw) using a 1H nuclear magnetic resonance (NMR) technique. Material-level biomechanical properties were calculated based on previously published whole bone mechanical tests.
Results
Cortical bone from animals with high turnover disease had lower Pyd and Dpd crosslink levels (−21% each), lower bw (−10%), higher PE (+71%), and higher pw (+46%), compared to NL. Animals with low turnover had higher Dpd, PE (+71%), and bw (+7%) along with lower pw (−60%) compared to NL. Both high and low turnover animals had reduced material-level bone toughness compared to NL animals as determined by three-point bending.
Conclusions
These data document an increase in skeletal PE with advanced CKD that is independent of bone turnover rate and inversely related to decline in kidney function. Although hydration changes occur in both high and low turnover disease, the data suggest that non-enzymatic collagen crosslinks may be a key factor in compromised mechanical properties of CKD.
Keywords: advanced glycation end products, pentosidine, bound water, bone material properties, toughness
INTRODUCTION
There are clear and consistent data showing that chronic kidney disease (CKD) is associated with an increased fracture risk compared to individuals without CKD [1-5]. More concerning is that individuals with CKD who experience a fracture are at greater risk of mortality compared to people without CKD who fracture [6, 7]. There are numerous challenges to reducing fracture risk in CKD patients because of the complexity in the underlying bone disease. CKD is associated with significant metabolic derangements, including secondary hyperparathyroidism, yet some patients have low levels of parathyroid (PTH) for unclear reasons [1, 8, 9]. At the skeletal level, CKD appears to have a preferential impact on cortical bone, leading to increased porosity, and likely playing a major role in fracture [10-14]. Preclinical studies have documented CKD-associated reductions in biomechanical properties of whole bones, as well as at the micro- and nano-scale levels of the hierarchical organization of bone [14-18]. Properties of the tissue, such as mineral content, have not shown clear differences between normal and diseased bone leaving a cloud of uncertainty as to what properties of the tissue are responsible for this compromised mechanical phenotype associated with CKD [16].
Increased attention to ‘bone quality’ (those aspects other than BMD that account for a bone’s resistance to fracture) has led to a greater understanding of various skeletal properties [19-21]. Collagen plays a vital role in bone with changes in collagen cross-links showing clear influence on bone mechanical properties [22-26]. CKD-induced collagen changes have been noted in serum and several soft-tissues [27] but data in bone are limited [28, 29]. A less often discussed bone quality variable, bone hydration, also affect bone mechanical properties [30-32], but only recently has it become clear that in vivo modulation of hydration can play a role in a bone’s fracture resistance capacity [33-35].
While there is an increasing awareness of the importance of bone quality in CKD [20, 21], few studies have explicitly examined material properties in animal models [14-16, 18, 29]. The goal of the present study was to examine two specific properties, collagen crosslinks and matrix hydration (bound and pore water) in a rat model with progressive development of CKD that can be manipulated to have either high or low turnover disease [14, 36]. We hypothesized that CKD adversely affects both collagen cross-links and matrix hydration, that these parameters would be most affected in high turnover disease, and there would be relationships between these properties and bone mechanical outcomes.
MATERIALS AND METHODS
Animal Model
The current study utilized a slowly progressive animal model of CKD, the Cy/+ rat, which has been described in detail several times [15, 16, 18, 36-38]. Tissues analyzed in the current study were from select groups of animals that were part of a larger study [14]. Specifically, the animals were in one of three groups: Cy/+ animals that were untreated and thus had high turnover bone disease, Cy/+ given calcium supplementation in their drinking water to suppress PTH starting at 25 weeks of age (low turnover disease), or normal littermate controls (n=8-10/gp). All animals were euthanized at 35 weeks of age and femora (used to study both outcome measures) were saved wrapped in saline soaked gauze at −20C. Prior to the assays reported in this paper, femora were tested in 3 point bending [14]. Blood was also collected at the end of the experiment for biochemical analyses. Dynamic histomorphometry (detailing turnover rates), 3 point bending, and biochemical assays have all been previously reported [14]. All procedures were conducted under the approval of Indiana University School of Medicine Institutional Animal Care and Use Committee protocol # 10479.
Collagen Cross-Linking
Segments of the femoral diaphysis (~3 mm in length) were processed for collagen cross-links as previously described [16]. Briefly, following demineralized in 20% EDTA (0.68 M, pH 7.4), demineralized bone was hydrolyzed. Each hydrolysate was resuspended in ultrapure water, split into two equal portions, and dried. Half the residue was resuspended in ultrapure water with an internal standard (5 × 10−6 g/L pyridoxine) and assayed by a high performance liquid chromatography (HPLC) system (Beckman-Coulter System Gold 168). Standards with varying concentrations of pyridinoline (Pyd) (Quidel), deoxypyridinoline (Dpd) (Quidel), pentosidine (PE) (International Maillard Reaction Society), and a constant amount of pyridoxine were also assayed. Using a Waters 2475 fluorescence detector (excitation/emission of 295/400 nm for Pyd and Dpd and 328/378 nm for PE), chromatograms were recorded to determine the amount of each crosslink. These amounts were then normalized by collagen content (hydroxyproline), which was determined from the other half of each hydrolysate by another HPLC assay. The calculated mass of hydroxyproline was then multiplied by 7.5 (assuming 13-14% of type I collagen by mass) and divided by the molecular weight of collagen (30,000 Da), thereby giving crosslink concentration as mol/mol of collagen. For each chromatogram, the area of the peak is calculated and divided by an internal standard. These values are then plotted onto a standard curve that plots the known masses of the standards to their given areas, providing an estimate of the mass of the given molecule in the unknowns (hydroxyproline, pyridinoline, deoxypyridinoline, and pentosidine).
1H Nuclear magnetic resonance spectroscopy (NMR)
A ~5 mm cross-section of the femur mid-shaft was placed into a low proton, loop-gap-style radio-frequency (RF) coil [39] along with a reference microsphere of water (T2 ~ 2 s). The NMR analysis to separate proton signals within the bone was then performed in a 4.7T horizontal-bore magnet (Varian Medical Systems, Santa Clara, CA) using 90°/180° RF pulses of ~ 9/18 μs duration and collecting Carr-Purcell-Meiboom-Gill (CPMG) measurements with 10,000 echoes at 100 μs spacing. To generate the spectrum of transverse relaxation time constants (T2), the echo magnitudes were fitted with multiple exponential decay functions [40]. Upon normalizing the integrated areas of bound water (T2 = 100 μs-800 μs) and pore water (T2 = 800 μs – 600 ms) to the area of the reference (T2 = 600 ms-10 s) [41], the volume of bound water and the volume of pore water were divided by the specimen volume (calculated from Archimedes’ principle) to give bound water (bw) and pore water (pw) volume fractions.
Material-level biomechanical properties
Whole bone material properties, determined using 3 point bending of the femora, have been previously reported [14]. Material-level properties were estimated using standard beam theory equations [30]. Bone diameters were measured at the mid-diaphysis using digital calipers while cross-sectional moment of inertia was measured using micro computed tomography.
Statistical Analysis
All analyses were performed using SAS software. Comparisons among the groups were made using student’s t-tests as the focus of this work was to independently assess high/lower turnover conditions versus control. Pearson product moment correlation tests were used to assess the relationship between variables across all treatments with the exception of hydration for which Spearman correlations were used, as the data were not normally distributed. A priori α-levels were set at 0.05 to determine significance.
RESULTS
High-turnover CKD model
Details about the phenotype of these animals with secondary hyperparathyroidism have been previous published [14]. Briefly, measures of kidney function and PTH were both significantly higher compared to normal (+200 and 2800%, respectively), indicative of active disease. Trabecular bone turnover, measured by dynamic histomorphometry, was more than 4-fold higher in compared normal; cortical porosity was more than doubled. Structural biomechanical properties, ultimate load and stiffness, were both significantly lower than controls with no difference in energy to fracture, a biomechanical property that is not independent of bone structure [14].
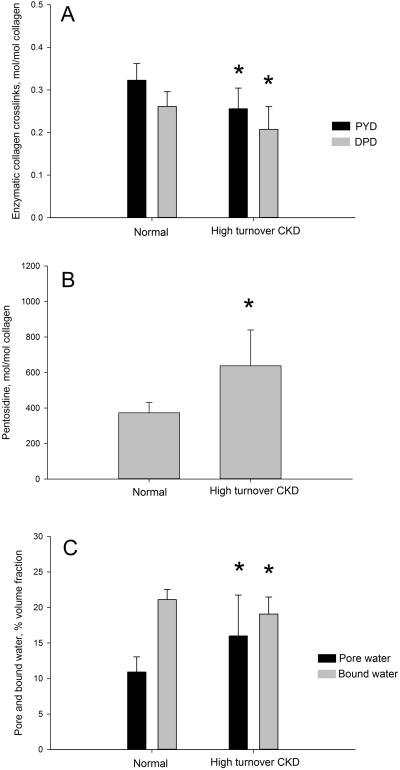
All measured forms of collagen cross-links were significantly different compared to normal. The two mature enzymatic cross-links, Pyd and Dpd were both significantly lower (−21%; p = 0.004 and 0.02) compared to control (Figure 1A). There was no significant difference in the Pyd/Dpd ratio. PE, an advanced glycation product, was 71% (p = 0.001) higher compared to normal (Figure 1B). Pore water volume fraction within the femoral cortex was higher (+46%: p = 0.024) while matrix bound water volume fraction was significantly lower (−10%: p = 0.04) compared to normal animals (Figure 1C).
Figure 1.
High-turnover CKD associated changes in skeletal collagen crosslinking and tissue hydration. (A) Enzymatic collagen cross-links (pyridinoline, PYD and deoxypyridinoline, DPD) were significantly lower in CKD compared to normal littermates. (B) Pentosidine, an advanced glycation end product, was found in significantly higher concentrations in CKD bone compared to normal. (C) Pore water fraction was significantly higher and bound water fraction significantly lower in CKD animals compared to normal. * p < 0.05 compared to normal animals.
Ultimate stress was significantly lower than normal animals (−22%; p=0.001) with no difference in modulus and a trend toward lower toughness (−21%; p = 0.11) (Table 1).
Table 1.
Femoral diaphysis morphology and calculated material-level biomechanical properties
| Normal | High turnover CKD |
Low turnover CKD |
|
|---|---|---|---|
| Bone diameter, mm | 3.7 ± 0.2 | 3.6 ± 0.2 | 3.7 ± 0.1 |
| Porosity, % | 0.1 ± 0.1 | 4.1 ± 5.4 * | 0.5 ± 0.2 * |
| Cross-sectional moment of inertia, mm4 |
10.1 ± 1.5 | 8.56 ± 0.9 * | 11.1 ± 1.4 |
| Ultimate stress, MPa | 180 ± 17 | 140 ± 25 * | 176 ± 27 |
| Modulus, MPa | 6777 ± 831 | 6609 ± 750 | 6701 ± 880 |
| Toughness, MJ/m3 | 5.3 ± 1.1 | 4.2 ± 1.8 | 3.9 ± 0.7 * |
Values are presented as mean ± standard deviation.
p < 0.05 versus normal controls.
Low-turnover CKD model
Details about the phenotype of these animals, where PTH is suppressed with the administration of calcium in the drinking water, have been previous published [14]. Briefly, measures of kidney function were significantly higher (+150%), while PTH was significantly lower (−75%) compared to normal. Trabecular bone turnover, cortical porosity, and structural properties were not different from controls.
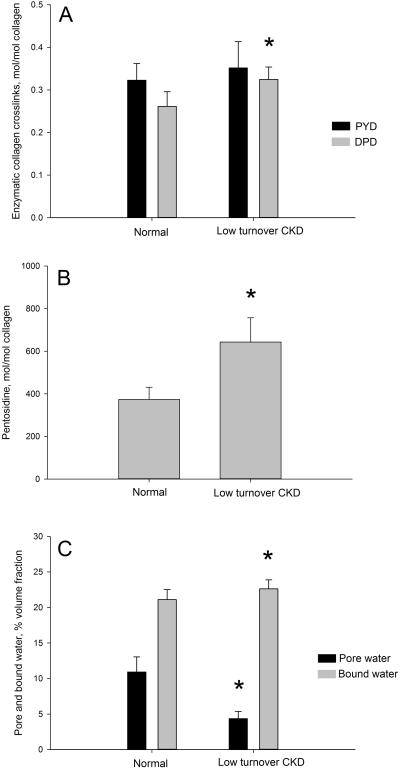
Levels of Pyd in the bone matrix were not different from controls, while Dpd levels were significantly higher (+24%; p = 0.002) compared to normal (Figure 2A). There was no significant difference in the Pyd/Dpd ratio. PE was 72% higher (p = 0.001 compared to normal (Figure 2B). The NMR-derived pore water within the femoral cortex was lower (−60%: p = 0.001), and bound water was significantly higher (7%: p = 0.04) compared to normal animals (Figure 2C).
Figure 2.
Low-turnover CKD associated changes in skeletal collagen crosslinking and tissue hydration. (A) The enzymatic collagen cross-link (deoxypyridinoline, DPD) was significantly higher than normal in low-turnover disease; pyridinoline, PYD was unaffected. (B) Pentosidine, an advanced glycation end product, was found in significantly higher concentrations in CKD bone compared to normal. (C) Pore water fraction was significantly lower and bound water fraction significantly higher in CKD animals compared to normal. * p < 0.05 compared to normal animals.
Toughness was significantly lower than normal animals (−27%; p = 0.005) (Table 1). Ultimate stress and modulus were similar to normal animals.
Correlations
To examine relationships between outcome measures, correlations were assessed across the entire data set (controls, low turnover CKD and high turnover CKD). Kidney function (assessed by BUN) was significantly correlated to PE (R = 0.68) (Figure 3A). PTH was significantly correlated to several outcomes including pore water (R= 0.81), bound water (R = −0.70), pyd (−0.58), Dpd (R = −0.72), and PE (R = 0.46). PE levels were negatively correlated to ultimate stress and toughness (R = −0.44 and −0.57, respectively) (Figure 3B).
Figure 3.
Correlations between skeletal pentosidine levels and kidney function (A) and mechanical properties (B). As both high and low turnover CKD models were shown to have higher pentosidine levels compared to normal we explored the relationship between kidney function and pentosidine. This relationship was statistically significant (r = 0.68, p < 0.001). Given the known role of pentosidine in modulation of mechanical properties we also explored the relationship between pentosidine and bone toughness (B). This relationship was statistically significant (r = −0.57, p = 0.002).
DISCUSSION
It is becoming clear that the increased risk of skeletal fracture associated with chronic kidney disease is multi-factorial and occurs due to changes in both bone quantity and quality [12, 20, 21, 42]. We have previously documented in an animal of progressive kidney disease, that compromised biomechanical properties can be quantified at the whole bone level as well as at the micro- and nano-scales [16]. These data illustrate that loss of bone mass, as has been repeatedly documented in both clinical and preclinical studies, is just one piece of the CKD fracture risk puzzle. Given the nano-scale changes in bone mechanical properties, our laboratory has focused on measuring properties of the tissue matrix that contribute to this level of mechanical integrity [16]. The results of the current work, in a preclinical animal model of progressive CKD, point to modification of both collagen crosslinking and bone hydration in late stage CKD. The changes in crosslinks confirm previous preclinical work in a different CKD model of high turnover bone [29], while the hydration data results represent novel data and a potential variable that could be used for non-invasive clinical assessment of bone quality [33, 43].
Collagen crosslinks in bone are either enzymatically-mediated (via lysyl oxidase) or non-enzymatically mediated (in the form of advanced glycation end products (AGEs) such as pendosidine) [22]. The predominant mature enzymatic crosslinks – pyridinoline and deoxypyridinoline (also known as hydroxylyslpyridinoline and lyslpyridinoline, respectively) – have been shown to be associated with changes in bone mechanical properties [22]. Previous work by our lab in the same animal model as the current study showed no difference in Pyd or Dpd in CKD animals at 30 weeks [16]. We have documented that this animal model has rapid disease progression, in high-turnover states, between 30 and 35 weeks of age [14, 15], leading us to hypothesize that major changes in collagen cross-links might manifest with progression to advanced disease. Although this turned out to be the case, the story is more complicated as the enzymatic crosslink changes were dependent of rate turnover; high turnover groups had reduced enzymatic cross-links while low turnover animals had higher than normal levels of Dpd.
The simplistic relationship between bone turnover and collagen crosslinks is an inverse one. High bone turnover leads to lower levels of mature enzymatic crosslinks because the mean tissue age is lower; while low turnover bone typically has high levels. Yet this relationship model assumes that the process of collagen crosslinks formation/maturation is not disrupted. Levels of lysyl oxidase, the key enzyme involved in enzymatic crosslinking, are tissue-specific. Kidney levels of lysyl oxidase have been shown to be higher in CKD and this is associated with increased fibrosis in renal tissue [44]. Serum measures of pyridinoline have been shown to be increased in patients with end stage renal disease, and these levels were higher in patients with high turnover compared to those with low turnover [45]. Yet these serum measures do not provide insight into skeletal levels, as the latter are determined both the rate of formation/breakdown (i.e., bone turnover) and the rate of crosslink maturation (i.e., divalent to trivalent bonded). Skeletal levels of mature crosslinks have been found to be lower in patients with high-turnover end stage renal disease [27], while animal models of low turnover have shown reductions in bone lysyl oxidase levels [28]. Reduction in lysyl oxidase would reduce enzymatic cross-links – although neither the clinical nor preclinical study measured both enzyme and crosslinks. Unfortunately, levels of lysyl oxidase were not assessed in the current work and thus definitive mechanisms underlying changes in mature cross-links cannot be ascertained.
Clinical studies have demonstrated that non-enzymatic cross-links (pentosidine, specifically) are increased in the circulation and soft tissue of patients with CKD [46]. Increased oxidative stress in CKD leads to increased production of AGE, and AGEs worsen oxidative stress creating a vicious cycle that is independent of blood glucose levels [47]. Skeletal levels of AGEs have been shown to be elevated in a small clinical study in patients on dialysis [27]. Although we have recently shown that at 30 weeks of age there is no difference in skeletal AGEs in our progressive CKD model, the current results show that at 35 weeks there is a striking difference (>70% higher than normal). This suggests that, like the enzymatic cross-links, major changes occur between 30 and 35 weeks in this animal model. More interesting is that the levels of AGEs in the bone are independent of bone remodeling rate – both high and low turnover models had similarly higher AGE levels compared to controls (Figures 1B and 2B). AGEs are typically linked to mean tissue age – since they accumulate in tissues over time when mean tissue age is higher AGEs are higher. It therefore follows that, in normal situations, bone with higher turnover rates will have lower AGEs compared to bone with lower turnover rates. This does not seem to hold true in the setting of CKD suggesting there is some intrinsic aspect of the disease that results in more AGE accumulation in bone independent of tissue age, perhaps due to ongoing AGE production in CKD [47] and reduced clearance by the kidney [48]. We hypothesize that elevations in oxidative stress may be underlying the accumulation of AGEs in this model. Thus, the severity of kidney disease may be more important than the level of bone turnover for determination of skeletal AGEs. The importance of the severity of kidney function is supported by a modest positive correlation between serum BUN and skeletal pentosidine (R = 0.679) in the current study (Figure 3A).
The influence of hydration on bone mechanical properties has long been appreciated as it pertains to ex vivo mechanical testing [30, 31]. More recently data have emerged showing modulation of hydration can occur in vivo [34], suggesting that it could play a role in disease-related bone mechanical phenotypes. The results of the current study show that in high turnover disease, pore water is significantly higher, while in low turnover disease pore water is lower, than normal animals. Changes in pore water track closely to changes in cortical porosity, which is high in high turnover animals and low in low turnover animals at 35 weeks of age [14]. The changes in bound water, which have been shown to have predominant mechanical effect on bone [34], were unexpected and the mechanisms through which bound water is modulated in vivo remain unclear. Bound water tends to be inversely related to mineralization and at least at 30 weeks of age mineralization characteristics (determined by Raman spectroscopy) are not different from controls [16]. It is possible that mineral has changed by week 35 in these animals yet unfortunately tissue is not available for such analyses. Also, as intracortical porosity increases, there is more pore water but less matrix interactions with water molecules. This is confirmed in the current work by the positive correlation between pore water and porosity (R = 0.69).
Both AGE levels and bone hydration have been documented as having significant effects on bone mechanical properties. Increasing AGE levels either in vitro, or in vivo, are inversely related to bone toughness – the ability of the bone tissue to absorb energy before fracture [23, 49]. Reductions in bone toughness reflect a bone that fractures more easily and in a more brittle fashion [50]. Conversely to AGEs, modulating bone hydration (specifically bound water) has been shown to have a positive relationship to bone toughness [34, 51]. Estimation of material-level mechanical properties in the current study shows that both high and low turnover disease lower bone toughness relative to normal animals, with only the lower turnover animals reaching statistical significance (Table 1). Interestingly, these two conditions had contrasting effects on pore and bound water, as well as enzymatically-mediated crosslinks. The only common feature of the two conditions was a significant increase in levels of AGEs (pentosidine). Skeletal pentosidine was negatively correlated to bone toughness (r = −0.57; Figure 3B). While we cannot definitively conclude that the higher AGE levels are fully responsible for the biomechanical phenotype, the results of this work suggest it is an outcome that should be a primary focus in future studies.
In conclusion, using an animal model of progressive chronic kidney disease, we have shown that both collagen crosslinking and skeletal hydration are affected in late-stage renal disease. Although both of these variables are known to play roles in bone mechanical properties, the data suggest that accumulation on non-enzymatic collagen crosslinks may be a key change that is responsible for altering mechanical properties associated with late-stage disease progression.
SUMMARY.
Chronic kidney disease (CKD) increases fracture risk. The results of this work point to changes in bone collagen and bone hydration as playing a role in bone fragility associated with CKD.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants AR58005 (SM), DL100093 (CN), AR063157 (JSN), and the Indiana Clinical Translational Science Institute grant TR000162 (CN). The cross-link analysis is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System. All authors were involved in the design, conduct and analyses of the study. The authors would like to thank Drew Brown, Shannon Roy, and Kali O’Neill for technical assistance. We would also like to acknowledge the late Dr. Vincent H. Gattone II (1951-2013), who was instrumental in developing this animal model.
Footnotes
CONFLICT OF INTEREST
Matthew R. Allen, Christopher L. Newman, Neal Chen, Mathilde Granke, Jeffry S. Nyman, and Sharon M. Moe declare they have no conflicts of interest related to this work.
REFERENCES
- 1.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 3.Ensrud KE, Lui L-Y, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167:133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Halter JB, Hazzard WR, et al. Prediction, Progression, and Outcomes of Chronic Kidney Disease in Older Adults. Journal of the American Society of Nephrology. 2009;20:1199–1209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 5.Jamal SA, West SL, Miller PD. Fracture risk assessment in patients with chronic kidney disease. Osteoporos Int. 2011;23:1191–1198. doi: 10.1007/s00198-011-1781-0. [DOI] [PubMed] [Google Scholar]
- 6.Nitsch D, Mylne A, Roderick PJ, et al. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant. 2009;24:1539–1544. doi: 10.1093/ndt/gfn678. [DOI] [PubMed] [Google Scholar]
- 7.Fusaro M, Gallieni M, Jamal SA. Fractures in chronic kidney disease: neglected, common, and associated with sickness and death. 2013;85:20–22. doi: 10.1038/ki.2013.302. [DOI] [PubMed] [Google Scholar]
- 8.Negrea LA. Biochemical Abnormalities in Chronic Kidney Disease–Mineral Bone Disease. Clinic Rev Bone Miner Metab. 2012;10:149–162. [Google Scholar]
- 9.Kiattisunthorn K, Moe SM. Chronic Kidney Disease-Mineral Bone Disorder: Definitions and Rationale for a Systemic Disorder. Clinic Rev Bone Miner Metab. 2011;10:119–127. [Google Scholar]
- 10.Ott SM. Bone histomorphometry in renal osteodystrophy. Semin Nephrol. 2009;29:122–132. doi: 10.1016/j.semnephrol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Terpstra AM, Kalkwarf HJ, Shults J, et al. Bone Density and Cortical Structure after Pediatric Renal Transplantation. Journal of the American Society of Nephrology. 2012;23:715–726. doi: 10.1681/ASN.2011050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28:1811–1820. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MA, Chin J, Miller SC, Fox J. Disparate effects of mild, moderate, and severe secondary hyperparathyroidism on cancellous and cortical bone in rats with chronic renal insufficiency. Bone. 1998;23:257–266. doi: 10.1016/s8756-3282(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 14.Moe SM, Chen NX, Newman CL, et al. A Comparison of Calcium to Zoledronic Acid for Improvement of Cortical Bone in an Animal Model of CKD. J Bone Miner Res. 2014;29:902–910. doi: 10.1002/jbmr.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen MR, Chen NX, Gattone VH, et al. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporos Int. 2012;24:1471–1481. doi: 10.1007/s00198-012-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman CL, Moe SM, Chen NX, et al. Cortical Bone Mechanical Properties Are Altered in an Animal Model of Progressive Chronic Kidney Disease. PLoS ONE. 2014;9:e99262. doi: 10.1371/journal.pone.0099262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwamoto J, Seki A, Sato Y, Matsumoto H. Vitamin K2 Improves Renal Function and Increases Femoral Bone Strength in Rats with Renal Insufficiency. Calcif Tissue Int. 2011;90:50–59. doi: 10.1007/s00223-011-9548-3. [DOI] [PubMed] [Google Scholar]
- 18.Moe SM, Radcliffe JS, White KE, et al. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011;26:2672–2681. doi: 10.1002/jbmr.485. [DOI] [PubMed] [Google Scholar]
- 19.Seeman E. Bone quality--the material and structural basis of bone strength and fragility. New England Journal of Medicine. 2006;354:2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 20.Malluche HH, Porter DS, Monier-Faugere MC, et al. Differences in Bone Quality in Low- and High-Turnover Renal Osteodystrophy. Journal of the American Society of Nephrology. 2012;23:525–532. doi: 10.1681/ASN.2010121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9:671–680. doi: 10.1038/nrneph.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2005;17:319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 23.Vashishth D, Gibson GJ, Khoury JI, et al. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19:1021–1026. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 25.Currey John D. Role of collagen and other organics in the mechanical properties of bone. Osteoporos Int. 2003;14(Suppl 5):S29–36. doi: 10.1007/s00198-003-1470-8. [DOI] [PubMed] [Google Scholar]
- 26.Oxlund H, Barckman M, Ørtoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:S365–S371. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 27.Mitome J, Yamamoto H, Saito M, et al. Nonenzymatic Cross-Linking Pentosidine Increase in Bone Collagen and Are Associated with Disorders of Bone Mineralization in Dialysis Patients. Calcif Tissue Int. 2011;88:521–529. doi: 10.1007/s00223-011-9488-y. [DOI] [PubMed] [Google Scholar]
- 28.Aoki C, Uto K, Honda K, et al. Advanced glycation end products suppress lysyl oxidase and induce bone collagen degradation in a rat model of renal osteodystrophy. 2013;93:1170–1183. doi: 10.1038/labinvest.2013.105. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone. 2011;48:1260–1267. doi: 10.1016/j.bone.2011.03.672. [DOI] [PubMed] [Google Scholar]
- 30.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 31.Dempster WT, Liddicoat RT. Compact bone as a non-isotropic material. Am J Anat. 1952;91:331–362. doi: 10.1002/aja.1000910302. [DOI] [PubMed] [Google Scholar]
- 32.Nyman JS, Gorochow LE, Adam Horch R, et al. Partial removal of pore and loosely bound water by low-energy drying decreases cortical bone toughness in young and old donors. Journal of the Mechanical Behavior of Biomedical Materials. 2013;22:136–145. doi: 10.1016/j.jmbbm.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical Bone Water: In Vivo Quantification with Ultrashort Echo-Time MR Imaging. Radiology. 2008;248:824–833. doi: 10.1148/radiol.2482071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallant MA, Brown DM, Hammond M, et al. Bone cell-independent benefits of raloxifene on the skeleton: A novel mechanism for improving bone material properties. Bone. 2014;61:191–200. doi: 10.1016/j.bone.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anumula S, Wehrli SL, Magland J, et al. Ultra-short echo-time MRI detects changes in bone mineralization and water content in OVX rat bone in response to alendronate treatment. Bone. 2010;46:1391–1399. doi: 10.1016/j.bone.2010.01.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75:176–184. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowley BD, Grantham JJ, Muessel MJ, et al. Modification of disease progression in rats with inherited polycystic kidney disease. YAJKD. 1996;27:865–879. doi: 10.1016/s0272-6386(96)90525-9. [DOI] [PubMed] [Google Scholar]
- 38.Cowley BD, Gudapaty S, Kraybill AL, et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43:522–534. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 39.Horch RA, Wilkens K, Gochberg DF, Does MD. RF coil considerations for short-T2 MRI. Magnetic Resonance Medicine. 2010;64:1652–1657. doi: 10.1002/mrm.22558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horch RA, Nyman JS, Gochberg DF, et al. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magnetic Resonance Medicine. 2010;64:680–687. doi: 10.1002/mrm.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horch RA, Gochberg DF, Nyman JS, et al. Non-invasive predictors of human cortical bone mechanical properties: T(2)-discriminated H NMR compared with high resolution X-ray. PLos ONE. 2011;6:e16359. doi: 10.1371/journal.pone.0016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonard MB. A Structural Approach to Skeletal Fragility in Chronic Kidney Disease. Semin Nephrol. 2009;29:133–143. doi: 10.1016/j.semnephrol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manhard MK, Horch RA, Harkins KD, et al. Validation of quantitative bound- and pore-water imaging in cortical bone. Magn Reson Med. 2014;71:2166–2171. doi: 10.1002/mrm.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Donato A, Ghiggeri GM, Di Duca M, et al. Lysyl Oxidase Expression and Collagen Cross-Linking during Chronic Adriamycin Nephropathy. Nephron. 1997;76:192–200. doi: 10.1159/000190168. [DOI] [PubMed] [Google Scholar]
- 45.Ureña P, Ferreira A, Kung VT, et al. Serum pyridinoline as a specific marker of collagen breakdown and bone metabolism in hemodialysis patients. J Bone Miner Res. 1995;10:932–939. doi: 10.1002/jbmr.5650100614. [DOI] [PubMed] [Google Scholar]
- 46.Suliman ME, Heimbürger O, Bárány P, et al. Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J Am Soc Nephrol. 2003;14:1614–1622. doi: 10.1097/01.asn.0000067413.32377.cf. [DOI] [PubMed] [Google Scholar]
- 47.Mallipattu SK, Uribarri J. Advanced glycation end product accumulation. Current Opinion in Nephrology and Hypertension. 2014;6:547–554. doi: 10.1097/MNH.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyata T, Ueda Y, Horie K, et al. Renal catabolism of advanced glycation end products: the fate of pentosidine. Kidney Int. 1998;53:416–422. doi: 10.1046/j.1523-1755.1998.00756.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang SY, Allen MR, Phipps R, et al. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burr D. Why bones bend but don’t break. 2011;11:270–285. [PubMed] [Google Scholar]
- 51.Bae WC, Chen PC, Chung CB, et al. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: Correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27:848–857. doi: 10.1002/jbmr.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]