Abstract
Changes in the extent of the post-translational modification glycosylation have been previously reported in several brain regions in schizophrenia. Quality control within the endoplasmic reticulum and Golgi, branching of glycans, intracellular trafficking and targeting, protein-protein interactions, and endocytosis are processes regulated by both N-linked and O-linked glycosylation. Previous studies in schizophrenia have found altered glycan biosynthesis and abnormal glycan levels in cerebrospinal fluid (CSF) and plasma, as well as altered expression in frontal cortex of glycosyltransferase transcripts encoding proteins associated with both N- and O-linked glycosylation. The N-acetylglucosaminyltransferases (GlcNAcTs) are glycosylating enzymes that play a key role in adding N-acetylglucosamine (GlcNAc) to substrates to facilitate their proper trafficking, intracellular targeting, and cellular function. Given previous results indicating abnormal glycosylation in schizophrenia, we hypothesized that these GlcNAcTs may be abnormally expressed in this illness. We measured protein expression of nine distinct GlcNAcTs by Western blot analysis in postmortem samples of dorsolateral prefrontal cortex (DLPFC) from twelve pairs of elderly patients with schizophrenia and comparison subjects. We found decreased protein expression of UDP-GlcNAc:BetaGal Beta-1,3 GlcNAcT 8 (B3GNT8) and mannosyl (alpha-1,3-)-glycoprotein beta-1,4 GlcNAcT (MGAT4A) expression in schizophrenia. These data provide further evidence that glycosylation is dysregulated in schizophrenia, and suggest a potential mechanism associated with alterations in protein function, trafficking, and intracellular targeting in this illness.
Keywords: B3GNT8, MGAT4A, N-glycosylation, O-glycosylation, posttranslational modifications, postmortem brain
1.0 Introduction
Past studies in postmortem brain of schizophrenia patients have focused on dysregulated transcript and protein levels, but more recently abnormalities in post-translational modifications (PTMs) of proteins have been identified. Glycosylation, ubiquitination, myristoylation, and phosphorylation are some of the PTMs that have recently been studied in schizophrenia brain (Bauer et al., 2010; Mueller et al., 2014; Pinner et al., 2014; Rubio et al., 2013; Tucholski et al., 2013a; Tucholski et al., 2013b).
One of the most common PTMs is glycosylation (Khoury et al., 2011). N-linked glycosylation is the process of systematically adding a glycan to asparagine residues of proteins via specific enzymes in a step-wise manner (Colley, 1997; Rini et al., 2009). It is a highly regulated process that is initiated in the endoplasmic reticulum (ER), with subsequent modification in the Golgi apparatus, and is necessary for glycoproteins to be properly trafficked within the cell. N-glycosylation plays a role in many intracellular processes including facilitating protein folding and subunit assembly, serving as a quality control signal in the ER and Golgi, regulating intracellular targeting and trafficking, modulating protein-protein interactions, and negatively regulating receptor and transporter endocytosis (Dennis et al., 2009; Helenius & Aebi, 2004; Ohtsubo & Marth, 2006; Parodi, 2000). In contrast, O-linked glycosylation is a process where sugars are added to proteins via attachment to the hydroxyl group of a peptidyl serine or threonine, forming an O-glycoprotein. O-glycosylation may be initiated within the cell or in the extracellular space, and forms the initial glycan structure to which subsequent monosaccharides may be sequentially added to produce branching glycans (Schachter, 2000).
Recent studies in postmortem cortex in schizophrenia have found alterations of N-linked glycosylation of the ionotropic glutamate receptor subunits GluA2 and GluK2, glutamate transporters EAAT1 and EAAT2, and γ-aminobutyric acid (GABA) A receptor subunits α1, β1, and β2 (Bauer et al., 2010; Mueller et al., 2014; Tucholski et al., 2013a; Tucholski et al., 2013b). In addition, abnormal expression of glycan structures in cerebrospinal fluid (CSF) and serum (Stanta et al., 2010), abnormal expression of sphingolipid metabolism genes (Kittler et al., 2002), and dysregulated cytoplasmic chondroitin sulfate proteoglycan expression (Macdonald et al., 2009) have all been reported in schizophrenia. These studies suggest that glycosylation pathways are dysregulated in schizophrenia.
While there is evidence for glycosylation abnormalities in schizophrenia, few studies have investigated protein expression of specific enzymes that catalyze N- and O-linked glycosylation which could give rise to previously reported glycosylation changes. In this study, we have characterized in schizophrenia and matched comparison subjects the expression of a set of key glycosyltranferases, the N-acetylglucosaminyltransferases (GlcNAcTs), focusing on the beta-1,3-GlcNAcTs (B3GNTs) and the monoaceylglycerol aceyltransferases (MGATs). The B3GNTs are responsible for both initiating O-glycosylation of proteins as well as extending existing glycan branches within N- and O-glycosylation pathways (Ishida et al, 2004), while the MGATs are responsible for initiating the production of bi-, tri-, and tetra-antennary N-linked sugar chains (Dennis et al., 2009). These enzymes transfer N-acetylglucosamine (GlcNAc) residues onto glycosides, and are involved in the synthesis of polysaccharides previously reported to be changed in CSF in schizophrenia (Stanta et al., 2010). This study aims to determine if expression of GlcNAcTs associated with both N- and O-linked glycosylation pathways are abnormal in prefrontal cortex in schizophrenia.
2.0 Experimental/Materials and methods
2.1 Human subjects
Samples of full thickness of grey matter removed at autopsy from the dorsolateral prefrontal cortex (DLPFC, Brodmann Areas 9 & 46) were obtained from the Mount Sinai Medical Center brain collection, as previously described (Hammond et al., 2010; Mueller et al., 2014). Tissue samples from all subjects were pulverized with small amounts of liquid nitrogen and stored at -80°C. Next of kin consent was obtained for each subject before being included in this collection. The medical and psychiatric history of each subject was reviewed extensively. All subjects included in the schizophrenia group met DSM-III-R criteria for the illness, were diagnosed by at least two clinicians, had documented psychosis before the age of forty, and at least ten years of hospitalization for schizophrenia (Powchik et al, 1998; Purohit et al, 1998). Neuropathological examination was conducted for all subjects and none were used in this study if there was evidence of any neurodegenerative disease. Any subject with coma greater than six hours, death by suicide, or documented history of drug or alcohol abuse was excluded from study. All comparison subjects were free of neurological or psychiatric disorders. Twelve pairs of schizophrenia and comparison subjects were matched for age, tissue pH, sex, and postmortem interval (Tables 1 & 2).
Table 1. Summary of subject demographics.
| Schizophrenia | Comparison | |
|---|---|---|
| N | 12 | 12 |
| Sex | 6M/6F | 6M/6F |
| Tissue pH | 6.4 ± 0.2 | 6.7 ± 0.3 |
| Postmortem interval (hours) | 14.9 ± 6.6 | 10.0 ± 7.5 |
| Age (years) | 75.8 ± 10.8 | 75.7 ± 9.4 |
| On/Off Rx | 7/5 | 0/12 |
Values reported as means ± standard deviation.
On/Off Rx: received antipsychotic medications within 6 weeks of death.
Table 2. Subject Pairs.
| Pair | Subject | Rx Antipsychotics | Sex/Age | pH | PMI (h) |
|---|---|---|---|---|---|
| 1 | Comparison | F/79 | 6.38 | 10.1 | |
| Schizophrenia | On | F/77 | 6.01 | 9.7 | |
| 2 | Comparison | M/70 | 6.10 | 6.7 | |
| Schizophrenia | On | M/73 | 6.50 | 7.9 | |
| 3 | Comparison | F/89 | 6.72 | 2.3 | |
| Schizophrenia | On | F/89 | 6.20 | 9.6 | |
| 4 | Comparison | M/93 | 6.28 | 4.2 | |
| Schizophrenia | On | M/97 | 6.50 | 9.3 | |
| 5 | Comparison | M/69 | 6.67 | 7.4 | |
| Schizophrenia | On | M/70 | 6.35 | 7.2 | |
| 6 | Comparison | M/7 | 6.43 | 5.0 | |
| Schizophrenia | Off | M/80 | 6.37 | 15.4 | |
| 7 | Comparison | M/5 | 6.67 | 20.4 | |
| Schizophrenia | On | M/57 | 6.40 | 20.7 | |
| 8 | Comparison | F/81 | 6.37 | 19.4 | |
| Schizophrenia | Off | F/81 | 6.67 | 15.1 | |
| 9 | Comparison | F/66 | 6.85 | 22.6 | |
| Schizophrenia | On | F/62 | 6.74 | 23.7 | |
| 10 | Comparison | F/78 | 6.97 | 16.0 | |
| Schizophrenia | Off | F/75 | 6.49 | 21.5 | |
| 11 | Comparison | F/73 | 6.98 | 3.0 | |
| Schizophrenia | Off | F/70 | 6.51 | 13.2 | |
| 12 | Comparison | M/76 | 6.32 | 2.9 | |
| Schizophrenia | Off | M/78 | 6.64 | 26.1 |
Abbreviations: Postmortem Interval (PMI). Hours (h).
On/Off Rx: received antipsychotic medications within 6 weeks of death.
2.2 Antipsychotic treated rats
Animal studies and procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Male Sprague-Dawley rats (∼250g) were housed in pairs for the duration of the study (9 months). Treatment was with either haloperidol decanoate (28.5 mg/kg, N = 8) or sesame oil (vehicle, N = 8) administered via intramuscular injection every three weeks. A total of twelve injections were given. This dose and duration of treatment has been previously described (Harte et al., 2005; Kashihara et al., 1986). The animals were sacrificed by decapitation, and brains were immediately harvested. Frontal cortex was dissected on wet ice, snap frozen on dry ice, and stored at -80°C.
2.3 Sample preparation
Tissue from both human and rat samples were reconstituted in cold 5 mM Tris-HCL pH 7.5, 0.32 M sucrose with a protease inhibitor tablet and a phosphatase inhibitor tablet (Complete Mini, EDTA-free and PhosSTOP both from Roche Diagnostics). A Power Gen 125 (Thermo Fisher Scientific) homogenizer was used at speed setting 5 for sixty seconds (Pinner et al., 2014). Protein concentration was determined using a BCA protein assay kit (Thermo Scientific). After homogenization, the samples were stored at -80°C until used for western blot analysis.
2.4 Western blot analysis
Homogenized samples were thawed on wet ice, denatured using 6× loading buffer (reduced with 2-mercaptoethanol) at 70°C for ten minutes, and stored at -20°C until use. All samples were loaded (15μg of total protein in 10μl per lane) onto NuPAGE 4-12% Bis-Tris 1 mm, 17 well gels (Invitrogen), electrophoresed, and transferred to 0.45 μM nitrocellulose membranes using a BioRad Semi-Dry Transblotter. A molecular mass standard was run on each gel (Novex Sharp Pre-stained Protein Standard, Life Technologies). Membranes were blocked for one hour at room temperature in blocking buffer before being incubated with the appropriate primary antibody diluted in blocking buffer containing 0.1% Tween-20 (Sigma) under conditions listed in Table 3. Valosin-containing protein (VCP) was chosen as an intralane loading control because its molecular weight is substantially different from the proteins of interest and has been shown to not be altered in schizophrenia (Bauer et al., 2009; Stan et al., 2006; Mueller et al., 2014). VCP antibodies from two different host species (mouse and rabbit) were used as needed to avoid cross-reactivity with the host species of primary antibodies for proteins of interest.
Table 3. Antibodies used for Western Blot Analysis.
| Antibody | Species | Dilution | Buffer | Incubation | Company (Cat.#) |
|---|---|---|---|---|---|
| MGAT1 | Mouse | 1:1,000 | Li-cor | 16 h 4°C | Proteintech (15103-1-AP) |
| MGAT4A | Mouse | 1:500 | 5% BSA | 16 h 4°C | Abnova (H00011320-M02) |
| MGAT4B | Mouse | 1:250 | 5% Milk | 16 h 4°C | Abnova (H00011282-M01) |
| MGAT4C | Rabbit | 1:250 | 5% Milk | 16 h 4°C | Sigma (HPA016418) |
| MGAT5 | Mouse | 1:1,000 | 5% Milk | 16 h 4°C | Abnova (H00004249-M09) |
| MGAT5B | Rabbit | 1:500 | Li-cor | 16 h 4°C | Proteintech (16993-1-AP) |
| B3GNT2 | Goat | 1:1,000 | Li-cor | 16 h 4°C | Abnova (PAB7522) |
| B3GNT3 | Mouse | 1:250 | 5% BSA | 16 h 4°C | Abnova (H00010331-A01) |
| B3GNT8 | |||||
| (B3GNTL1) | Rabbit | 1:2,000 | Li-cor | 16 h 4°C | Novus (NBP1-90838) |
| VCP | Mouse | 1:25,000 | Li-cor | 1 h RT | Abcam (AB11433) |
| VCP | Rabbit | 1:25,000 | Li-cor | 1 h RT | Abcam (AB36047) |
Abbreviations: Bovine serum albumin (BSA); Li-cor Odyssey blocking buffer (Li-cor)
Membranes were washed three times in phosphate-buffered saline containing 0.1% Tween-20 (PBST) for five minutes each before being incubated with the appropriate IR-dye labeled secondary antibody diluted in blocking buffer (Table 3). The membranes were washed again three times each in PBST for five minutes. Membranes were then placed in MilliQ water before being scanned with an Odyssey Infrared Imaging System (Li-Cor Biosciences) at a resolution of 169μm and intensity level of 3.
2.5 Data Analysis
Image Studio 4.0 analytical software (Li-Cor Biosciences) was used to determine the expression of each protein of interest. Relative protein expression values were normalized to intralane labeling of VCP. Integrated intensity values for VCP were not different between schizophrenia and comparison groups, consistent with previous reports in prefrontal cortex (Bauer et al., 2009; Stan et al., 2006). All data were confirmed to be normally distributed using the D'Agostino & Pearson omnibus normality test. Correlational analyses were performed using Statistica 7 (StatSoft) to assess for potential associations between dependent measures and age, pH, or postmortem interval (PMI). None of these potential covariates were correlated with any dependent measures. Human data were analyzed using Prism 6.04 (GraphPad) with paired Student's t-tests, and rat data were analyzed using unpaired t-tests. For all statistical tests, α = 0.05.
3.0 Results
Protein levels for nine GlcNAcTs were assayed in schizophrenia and comparison subjects. Both UDP-GlcNAc:BetaGal Beta-1,3 GlcNAcT 8 (B3GNT8) (t(11) = 2.6, p = 0.025) and mannosyl (alpha-1,3-)-glycoprotein beta-1,4 GlcNAcT (MGAT4A) (t(11) = 2.7, p = 0.022) expression were decreased in schizophrenia relative to matched comparison subjects (Table 4 and Fig. 2); expression of the other enzymes was not changed (Table 4).
Table 4. N-acetylglucosaminyltransferase expression in schizophrenia and comparison subjects.
| Enzyme | Schizophrenia | Comparison | t | p |
|---|---|---|---|---|
| MGAT1 | 0.021± 0.017 | 0.014 ± 0.010 | 1.69 | |
| MGAT4A | 5.669 ± 4.907 | 7.941 ± 6.040 | 2.66 | 0.022 |
| MGAT4B | 0.004 ± 0.006 | 0.004 ± 0.003 | 0.31 | |
| MGAT4C | 0.027 ± 0.029 | 0.024 ± 0.014 | 0.3 | |
| MGAT5 | 0.003 ± 0.002 | 0.002 ± 0.001 | 0.55 | |
| MGAT5B | 1.127 ± 1.041 | 1.691 ± 2.365 | 0.96 | |
| B3GNT2 | 4.172 ± 4.350 | 4.641 ± 4.134 | 0.66 | |
| B3GNT3 | 0.009 ± 0.005 | 0.009 ± 0.004 | 0.01 | |
| B3GNT8 | 44.53 ± 68.46 | 153.52 ± 192.76 | 2.58 | 0.025 |
Data are expressed as ratios of signal intensity of target protein divided by signal for VCP, and reported as means ± standard deviation.
Figure 2.
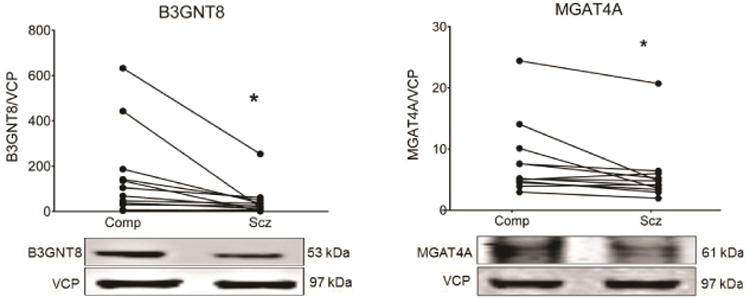
Expression of B3GNT8 and MGAT4A in dorsolateral prefrontal cortex from paired schizophrenia (Scz) and comparison subjects (Comp). Data are expressed as the ratio of signal intensity of each protein of interest divided by the signal of valosin-containing protein (VCP) from the same lane of the same blot. Both B3GNT8 and MGAT4A are reduced in schizophrenia. *p < 0.05
To determine if the changes in these two enzymes might be due to chronic antipsychotic treatment, a post-hoc analysis was performed to compare schizophrenia subjects receiving and not receiving antipsychotic medications within six weeks of death. Given that these patients had a longstanding history of schizophrenia, all had been exposed over their lifetime to a variety of antipsychotics including both first and second generation medications, although five of the twelve patients had not received antipsychotics at the time of death. There was no difference in expression of either enzyme in the antipsychotic free vs. treated subgroups. In addition, experiments in rats chronically treated with haloperidol decanoate were performed. Treatment of rats with haloperidol for nine months did not affect the expression in frontal cortex of either B3GNT8 or MGAT4A (Fig. 3).
Figure 3.
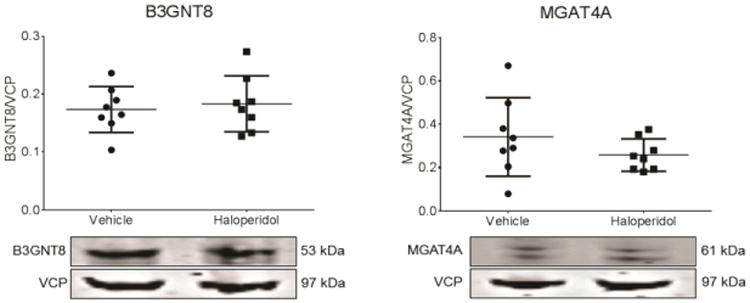
Expression of B3GNT8 and MGAT4A in frontal cortex from male rats treated for nine months with haloperidol decanoate or vehicle. Data are presented as the ratio of signal intensity of each target protein to intensity of valosin-containing protein (VCP) labeling from the same lane of the same blot. Bars represent means ± standard deviation. Chronic haloperidol treatment did not change expression of either B3GNT8 or MGAT4A.
4.0 Discussion
In this study, we measured the protein expression of a set of N-acetylglucosaminyltransferases (Fig. 1) and found decreased expression of B3GNT8 and MGAT4A in DLPFC in schizophrenia. These data are consistent with previous studies that found down-regulation of β-1,4-mannosyl-glycoprotein 4-β GlcNAcT 3 and β-galactosidase α-2,3/6-sialyltransferase in plasma from patients with schizophrenia (Maguire et al., 1997), and extend our earlier reports of abnormalities of N-linked glycosylation of neurotransmitter receptor subunits and transporters in frontal cortex in schizophrenia (Bauer et al., 2010; Drummond et al., 2013; Mueller et al., 2014; Tucholski et al., 2013a; Tucholski et al., 2013b).
Figure 1.
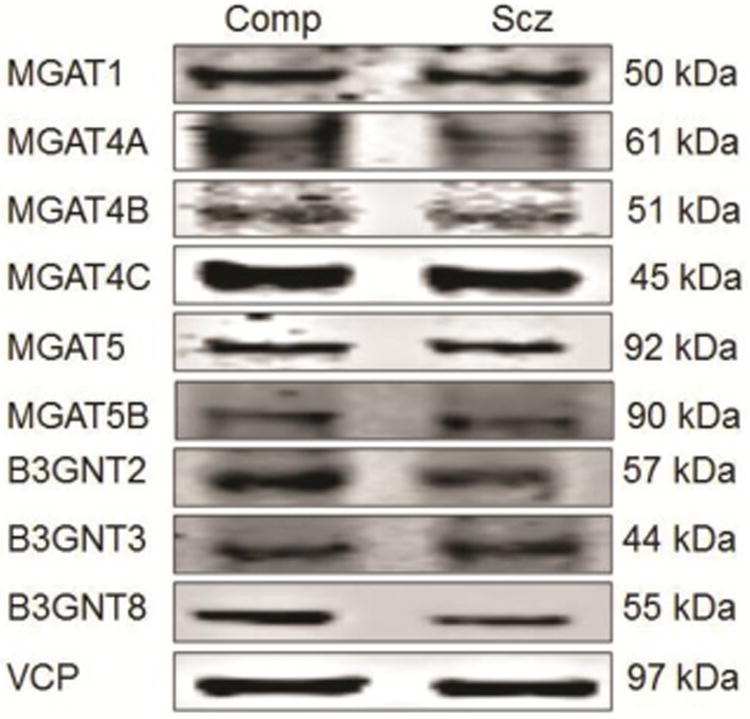
Representative western blot images of assayed proteins in a comparison (Comp) and schizophrenia (Scz) subject.
Posttranslational protein modifications, such as glycosylation, may represent common mechanisms underlying dysregulation of multiple neurotransmitter systems and pathways in schizophrenia. Protein folding, trafficking, localization, and recycling are all affected by glycosylation (Dennis et al., 2009; Helenius & Aebi, 2004; Ohtsubo & Marth, 2006; Parodi, 2000), and alterations of these processes in schizophrenia may be linked to abnormalities of glycosylation pathways. Multiple studies have found abnormal subcellular distribution of neurotransmitter receptors and transporters as well as other synaptic proteins in the brain in schizophrenia (Thompson et al., 1998; Talbot et al., 2011; Deo et al., 2012; Hammond et al., 2010; Kristiansen et al., 2010; Shan et al., 2014; Banerjee et al., 2014; Mueller et al., 2015). Interestingly, several studies have shown that neurotransmitter receptors containing abnormally N-glycosylated subunits exhibit abnormal subcellular distribution in schizophrenia brain. AMPA receptor subunits as well as the glutamate transporters have both altered subcellular expression (Hammond et al., 2010; Shan et al., 2014) and abnormal patterns of N-glycosylation (Bauer et al., 2010; Tucholski et al., 2013a). The β2 subunit of the GABAA receptor has altered N-glycosylation and abnormal expression in the endoplasmic reticulum and at the synapse in schizophrenia (Mueller et al., 2014; Mueller et al., 2015). Interestingly, fucosyltransferase (Fut8) knockout mice exhibit schizophrenia-like behaviors, increased AMPA receptor expression in the post-synaptic density, but no change in total AMPA receptor expression levels (Gu et al., 2015), suggesting that glycosyltransferases similar to the GlcNAcTs can affect the subcellular localization of neurotransmitter receptors. Accordingly, abnormal glycosylation may underlie the disruption of subcellular processing, targeting, and localization of multiple neurotransmitter systems in schizophrenia.
Previous research showing dysregulation in schizophrenia of glycan levels and glycan biosynthesis in CSF and serum, and down-regulation of glycosyltransferases in transcriptomic studies, provided initial evidence suggesting decreased protein expression of GlcNAcTs (Narayan et al., 2009; Stanta et al., 2010). Glycosyltransferases are type 2 transmembrane proteins that contain a luminal catalytic domain that can be cleaved by secretory proteases resulting in secretion into CSF and other body fluids (McCaffrey and Jamieson, 1993). Disruption of these GlcNAcTs, and subsequently changes in the glycan branching structures they synthesize on proteins (as well as lipids), may be associated with aberrant protein handling and provide a mechanism behind alterations in multiple neurotransmitter systems and pathways previously implicated in schizophrenia.
Multiple glycosylation pathways play roles in regulating trafficking and cellular localization of proteins by enzymatically pruning and subsequently attaching glycans in a specific, step-wise manner. Glycoprotein production relies on sugar branches formed by previous enzyme reactions to produce the correct substrate for sequential addition of the next glycan (Schachter, 2000). For correct GlcNAcT mediated glycan branching on proteins and lipids to occur, the molecules to be glycosylated, GlcNAcTs, and nucleotide sugar transporters must all co-localize to the same Golgi compartment (Moremen et al., 2012). A single GlcNAcT can modify multiple seemingly unrelated glycosides because they glycosylate a growing glycan branching structure rather than the protein or lipid to which the glycan “tree” is attached.
The process of enzymatically extending glycan structures on proteins has been shown to initiate proper intracellular signaling, nuclear signaling, and cytoskeleton formation, cellular functions which have previously been shown to be dysregulated in schizophrenia (Tang et al., 2012; de Bartolomeis et al., 2014: Lipska et al., 2006). Accordingly, down-regulation of a single GlcNAcT such as B3GNT8 or MGAT4A could affect the modification of multiple proteins associated with that specific GlcNAcT (Ohtsubo & Marth, 2006) including neurotransmitter receptor subunits, transporters, and their respective chaperone proteins. For example, the MGAT family initiates glycan branching in a sequential manner beginning with the addition of GlcNAc by MGAT1, and continuing until MGAT3 synthesizes bisecting glycan branches in competition with MGAT4 and MGAT5, which form tri- and tetra-antennary glycan structures (Schachter et al, 1986). This competition between GlcNAcTs can influence glycan branching and may affect binding affinity of glycoproteins to endogenous lectins (Dennis et al., 2009; Moremen et al., 2012; Ohtsubo & Marth, 2006). Lectins are proteins that selectively bind to glycosides and recognize specific glycan branching structures; lectin binding can affect glycoprotein folding and trafficking (Goldstein et al., 2002). Glycosylation can directly modulate transmembrane protein cell surface half-life, organization in the plasma membrane, intracellular signaling process, and endocytic cycling by increasing the binding affinity of glycoproteins to associated lectins (Ohtsubo & Marth, 2006).
Many transmembrane receptor subunits and transporters are glycosylated in the Golgi, which serves as a quality control step before lectin binding occurs in the early secretory pathway (Dennis et al., 2009). Proper glycan branch structure can increase lectin affinity and oppose the internalization of cell surface receptors and transporters by indirectly inhibiting endocytosis to the early endosome (Lau et al., 2008). The down-regulation of MGAT4A in schizophrenia we report here could decrease the tri-antennary and subsequently the tetra-antennary glycan branching structure of glycoproteins which may result in dysregulated lectin binding of receptor subunits and transporters at the plasma membrane. A previous study has shown that decreased MGAT5, which requires the prior activity of MGAT1-4C, leads to increased receptor content in the early endosome by affecting lectin binding affinity (Partridge et al., 2004). Decreased expression of MGAT4A could similarly lead to shorter plasma membrane residency of receptor subunits and transporters by initiating their endocytosis into the early endosome. Interestingly, GluA1 is glycosylated in human cortex (Tucholski et al., 2014) and binds multiple lectins including Lycopersicon esculentum (LEL), which specifically recognizes GlcNAc moieties that B3GNTs and MGATs transfer. We have previously reported that the GluA1 AMPA receptor subunit has increased expression in the early endosome (Hammond et al., 2010) but decreased expression in the synapse (unpublished data) in schizophrenia. Our finding of decreased expression of B3GNT8 and MGAT4A may represent an underlying mechanism contributing to increased expression of GluA1 in the early endosome and in turn alterations in AMPA mediated signaling in schizophrenia.
Glycosylation by GlcNAcTs may also protect some proteins from proteolytic degradation (Sola and Griebenow, 2009). The down-regulation of B3GNT8 and MGAT4A may lead to increased glycoprotein degradation given the role of glycosylation in protein quality control mechanisms. B3GNT8 is known to interact with ubiquitin c (Wagner et al., 2011), which plays a role in endoplasmic reticulum-associated degradation (ERAD), DNA repair, and lysosomal degradation (Eletr and Wilkinson, 2014). If ubiquitin c is not glycosylated properly, it may become misfolded and alternatively trafficked rather than being targeted to the ER, where it marks proteins for degradation. Such a disruption in protein glycosylation could result in extensive downstream effects (Narayan et al., 2009).
As with most human postmortem studies in psychiatric illnesses, there are several limitations to this work. The subjects used in this study were elderly, so our results may not generalize to a younger cohort. We were able to pair subjects for sex, age, pH, and where possible PMI; however, there was a difference of PMI between the comparison and schizophrenia groups. To address this difference, we performed post hoc analyses with PMI as a covariate for the two significant dependent measures, which did not change our results. In addition, PMI was not correlated with protein expression of either enzyme. Potential effects of chronic antipsychotic treatment are a concern in these types of studies in schizophrenia. We assessed the expression of B3GNT8 and MGAT4A in frontal cortex of rats chronically treated with haloperidol and found no difference in these protein levels. Accordingly, it is unlikely that the changes in protein expression that we report in schizophrenia are due to chronic antipsychotic treatment.
In summary, we found that two N-acetylglucosaminyltransferases, B3GNT8 and MGAT4A, are down-regulated in postmortem DLPFC from patients with schizophrenia. These findings are consistent with dysregulated glycosylation, which could lead to aberrant glycan branching, disrupted protein folding and localization, and altered transmembrane protein internalization dynamics at the plasma membrane. These data add to the growing body of evidence that multiple glycosylation pathways are dysregulated in schizophrenia, and may represent a potential underlying mechanism linking abnormalities observed in multiple neurotransmitter systems in this illness.
Acknowledgments
The authors have no acknowledgments.
Funding body agreements and policies: This work was funded by the National Institutes of Health Grants MH53327 (JHMW), MH064673 (VH), and MH066392 (VH).
Footnotes
Contributors: JMK, TMM and JHMW designed the study. JMK performed the experiments and statistical analyses, and wrote the first draft of the manuscript. VH provided the human tissue. All authors contributed to and have approved the final manuscript.
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee A, Wang HY, Borgmann-Winter KE, MacDonald ML, Kaprielian H, Stucky A, Kvasic J, Egbujo C, Ray R, Talbot K, Hemby SE, Siegel SJ, Arnold SE, Sleiman P, Chang X, Hakonarson H, Gur RE, Hahn CG. Src kinase as a mediator of convergent molecular abnormalities leading to NMDAR hypoactivity in schizophrenia. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.115. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7(1):1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Latte G, Tomasetti C, Iasevoli F. Glutamatergic Postsynaptic Density Protein Dysfunctions in Synaptic Plasticity and Dendritic Spines Morphology: Relevence to Schizophrenia and Other Behavioral Disorders Pathophysiology and Implications for Novel Therapeutic Approaches. Mol Neurobiol. 2014;49:484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- Deo A, Cahill M, Li S, Goldszer I, Henteleff R, Vanleeuwen J, Rafalovich I, Gao R, Stachowski E, Sampson A, Lewis D, Penzes P, Sweet R. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: its role in dendritic pathology. Neurobiol Dis. 2012;45(2):796–803. doi: 10.1016/j.nbd.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10(11):1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophr Res. 2013;147(1):32–38. doi: 10.1016/j.schres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletr ZM, Wilkinson KD. Regulation of Proteolysis by Deubiquitinating Enzymes. Biochim Biophys Acta. 2014;1843(1) doi: 10.1016/j.bbamcr.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Fukuda T, Isaji T, Hang Q, Lee H, Sakai S, Morise J, Mitoma J, Higashi H, Taniguchi N, Yawo H, Oka S, Gu J. Loss of α1,6-fucosyltransferase decreased hippocampal long-term potentiation: implications for core fucosylation in the regulation of AMPA receptor heteromerization and cellular signaling. J Biol Chem. 2015 doi: 10.1074/jbc.M114.579938. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IJ. Lectin structure-activity: the story is never over. J Agric Food Chem. 2002;50:6583–6585. doi: 10.1021/jf0201879. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35(10):2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005;75(2-3):303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annual Review of Biochemistry. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Ishida H, Togayachi A, Sakai T, Iwai T, Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, Shoda J, Tanaka N, Narimatsu H. A novel β1,3-N-acetylglucosaminyltransferase (β3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Letters. 2004;579(2005):71–78. doi: 10.1016/j.febslet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biological Psychiatry. 1986;21(7):650–6. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. 2011;1(90) doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assmebly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26(2-3):251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64(7):495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Lau KS, Dennis JW. N-glycans in cancer progression. Glycobiology. 2008;18(10):750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Mitkus SN, Mathew SV, Fatula R, Hyde TM, Weinberger DR, Kleinman JE. Functional genomics in postmortem human brain: abnormalities in a DISC1 molecular pathway in schizophrenia. Dialogues Clin Neurosci. 2006;8:353–357. doi: 10.31887/DCNS.2006.8.3/blipska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Schultz SC. What we know: findings that every theory of schizophrenia should explain. Schizophrenia Bulletin. 2009;35(3):493–508. doi: 10.1093/schbul/sbp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biological Psychiatry. 1997;41(11):1131–1136. doi: 10.1016/S0006-3223(96)00223-5. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Jamieson JC. Evidence for the role of a cathepsin D-like activity in the release of Gal beta 1-4GlcNAc alpha 2-6sialtransferase from rat and mouse liver in whole-cell systems. Comp Biochem Physiol. 1993;104:91–94. doi: 10.1016/0305-0491(93)90342-3. [DOI] [PubMed] [Google Scholar]
- Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology. 2014;39(3):528–537. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Remedies CE, Haroutunian V, Meador-Woodruff JH. Abnormal Subcellular Localization of GABAA Receptor Subunits in Schizophrenia Brain. Transl Psychiatry. 2015 doi: 10.1038/tp.2015.102. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Head SR, Gilmartin TJ, Dean B, Thomas EA. Evidence for disruption of sphingolipid metabolism in schizophrenia. Journal of neuroscience research. 2009;87(1):278–288. doi: 10.1002/jnr.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Parodi AJ. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem J. 2000;348(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granocsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- Pinner AL, Haroutunian V, Meador-Woodruff JH. Alterations of the myristoylated, alanine-rich C kinase substrate (MARCKS) in prefrontal cortex in schizophrenia. Schizophr Res. 2014;154(1-3):36–41. doi: 10.1016/j.schres.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophrenia Bulletin. 1998;24(3):325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Archives of general psychiatry. 1998;55(3):205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Rini J, Esko J, V A. Glycosyltransferases and Glycan-processing Enzymes. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2009. [PubMed] [Google Scholar]
- Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38(10):1910–20. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biology. 1986;64:163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Schachter H. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj J. 2000;17:465–483. doi: 10.1023/a:1011010206774. [DOI] [PubMed] [Google Scholar]
- Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014;154(1-3):1–13. doi: 10.1016/j.schres.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98(4):1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M, Rahmoune H, Levin Y, Guest PC, Bahn S, Rudd PM. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J Proteome Res. 2010;9(9):4476–4489. doi: 10.1021/pr1002356. [DOI] [PubMed] [Google Scholar]
- Tang J, Liao Y, Zhou B, Tan C, Liu W, Wang D, Liu T, Hao W, Tan L, Chen X. Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS. 2012;7(7):e40247. doi: 10.1371/journal.pone.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Louneva N, Cohen JW, Kazi H, Blake DJ, Arnold SE. Synaptic dysbindin-1 reductions in schizophrenia occur in an isoform-specific manner indicating their subsynaptic location. PLoS. 2011;6(3):e16886. doi: 10.1371/journal.pone.0016886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Sower AC, Perrone-Bizzozero NI. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry. 1998;34(4):239–243. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr Res. 2013a;146(1-3):177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013b;24(12):688–691. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Pinner AL, Simmons MS, Meador-Woodruff JH. Evolutionarily conserved pattern of AMPA receptor subunit glycosylation in mammalian frontal cortex. PLoS. 2014;9(4):e94255. doi: 10.1371/journal.pone.0094255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, Choudhary C. A Proteome-wide, Quantitative Survey of In Vivo Ubiquitylation Sites Reveals Widespread Regulatory Roles. Mol Cell Proteomics. 2011;10(10):M111.013284. doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]


