Abstract
PURPOSE
To estimate quality-of-life (QOL)-adjusted cost-utility with addition of bevacizumab (B) to intravenous paclitaxel/carboplatin (PC) for primary treatment of advanced-stage epithelial ovarian cancer.
METHODS
A modified Markov state transition model of 3 regimens evaluated in GOG 218 (PC, PC + concurrent B [PCB], and PCB + maintenance B [PCB+B]) was populated by prospectively collected survival, adverse event, and QOL data from GOG 218. Progression-free survival (PFS) and overall survival (OS) were modeled using primary event data. Costs of grade 4 hypertension, grade 3-5 bowel events, and growth factor support were incorporated. QOL scores were converted to utilities and incorporated into the model. Monte Carlo probabilistic sensitivity analysis was performed to account for uncertainty in estimates.
RESULTS
PC was the least expensive ($4,044) and least effective (mean 1.1 quality-adjusted progression-free years [QA-PFY]) regimen. PCB ($43,703 and 1.13 QA-PFY) was dominated by a combination of PC and PCB+B. PCB+B ($122,700 and 1.25 QA-PFY) was the most expensive regimen with incremental cost-effectiveness ratio of $792,380/QA-PFY compared to PC. In a model not incorporating QOL, the incremental cost-effectiveness ratio (ICER) of PCB+B was $632,571/PFY compared to PC.
CONCLUSIONS
In this cost-utility model, incorporation of QOL into an analysis of GOG 218 led to less favorable ICER (by >$150,000/QA-PFY) in regimens containing B compared with those that do not include B. Continued investigation of populations with ovarian cancer in whom the efficacy of treatment with bevacizumab is expected to be increased (or in whom QOL is expected to increase with use) is critical.
INTRODUCTION
Ovarian cancer is the most lethal gynecologic cancer, with 14,270 women expected to die from the disease in 2014 [1]. With the current standard of care, patients undergo surgical cytoreduction followed by platinum-based cytotoxic chemotherapy. Despite this aggressive treatment approach, most women diagnosed with advanced ovarian cancer will have a recurrence and die of their disease. Recent advancements in the treatment of this disease have included the incorporation of intraperitoneal chemotherapy into the front-line therapy of optimally resected disease. Despite improved survival with this strategy, increased rates of complications compared with intravenous therapy preclude its use in certain patients [2]. Continued investigations of alternative treatment strategies are necessary to maximize survival while minimizing toxicity. In 2011, the Gynecologic Oncology Group (GOG) published the results of a randomized phase III clinical trial investigating the use of bevacizumab with the standard intravenous agents carboplatin and paclitaxel, GOG 218 [3]. The results demonstrated an additional 3.8 months of median progression-free survival (PFS) when maintenance bevacizumab was added following carboplatin, paclitaxel and bevacizumab (14.1 months median PFS), as compared with the control arm without any bevacizumab (10.3 months median PFS). In the quality of life (QOL) analysis of this trial published in 2013, bevacizumab was found to compromise QOL during chemotherapy, as measured by the Trial Outcome Index (TOI) of the Functional Assessment of Cancer Therapy-Ovary (FACT-O) [FACT-O TOI] (before cycle four, approximately three point decrease in QOL with bevacizumab compared to the control arm), but had no impact after chemotherapy was completed [4]. While generally well tolerated, bevacizumab has been associated with an increased risk of serious adverse events such as intestinal perforation, hemorrhage, proteinuria, thrombosis, central nervous system disorders, increased bone marrow suppression, and delayed wound healing. Furthermore, the use of bevacizumab is associated with increased cost, both directly related to the cost of the drug as well as the costs associated with its toxicity. Recently, groups have been interested in the costs associated with novel therapeutics, leading to studies and reviews of the pharmacoeconomics of drugs such as bevacizumab [5]. A previously reported cost-effectiveness model (based on the GOG 218 data presented in abstract form) initiated the discussion of the cost-effectiveness of bevacizumab in treating patients with newly diagnosed ovarian cancer. The authors challenged patients, providers, regulators, and the pharmaceutical industry to begin to think about the responsibility of controlling the escalating cost of healthcare in the United States [6]. However this prior cost-effectiveness study did not take into account quality of life in different treatment arms. Standard methods for cost-effectiveness include incorporation of quality of life, when available [7]. The incorporation of health related QOL into a cost-effectiveness analysis results in a cost-utility analysis. This methodology considers lower QOL to constitute a decrement in overall effectiveness, and may lead to a substantially different interpretation of cost-effectiveness than without consideration of QOL.
Following the recently released results of the QOL data from GOG 218, we performed a cost utility analysis of the use of bevacizumab as prescribed in GOG 218, and aimed to determine the impact of bevacizumab on the potential costs associated with the treatment of advanced ovarian cancer.
METHODS
Model structure
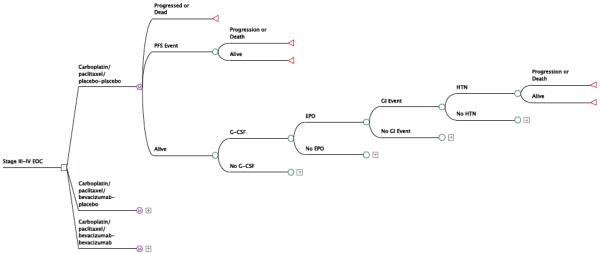
We constructed a cost-utility model [8]] using a modified Markov state transition structure (Figure 1) to compare the three regimens evaluated in GOG 218: (1) paclitaxel/carboplatin (PC), (2) PC + concurrent bevacizumab (PCB), and (3) PCB+ maintenance bevacizumab (PCB+B). A third party payer perspective was employed. The model’s time horizon was 60 months; one Markov cycle was set equivalent to three months. Discounting of costs and effectiveness was performed at 3% annually. The model was populated by prospectively collected survival, adverse event, and QOL data (Table 1) as reported in GOG 218 [3,4], including standard deviations and classification of distributions.
Figure 1.
Decision tree structure for the cost utility model of GOG 218.
Table 1.
Clinical Estimates
| Parameter | PC | PCB | PCB+B | Distribution Type |
|---|---|---|---|---|
| Median PFS (months) | 10.3 | 11.2 | 14.1 | beta |
| Median OS (months) | 39.3 | 38.7 | 39.7 | beta |
| Erythropoietin use, % of all cycles including placebo |
6.5 | 4.5 | 4.2 | beta |
| Granulocyte colony-stimulating factor use, % of all cycles |
5.6 | 4.9 | 5.5 | beta |
| Grade 3-5 intestinal events, %* | 1.4 | 3.2 | 3.3 | beta |
| Grade 4 hypertension, % | 0 | 0.3 | 0.5 | beta |
| Quality of life-related utility [mean value (standard deviation)] |
||||
| Baseline | 0.79 (0.118) |
0.79 (0.116) |
0.79 (0.119) |
** |
| Cycle 4 | 0.82 (0.115) |
0.80 (0.115) |
0.79 (0.058) |
normal |
| Cycle 7 | 0.83 (0.057) |
0.81 (0.111) |
0.81 (0.114) |
normal |
| Cycle 13 | 0.86 (0.108) |
0.85 (0.106) |
0.85 (0.109) |
normal |
| Cycle 21 | 0.85 (0.152) |
0.86 (0.098) |
0.85 (0.052) |
normal |
| 6 months following completion of treatment | 0.84 (0.095) |
0.85 (0.094) |
0.85 (0.147) |
normal |
Includes gastrointestinal (GI) perforation, GI leak, GI fistula, GI necrosis, and GI hemorrhage
Pre-treatment QOL was not modeled
PFS, progression-free survival
OS, overall survival
PC, paclitaxel/carboplatin
PCB, paclitaxel/carboplatin/bevacizumab
PCB+B, paclitaxel/carboplatin/bevacizumab + maintenance bevacizumab
Costs
Costs were assigned to treatment regimens, growth factor support, and severe adverse events (assigned within the three-month cycle in which they were incurred), and were discounted accordingly (Table 2 and Supplemental Table 1). All costs were inflated to 2013 US dollars using medical inflation rates (http://www.halfhill.com/inflation.html).
Table 2.
Costs
| Item | J Code* | Cost ($) | Source |
|---|---|---|---|
| Chemotherapy Regimens, per cycle | |||
| Paclitaxel/carboplatin (PC) | See Supplemental Table | 449 | CMS.gov |
| Paclitaxel/carboplatin/bevacizumab (PCB) |
See Supplemental Table | 7,127 | CMS.gov |
| Paclitaxel/carboplatin/bevacizumab + bevacizumab maintenance (PCB+B) |
See Supplemental Table | 7,127+6,999** | CMS.gov |
| Growth Factors, per cycle | |||
| Darbepoetin alfa | J0881 | 1,670 | CMS.gov |
| Pegfilgrastim | J2505 | 2,940 | CMS.gov |
| Severe Adverse Events | |||
| ICD-9 code*** | Cost (SD), $ | Source | |
| Hypertensive crisis | 401 | 5,756 (123) | AHRQ.gov |
| Bowel perforation | 569.83 | 29,375 (925) | AHRQ.gov |
Healthcare Common Procedure Coding System J-codes for medications
Indicates additional cost per cycle of bevacizumab alone
International Classification of Diseases Ninth Edition (ICD-9) codes for diagnoses.
Treatment
Costs of chemotherapy and common support drugs were assigned using Medicare J code reimbursements. Outpatient administration fees were assigned using Medicare reimbursements (www.cms.gov).
Growth factors
We incorporated the costs of erythropoietin and granulocyte colony stimulating factor based on the actual number of cycles in which these drugs were administered in each study arm.
Adverse Events
For simplicity, we assigned costs to the following major adverse events: grade 4 hypertension, grade 3-5 bowel perforation, necrosis, hemorrhage, leak and fistula events. Costs of adverse events were modeled as normal distributions using inpatient cost data from the Agency for Healthcare Research and Quality’s publically accessible Healthcare Cost and Utilization Project Nationwide Inpatient Sample (http://hcupnet.ahrq.gov) (GI perforation, ICD-9 code 569.83; hypertensive crisis, ICD-9 code 410). Hospitalizations were not reported separately given that the majority of the toxicities captured in this analysis would require inpatient care; thus, assigning a separate cost to hospitalization risks over-representing the cost of these toxicities.
Survival
The primary outcome of GOG 218 was progression-free survival (PFS). Given the individualization of treatment, the costs of treating recurrent ovarian cancer, the lack of available data on events past recurrence, and the lack of QOL data between progression and death, we used PFS to calculate the primary effectiveness metric in the model. Overall survival (OS) was evaluated as a secondary endpoint of the parent trial and was used to calculate the secondary effectiveness metric in the model. The model’s primary cost-effectiveness metric was therefore quality-adjusted progression-free years (QA-PFY), and the secondary metric, quality-adjusted life years (QALY). PFS and OS in each treatment group were modeled as beta distributions using primary event data at three-month intervals to the 60-month time horizon. In order to ensure validity, the survival data was replicated using the model described.
Quality of life
QOL scores were collected in the clinical trial using the Trial Outcome Index (TOI) of the Functional Assessment of Cancer Therapy-Ovary (FACT-O) [FACT-O TOI] instrument at baseline, prior to cycle 4, cycle 7, cycle 13, cycle 21, and 6 months following completion of treatment [9]. FACT subscale scores were converted to utilities using the Dobrez method and modeled as normal distributions [10,11]. As there was no QOL data beyond the 6-month post treatment time point, QOL was assumed to be equivalent between treatment arms from the final measured time point to the date of progression or 60 months.
Sensitivity analysis
All trial data were entered as appropriate mathematical distributions such that the primary model was run as a Monte Carlo probabilistic sensitivity analysis to simultaneously account for uncertainty in all model parameters. Cost and effectiveness results were therefore reported with surrounding 95% confidence intervals. Extensive one-way sensitivity analyses were additionally performed around the cost of bevacizumab, QOL in the PCB+B arm, and the possible additional costs of earlier disease progression.
Cost-effectiveness analysis
Effectiveness was defined primarily as months of QA-PFY and costs were calculated as total costs per strategy. Cost-effectiveness ratios were defined as the cost per year of PFS. Incremental cost-effectiveness ratios (ICER) were defined as the costs per QA-PFY saved in the primary analysis and cost per QALY saved in the secondary analysis.
The ICER for a given strategy was compared to the next less costly strategy. A strategy that is more costly but more effective than an alternative strategy has traditionally been considered cost-effective if its ICER is below $50,000/QALY, with more recent analyses considering $100,000/QALY acceptable [12]. Despite the controversy regarding this commonly used “willingness-to-pay (WTP) threshold” for cost-effectiveness analyses evaluating preventive health measures, this convention was used to guide the interpretation of the model rather than to conclude that one intervention should or should not be utilized [13].
Alternative scenario analysis
We performed several alternative scenario analyses. First, we modeled a scenario in which QOL was not incorporated into the model (with the cost-effectiveness metric being cost/progression-free year (PFY) saved). In a second alternative scenario, we incorporated QOL, but assumed that it was uniform across all three regimens. In this scenario, utility values for the PC arm were used to model QOL in all three arms of the model (with the metric being cost/QA-PFY). Finally, to determine whether a large improvement in QOL could potentially make the PCB+B comparator arm cost-effective compared to PC, we assumed perfect QOL (utility = 1 at all time points) in the PCB+B arm throughout the trial.
RESULTS
Base case model
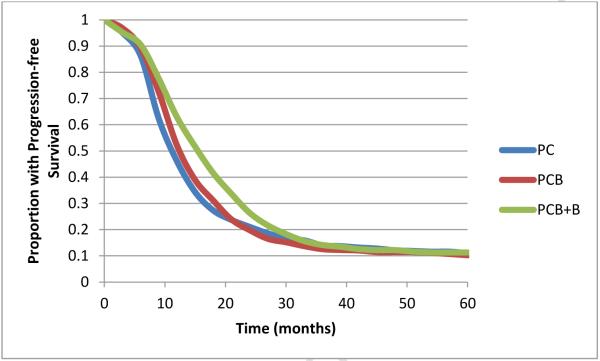
PFS output from the primary model is shown in Figure 2. PC was the least expensive regimen at a mean cost of $4,044 (95% CI $3,823-$4,296), followed by PCB at $43,703 ($42,342-$44,087) and PCB+B at $122,700 ($120,572-$124,784). PC was least effective at 1.1 QA-PFY (95% CI 1.04-1.16), followed by PCB at 1.13 QA-PFY (1.07-1.18) and PCB+B at 1.25 QA-PFY (1.19-1.30). PCB was less effective and more expensive than a combination of PC and PCB+B (known as extended dominance), and was therefore excluded from subsequent cost-effectiveness comparisons. PCB+B had an ICER of $792,380/QA-PFY compared to PC. In an acceptability curve analysis with a WTP threshold of $100,000/QA-PFY, PC was the treatment of choice in 100% of simulations. PC remained the treatment of choice in 100% of simulations unless the WTP threshold exceeded $360,000/QA-PFY.
Figure 2.
Modeled PFS. The curves are identical to the curves from GOG 218, demonstrating external validity of the model.
Sensitivity analysis on QOL
In a model not incorporating QOL, the ICER of PCB+B was $632,571/PFY compared to PC. In a model incorporating QOL, but assuming identical QOL for all three regimens, the ICER of PC+B compared to PC was $746,171/QA-PFY. Assumption that a perfect QOL (utility=1) was achieved in the PCB+B arm, the ICER was $305,772/QA-PFY compared to PC.
Sensitivity analysis on cost
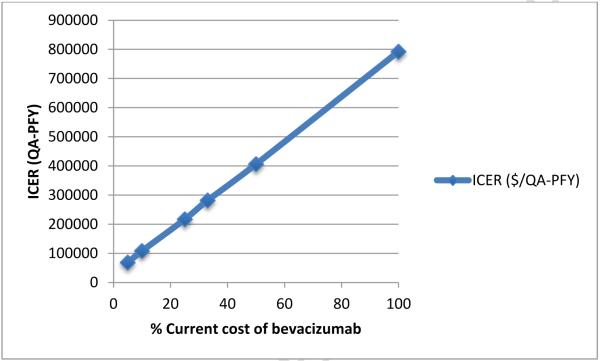
When the cost of bevacizumab was decreased from baseline over a wide range of estimates, reduced ICERs were seen compared with the baseline (100% cost) estimates (Figure 3). When the cost of bevacizumab was reduced to 50%, 33%, and 25% of baseline, the ICER for PCB+B compared to PC was $405,898, $281,395, and $216,635/QA-PFY, respectively. When the cost of bevacizumab was less than 10% of baseline, the ICER of PCB+B fell below $100,000 per QA-PFY.
Figure 3.
Cost-effectiveness of PCB+B compared to PC at different bevacizumab costs. ICER, incremental cost-effectiveness ratio; QA-PFY, quality-adjusted progression-free year.
Alternative analysis accounting for bevacizumab at progression
In an alternative scenario analysis, we incorporated a single, discounted cost of the first treatment for recurrence. Based on the assumption that bevacizumab would likely not be re-initiated in women with recurrent disease who were originally randomized to the PCB or PCB+B arms, we assumed a cost of the regimen for first recurrence of $10,000 in those patients previously treated with bevacizumab. For women randomized to the PC arm, we assumed that the first treatment for recurrence would contain bevacizumab and evaluated a range of $21,000 (3 cycles bevacizumab) to $105,000 (15 cycles bevacizumab). Assuming treatment with up to 7 cycles of bevacizumab for treatment of recurrence in patients randomized to the PC arm, the model’s results were unchanged, with PC remaining the preferred strategy. When greater than 7 cycles of bevacizumab were used for treatment of recurrence in patients randomized to the PC arm, PC was dominated (less effective and more costly) by PCB. PCB+B was not cost-effective over this range, with an ICER between $600,000 and $700,000/QA-PFY compared to PCB.
Secondary endpoint analysis
When using overall survival as the effectiveness endpoint, PCB was less effective and more expensive than a combination of PC and PCB+B (extended dominance). When excluding PCB as a dominated strategy, PCB+B had an ICER of $2,523,405/QALY compared to PC.
DISCUSSION
In this analysis of patients with advanced ovarian cancer treated on GOG 218, we demonstrate that the addition of bevacizumab to standard carboplatin and paclitaxel is not cost-effective. While the regimen of concurrent and maintenance bevacizumab (PCB+B) had the longest progression free survival (compared with PC or PCB), the decrement in quality of life and the additional cost of bevacizumab led to a high incremental cost-effectiveness ratio (ICER) compared to PC, far exceeding usual willingness to pay thresholds.
A previous cost-effectiveness analysis of GOG 218 (modeled on data presented in abstract form and without inclusion of quality of life data) demonstrated similar findings, with bevacizumab-containing arms having an ICER that exceeded usual willingness to pay thresholds [6]. One of the greatest weaknesses of this prior study, however, was the lack of inclusion of QOL data in the model, and the uncertainty of the impact of this data on the cost-effectiveness of the use of bevacizumab. The information used to inform the current model is more comprehensive than that used to develop the previous model in that the QOL data is included, thus the absolute results are different (for example, PCB+B had an ICER of $401,088 per PFY in the prior study compared with $792,380 per QA-PFY in the current model). However, no data exists regarding the impact of treatment with adjuvant and maintenance bevacizumab on lost patient and caregiver time (and thus the cost of this lost time). For this reason, we constructed this model from a third party payer, rather than a societal, perspective. Future clinical trials should be designed to collect this information to provide the most comprehensive assessment of the overall societal costs of cancer treatment. In an effort to determine whether this difference is, in part, due to the inclusion of the QOL data in the current model, we performed a secondary analysis in which QOL was not incorporated into the model, and found that, as expected, the ICER was reduced, but only to $632,571/PFY. In a previous investigation, there was a high correlation between cost/QALY and cost/LY (the equivalent of the reporting of PFY and QA-PFY in this model), adjusting for QOL leads to an increased ICER, but not to the extent that it substantively impacts the cost per life-year ratios for the vast majority of cancer-related interventions [14]. Nonetheless, the importance of adjusting for the impact on QOL would be expected to be even more important in cases where improvements in QOL are realized by interventions that have only a modest impact on survival. The 3.8 month PFS advantage of PCB+B compared with PC justifies adjustment for QOL; however, the reduction in QOL with the addition of bevacizumab in GOG 218 led to an ICER that was even further from usual willingness to pay thresholds. In a similar randomized trial of standard chemotherapy with or without bevacizumab (though at a reduced dose compared with GOG 218), results of ICON 7 demonstrated modest improvements in progression free survival, an improvement in overall survival in a subgroup analysis of the population at highest risk for recurrence, and a small decrease in QOL with bevacizumab [15,16]. Given the reduced dose of bevacizumab used in ICON7, one could hypothesize that the use of bevacizumab could be more cost-effective than in GOG 218; however, modeling studies demonstrate that even using more selective criteria such as treatment of only the high risk subgroup, the addition of bevacizumab is not cost-effective, and results are most strongly influenced by the cost of bevacizumab [17] as highlighted in Figure 3. Recently, an alternative cost-effectiveness model of GOG 218 and ICON 7 data suggested that in very specific circumstances (treatment with ICON 7 schedule, in patients at highest-risk for recurrence and at a 30% reduced cost of bevacizumab and at a WTP threshold of $150,000), the use of bevacizumab was cost-effective [18]. Continued investigation of the clinical scenarios in which the use of bevacizumab becomes cost-effective is critical.
Quality of life data have the potential to significantly impact the results of any cost utility analysis. In the QOL analysis of GOG 218, the FACT-O TOI, which measures physical and functional well-being together with ovarian cancer-specific concerns, improved over time during treatment for all three treatment arms, as would be expected for a population of women following cytoreduction with limited-volume residual (thus likely asymptomatic) ovarian cancer. However, as mentioned previously, significantly less improvement was evident in the treatment arms containing bevacizumab, leading to statistically significant (though possibly not clinically significant) treatment differences during chemotherapy that did not persist during the maintenance phase of the trial. With respect to abdominal discomfort, all arms of the trial improved over time. While no QOL differences were observed between bevacizumab-containing and control arms on this outcome measure, study-associated placebo treatments may have negatively influenced QOL in the PC arm and contributed to the lack of difference in QOL observed during the maintenance phase.
The improvement in PFS seen in women receiving PCB+B compared to PC is a signal of activity of bevacizumab, but the modest difference may have been due to the dilution of patients with highly favorable long-term benefit by those who did not respond. Thus, identification of prognostic or predictive factors that would indicate a high likelihood of response to an intervention could strongly impact the effectiveness (and thus, the cost-effectiveness) of a novel therapeutic like bevacizumab. Recently, a theoretical model was developed aiming to evaluate alternative strategies to maximize the cost-effectiveness of treating advanced ovarian cancer patients with bevacizumab, and included the impact of a test that would potentially predict response to bevacizumab (a predictive biomarker) [17]. Compared with a strategy of treating all patients with ovarian cancer and a strategy of treating only those patients at the highest risk for recurrence (due to larger volume residual disease), the strategy that used the predictive biomarker had the lowest incremental cost-effectiveness ratio ($129,000 per QALY) compared with chemotherapy alone and dominated other bevacizumab treatment strategies. While no strategy that included bevacizumab fell within usual willingness to pay thresholds, this theoretical model of incorporating a predictive biomarker is intriguing and deserves further exploration in an effort to optimize the use of bevacizumab in the treatment of advanced ovarian cancer. Examples of using a predictive biomarker in an economic analysis do exist in the literature; the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) investigated erlotinib in previously treated lung cancer, which demonstrated an overall survival advantage to erlotinib of 2 months (6.7 months versus 4.7 months with placebo)[19]. A concurrent correlative study of biomarkers predicting response to erlotinib showed that the presence of an EGFR mutation increased responsiveness to the agent, but without improved survival [20]. A follow up study by the NCIC-CTG Working Group on Economic Analysis five years later demonstrated that, when stratified by EGFR copy number, the use of erlotinib was more cost-effective with a high EGFR copy number (ICER $33,353 per LYS) compared with a low EGFR copy number (ICER $109,792 per LYS) [21]. Identification of such a marker for bevacizumab response, albeit beyond the scope of this discussion, will be imperative to provide a cost-effective strategy for its use.
Our analysis is limited by the modeling and assumptions that are required to build the model. In particular, QOL assessments were not performed beyond 6 months past the last treatment, limiting our ability to quantify the QOL decrement that is likely associated with the onset of progressive disease. If QOL utilities could be applied to post-progression survival, then direct estimation of QALs would be possible. Future clinical trials will hopefully be designed with consideration of collecting QOL as well as cost events after the date of progression to improve the ability to perform comprehensive and accurate cost utility analyses. Because the standard for judging cost-effectiveness is cost/QALY, not cost/QA-PFY, this would be an important step forward for economic assessments of cancer treatments. In addition, the conversion of QOL scores from the FACT-O instrument to a utility has not been extensively validated, and future prospectively collected studies will address this issue by including both the FACT and a more standard instrument for measuring utilities such as the EQ-5D [22]. Nonetheless, regardless of how utilities are measured, use of a QALY from a third party payer perspective does not always reflect patient preferences for cancer care, which may not always be consistent over time. Furthermore, given that patients not receiving bevacizumab in the frontline setting may be more likely to receive it (with or without cytotoxic chemotherapy) at the time of recurrence, our alternative analysis suggests that the use of bevacizumab could be more cost-effective if one considers the expense of treating recurrences with the same drug. However, this model demonstrates that even in the alternative strategy accounting for bevacizumab at progression, PCB+B is never cost-effective (while PCB becomes the preferred strategy when more than 7 cycles salvage bevacizumab are assumed in the PC arm). Additionally, the PFS endpoint used in GOG 218 could influence the interpretation of this CUA. The United Kingdom’s National Institute for Health and Clinical Excellence (NICE, an organization that serves to provide guidance to ensure quality and value for money in healthcare) provided its opinion on the clinical effectiveness and cost effectiveness of bevacizumab in combination with paclitaxel and carboplatin in the front-line treatment of advanced ovarian cancer, and noted that the uncertainty related to the percentage of patients who received bevacizumab after progression precludes an adequate cost effectiveness analysis with OS as a primary endpoint, and accepted the PFS endpoint for their technology appraisal [23]. In the current study, we provide alternative scenario analyses, both using OS as an endpoint as well as varying the number of cycles of bevacizumab for treatment of recurrence after chemotherapy without bevacizumab. Neither of these alternative analyses provides evidence of the use of bevacizumab being cost effective. We do acknowledge the limitations of this alternative model in that we have not specified an explicit treatment strategy. However, our implementation of a $10,000 cost for a recurrence regimen that does not include bevacizumab is intentionally conservative given prior cost-effectiveness study of recurrent ovarian cancer where the costs of cytotoxic recurrence strategies ranged from $4,700 to $20,000 [2014 dollars] [24].
In summary, bevacizumab is not a cost-effective treatment in the front-line management of advanced ovarian cancer, even when consideration of the impact of QOL is incorporated into a cost utility analysis. Given that inclusion of the QOL scores increased the ICER (by over $150,000 in this model), the modest PFS advantage and the high cost of drug in the PCB+B arm observed in GOG 218 means that no QOL benefit would reduce the ICER to within commonly accepted willingness to pay thresholds. As such, identification of populations in whom treatments would be most likely to be effective is imperative, and continued investigation of the development (and exploration of cost-effectiveness) of predictive biomarkers is critically important.
Supplementary Material
Research Highlights.
Bevacizumab is not cost effective in the adjuvant and maintenance treatment of advanced ovarian cancer
Prospective collection of cost and quality of life data is critical to a well-executed cost utility analysis
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517) and NRG Oncology Grant number: 1 U10 CA180822. The clinical trial upon which this manuscript is based was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI), under the Collaborative Research and Development Agreement (CRADA) for bevacizumab between NCI and Genentech, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this study was presented in part at the 45th Annual Meeting on Women’s Cancer, Society of Gynecologic Oncologists, March 22-25, 2014, Tampa, FL.
Registration number at ClinicalTrials.gov: NCT00262847
Conflict of Interest
The authors wish to disclose that there are no conflicts of interest.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Bundy B, Wenzel L, et al. Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Brady MF, Bookman MA, et al. Gynecologic Oncology Group Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. F. 29. [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Huang HQ, Burger RA, et al. Patient reported outcomes of a randomized, placebo-controlled trial of bevacizumab in the front-line treatment of ovarian cancer: A Gynecologic Oncology Group Study. Gynecol Oncol. 2013;128:573–78. doi: 10.1016/j.ygyno.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansman FG, Postma MJ, Brouwers JR. Cost considerations in the treatment of colorectal cancer. Pharmacoeconomics. 2007;25:537–62. doi: 10.2165/00019053-200725070-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cohn DE, Kim KH, Resnick KE, et al. At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29:1247–51. doi: 10.1200/JCO.2010.32.1075. 1. [DOI] [PubMed] [Google Scholar]
- 7.Gold MR, Seigel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 8.Havrilesky LJ, Secord AA, Darcy KM, Armstrong DK, Kulasingam S, Gynecologic Oncology Group Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2008 Sep 1;26(25):4144–50. doi: 10.1200/JCO.2007.13.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19:1809–17. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 10.Dobrez D, Cella D, Pickard AS, et al. Estimation of patient preference-based utility weights from the functional assessment of cancer therapy - general. Value Health. 2007;10:266–72. doi: 10.1111/j.1524-4733.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 11.Hess LM, Brady WE, Havrilesky LJ, et al. Comparison of methods to estimate health state utilities for ovarian cancer using quality of life data: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;128:175–80. doi: 10.1016/j.ygyno.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann PJ, Sandberg EA, Bell CM, et al. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19:92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 13.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg D, Neumann PJ. Does adjusting for health-related quality of life matter in economic evaluations of cancer-related interventions? Expert Rev Pharmacoecon Outcomes Res. 2011;11:113–19. doi: 10.1586/erp.11.1. [DOI] [PubMed] [Google Scholar]
- 15.Perren TJ, Swart AM, Pfisterer J, et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 16.Stark D, Nankivell M, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial. Lancet Oncol. 2013;14:236–43. doi: 10.1016/S1470-2045(12)70567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett JC, Alvarez Secord A, Cohn DE, et al. Cost effectiveness of alternative strategies for incorporating bevacizumab into the primary treatment of ovarian cancer. Cancer. 2013;119:3653–61. doi: 10.1002/cncr.28283. [DOI] [PubMed] [Google Scholar]
- 18.Mehta DA, Hay JW. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol Oncol. 132:677–83. doi: 10.1016/j.ygyno.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. 14. [DOI] [PubMed] [Google Scholar]
- 20.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. 14. [DOI] [PubMed] [Google Scholar]
- 21.Bradbury PA, Tu D, Seymour L, et al. Economic analysis: randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer. J Natl Cancer Inst. 2010;102:298–306. doi: 10.1093/jnci/djp518. [DOI] [PubMed] [Google Scholar]
- 22.Rabin R, de Charro F. EQ-5D. A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 23.NICE Technology Appraisal Guide TA284, Bevacizumab in combination with paclitaxel and carboplatin for first-line treatment of advanced ovarian cancer. doi: 10.1016/S1470-2045(13)70248-1. issued May 2013, guidance.nice.org.uk/ta284. [DOI] [PubMed] [Google Scholar]
- 24.Havrilesky LJ, Secord AA, Kalasingam S, Myers E. Management of platinum-sensitive recurrent ovarian cancer: a Cost-effectiveness analysis. Gynecol Oncol. 2007;107:211–8. doi: 10.1016/j.ygyno.2007.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.