Abstract
Background
Computed tomography (CT) scans are commonly used to diagnose acute diverticulitis, but there are overlapping features between diverticulitis and colorectal cancer (CRC) on imaging studies. Hence, colonoscopy is typically recommended after an episode of acute diverticulitis to rule out underlying malignancy. Currently, 64-slice multidetector CT scanners are capable of providing higher-resolution images and may be able to distinguish malignancy from diverticular inflammation. We aimed to determine the prevalence of CRC among patients with CT-diagnosed acute diverticulitis.
Methods
We performed a retrospective study of patients with acute diverticulitis diagnosed on CT scan between December 2005 and December 2010 at St. Paul’s Hospital, Vancouver, BC. Nonresidents were excluded. We reviewed CT scan reports that included the term “diverticulitis,” reports of follow-up colonic evaluation within 1 year of diagnosis and pathology results. We queried the provincial cancer registry to ensure no cases of CRC were missed.
Results
A total of 293 patients had acute diverticulitis diagnosed on CT scan, but 8 were nonresidents and were excluded. Of the 285 included in the analysis, the mean age was 59.4 ± 15.1 years, and 167 (58.6%) were men. Among the 114 patients who underwent follow-up evaluation, malignancy was diagnosed in 4 (3.5%). The overall prevalence of malignancy among patients with CT-diagnosed diverticulitis was 1.4%.
Conclusion
Routine endoscopic evaluation after an episode of diverticulitis diagnosed with high-resolution CT scan does not appear to be necessary. Selective approach in patients with protracted clinical course or those with mass lesion/obstruction on CT scan may be of benefit.
Abstract
Contexte
La tomodensitométrie (TDM) est couramment utilisée pour le diagnostic de la diverticulite aiguë, mais des caractéristiques sont communes à la diverticulite et au cancer colorectal (CCR) aux épreuves d’imagerie. On recommande donc en général la coloscopie après un épisode de diverticulite aiguë pour écarter un diagnostic de cancer sous-jacent. À l’heure actuelle, les appareils de TDM multidétecteurs à 64 barrettes peuvent fournir des images de haute résolution et permettent même de distinguer le cancer d’une inflammation diverticulaire. Nous avons voulu déterminer la prévalence du CCR chez les patients ayant présenté une diverticulite aiguë diagnostiquée par TDM.
Méthodes
Nous avons procédé à une étude rétrospective sur des patients porteurs d’une diverticulite aiguë diagnostiquée à l’aide de TDM entre décembre 2005 et décembre 2010 à l’Hôpital St. Paul’s de Vancouver, en Colombie-Britannique. Les non-résidents ont été exclus. Nous avons examiné les rapports de TDM incluant le terme « diverticulite », les rapports d’examens du côlon au cours de l’année suivant le diagnostic et les rapports d’anatomopathologie. Nous avons interrogé le registre provincial sur le cancer pour nous assurer qu’aucun cas de CCR ne nous avait échappé.
Résultats
En tout, 293 patients ont reçu un diagnostic de diverticulite à l’aide de la TDM; 8 étaient des non-résidents et ont été exclus. Parmi les 285 patients inclus dans l’analyse, l’âge moyen était de 59,4 ± 15,1 ans et 167 (58,6 %) étaient des hommes. Parmi les 114 patients qui ont subi un examen de suivi, le cancer a été diagnostiqué chez 4 (3,5 %). La prévalence globale du cancer chez les patients porteurs d’un diagnostic de diverticulite posé par TDM était de 1,4 %.
Conclusion
L’évaluation endoscopique de routine après un épisode de diverticulite diagnostiquée à l’aide d’une TDM de haute résolution ne semble pas nécessaire. Une approche sélective chez les patients qui présentent une évolution clinique lente ou ceux qui présentent une lésion ou obstruction tumorale à la TDM pourrait être utile.
It is estimated that up to 20%–25% of patients with colonic diverticula will progress to diverticulitis.1,2 The diagnosis of acute diverticulitis is typically made using a combination of history, clinical exam, biochemical investigations and diagnostic imaging. Computed tomography (CT) has emerged as the imaging modality of choice given its high sensitivity and specificity for the diagnosis of diverticulitis, with some studies reporting up to 100% in either measure.3–8 Computed tomography findings of diverticula, inflammation of pericolic fat, bowel wall thickness greater than 4 mm and/or pericolic fluid/abscess are highly suggestive of acute diverticulitis.1,8,9 A CT scan also provides prognostic information and guides management by determining whether the diverticulitis is complicated by abscess, fistula formation, stricture/obstruction or free rupture. While most cases of uncomplicated diverticulitis respond well to conservative treatment, complicated diverticulitis requires surgical intervention.
There is overlap in the findings of acute diverticulitis and colorectal cancer (CRC), and CT findings alone are insufficient to exclude malignancy approximately 10% of the time.1,10 As a result, the American Society of Colon and Rectal Surgeons recommend performing a colonoscopy to exclude a potential malignancy after an episode of acute diverticulitis has resolved.1,9,11 However, since the advent of multidetector CT scanners that are capable of capturing images more quickly and thus reduce motion artifacts, the image qualities and diagnostic accuracy of CT scans have improved substantially. With increased resolution of the newer 64-slice CT scans that are currently in widespread use, acute diverticulitis can be diagnosed more accurately,12,13 and it may be possible to adequately distinguish acute diverticulitis from malignancy based on radiological features alone. Routine follow-up colonoscopy may no longer be required in patients with acute diverticulitis. The primary aim of this study was to determine the prevalence of colon cancer in patients with diverticulitis diagnosed on high-resolution CT scan to determine the need for follow-up colonoscopy in this patient population.
Methods
We performed a retrospective chart review of all patients with acute diverticulitis diagnosed on CT scan between December 2005 and December 2010 at St. Paul’s Hospital, Vancouver, BC, a university-affiliated tertiary care centre. We queried the CT scan report database for the term “diverticulitis” and then reviewed reports for findings consistent with acute diverticulitis. The CT images were obtained with the patients in the supine position using a LightSpeed VCT Scanner (General Electric). Images were acquired with the following specifications: collimation 40 mm, pitch 1.375:1, matrix 512 × 512, field of view to fit the patient, MA Noise index 35, tube rotation 0.5 s and peak voltage 120 kV. The images were reconstructed using a standard algorithm with thicknesses of 1.25–2.50 mm. Intravenous and oral contrast dye were administered unless contraindicated owing to renal insufficiency or a documented allergy to contrast dye, or if the imaging was initially indicated to rule out nephrolithiasis. Rectal contrast was not routinely administered.
Patients who were not residents of British Columbia were excluded owing to lack of availability of medical records. We collected baseline demographic and pathology reports from the hospital’s electronic medical record system. Reports of lower endoscopy performed before and after CT scan were retrieved from the hospital’s electronic medical record and/or requested from the patient’s family physician. We compared the findings identified at colonoscopy and the CT scan reports of the included patients to determine the prevalence of colonic neoplasia in patients with acute diverticulitis. Because not all patients had available follow-up colonoscopy reports, we queried the provincial cancer registry at the British Columbia Cancer Agency (BCCA) in February 2014 to capture all incident cases of CRC after the diagnosis of diverticulitis. Our study was approved by the University of British Columbia Providence Health Research Ethics Board.
Statistical analysis
Baseline demographic characteristics are summarized as means with standard deviations or medians with interquartile ranges (IQR) for continuous variables. Categorical variables are expressed as frequencies and percentages. We performed 2-sample t tests using Microsoft Excel 2007. Where statistical analyses were not appropriate, the results of this retrospective chart review were analyzed descriptively.
Results
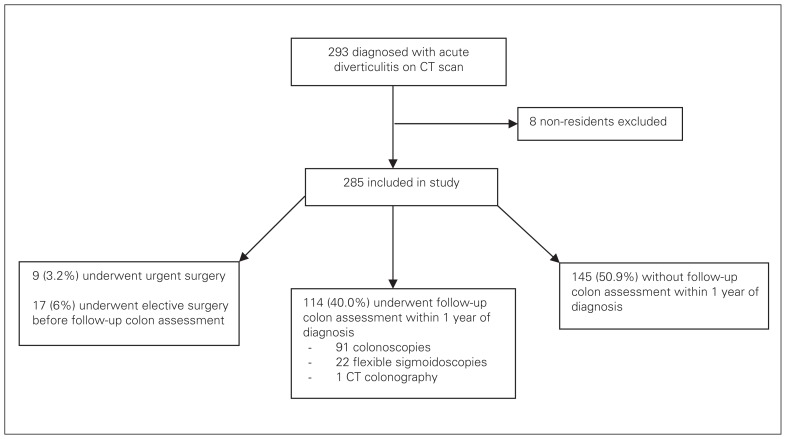
Between December 2005 and December 2010, 293 patients had acute diverticulitis diagnosed on CT scan; 8 of them were not residents of British Columbia and were thus excluded from analysis (Fig. 1). Of the remaining 285 patients, 58.6% (n = 167) were men, and the mean age of patients was 59.4 ± 15.1 years. The majority of the CT scans were performed using intravenous contrast media (n = 227, 79.6%; Table 1). Diverticulitis involving the sigmoid colon accounted for 74.4% (n = 212) of the cases.
Fig. 1.
Flow of patients through the study. CT = computed tomography.
Table 1.
Demographic and clinical characteristics of study sample
| Characteristic | No. (%)* |
|---|---|
| Age, yr | |
| Mean ± SD | 59.4 ± 15.1 |
| Median (IQR) | 61 (49–70) |
| Male sex | 167 (58.6) |
| Female sex | 118 (41.4) |
| CT scan contrast | |
| Intravenous | 227 (79.6) |
| Oral/rectal | 22 (7.7) |
| None | 36 (12.6) |
CT = computed tomography; IQR = interquartile range; SD = standard deviation.
Unless otherwise indicated.
Nine patients (3.2%) required emergent surgery within the same hospital admission. Seven of them underwent surgery owing to severe perforated diverticulitis, and 2 underwent surgery owing to worsening clinical status despite initial conservative treatment. Seventeen patients (6.0%) underwent nonurgent resection of the affected colonic segment before any follow-up endoscopic evaluation (Fig. 1). All surgical pathology reports were negative for malignancy.
A total of 114 patients (40%) underwent further evaluation of the colon within 1 year of the CT scan: 91 had colonoscopies, 22 had flexible sigmoidoscopies and 1 underwent CT colonography. The median time from CT scan to subsequent colonic evaluation was 3 (IQR 2.0–5.5) months. Within this cohort of patients, the mean age was 56.8 ± 15.2 years, and 71 (62.3%) were men. Colonic polyps/masses were identified in 42 patients. No adverse events from postdiverticulitis endoscopic evaluation were identified.
Four patients (3.5%; 3 women and 1 man) were found to have colorectal adenocarcinoma at the location identified on CT scan. The mean age of the patients with malignancy was 67.3 ± 14.8 years, which was not significantly different from that of the patients without malignancy (59.3 ± 15.1 yr, p = 0.15; Table 2) None of these 4 patients had undergone any prior CRC screening by endoscopic evaluation.
Table 2.
Endoscopic findings in the whole study sample (n = 285)
| Finding | No. (%) patients | Sex, no. (%) male | Age, mean ± SD, yr |
|---|---|---|---|
| Adenocarcinoma | 4 (1.4) | 1 (25) | 67.3 ± 14.8 |
| Noncancerous lesion | 281 (98.6) | 166 (59.1) | 59.3 ± 15.1 |
| Premalignant lesion | 23 (8.1) | 18 (78.3) | 61.5 ± 12.8 |
| Non-neoplastic lesion | 258 (90.5) | 148 (57.4) | 59.1 ± 15.3 |
SD = standard deviation.
Twenty-three patients (20.2%) had premalignant polyps: 2 had sessile serrated adenoma, 17 had tubular adenoma, 1 had villous adenoma and 3 had tubulovillous adenoma. Of the 17 patients with tubular adenoma, 4 had lesions 1 cm or larger and none had high-grade dysplasia. The mean age of patients with premalignant findings was 61.5 ± 12.8 years, which was not significantly different from that of the patients who did not have premalignant findings (59.1 ± 15.3 yr, p = 0.45; Table 3). Of the remaining patients, 11 had hyperplastic adenoma, 1 had benign colon mucosa (n = 1), and the pathology result was unavailable for 4.
Table 3.
Malignant and premalignant findings by colonic segment
| Finding* | Detection rate, no. | Location, no. | ||
|---|---|---|---|---|
| Cecum/ascending colon | Transverse colon | Descending colon/sigmoid colon/rectum | ||
| Adenocarcinoma | 4 | 0 | 1 | 3 |
| Tubular adenoma | 17 | 4 | 2 | 13 |
| Sessile serrated adenoma | 2 | 1 | 0 | 1 |
| Tubulovillous adenoma | 3 | 0 | 0 | 3 |
| Villous adenoma | 1 | 0 | 0 | 1 |
| Hyperplastic polyp | 11 | 1 | 1 | 10 |
Endoscopic finding classified based on highest-grade lesion.
Querying the provincial cancer registry identified the same 4 patients who were found to have malignancy on follow-up endoscopy. There were no additional cases of CRC among the remaining 281 patients, resulting in an overall prevalence of colon cancer of 1.4% in this group of patients with acute diverticulitis.
Discussion
Although there is no definitive evidence to suggest patients with diverticular disease are at higher risk of cancer,14,15 historically the overlapping features of diverticulitis and colon cancer on CT scan made exclusion of malignancy difficult.1 As a result, endoscopic evaluation of the colon after an episode of acute diverticulitis is currently recommended.1,9,11 The advent of high-resolution CT scanning has improved its diagnostic accuracy and has challenged the requirement for routine colonoscopy after CT-diagnosed acute diverticulitis.
In our study, initial analysis showed a colon cancer prevalence of 3.5% among the 114 patients who underwent colonic evaluation within 12 months of receiving a CT scan diagnosing diverticulitis. To minimize selection bias, we included patients who underwent surgical resection owing to recurrent/persistent disease and patients who either had a follow-up investigation elsewhere or who had none at all. As none of the patients who underwent surgery had any evidence of malignancy, the prevalence further decreased to 2.9% among those who had direct evaluation of the affected colon segment (i.e., endoscopic and surgical specimen). In order to capture the data for patients who did not undergo direct colonic evaluation and patients whose endoscopic results were not available, we further queried the provincial cancer registry and did not identify any additional cases of malignancy. The combination with population data allowed a nearly 100% follow-up rate for patients included in our study, thus providing an accurate assessment of the prevalence of malignancy among those with CT-diagnosed diverticulitis. The overall CRC prevalence of 1.4% and the adenoma detection rate of 20.2% among those who underwent endoscopic evaluation are both comparable to rates reported previously.16
It is worth noting that, although only 91 of the follow-up examinations were full colonoscopies, the patients who underwent flexible sigmoidoscopies were known to have sigmoid/descending colon diverticulitis. One patient underwent CT colonography, which may be a reasonable alternative to endoscopy for colonic evaluation following acute diverticulitis.17 Of the 4 patients with malignancy identified, 3 had lesions located in the sigmoid colon and 1 patient had synchronous lesions in the transverse colon and cecum. The locations of the lesions during endoscopic examination matched well with those seen on the CT scans. The CT scan findings suggestive of obstruction due to mass-like lesions were present in all 4 patients. Among the patients found to have premalignant adenoma, colonoscopy after diverticulitis was of benefit because none of these patients had undergone any prior colonoscopic evaluations. Given that the mean age of these patients was 61.5 years, it is conceivable that had they undergone age-appropriate screening, endoscopic evaluation after the diverticulitis was diagnosed would not have had added benefit.
Recently, several systematic reviews and meta-analyses have demonstrated similar findings to ours, leading the authors of these studies to conclude that routine colonoscopy after an episode of acute diverticulitis is not necessary.18–21 In these studies, the prevalence of CRC among patients with radiologically diagnosed diverticulitis ranged from 1.0% to 2.1%. However, 3 of these studies excluded patients with complicated diverticulitis and patients who underwent surgical management for their diverticular disease.18–20 The exclusion of patients with complicated diverticulitis may introduce selection bias, as the prevalence of CRC was determined only in patients with uncomplicated cases who underwent follow-up colonoscopy. The prevalence of CRC was found to be higher in patients with complicated diverticulitis diagnosed radiologically, leading some authors to conclude that only complicated diverticulitis requires follow-up colonoscopy. Two systematic reviews also included diverticulitis diagnosed on ultrasound,18,19 which is operator-dependent and generally considered inferior to CT scans in terms of diagnostic accuracy, particularly for complicated disease.13,22 It is also not clear whether all the CT scans in the included studies were 64-slice high-resolution scans, as used in our study, which may affect the ability to distinguish malignancy from inflammatory changes.
Brar and colleagues23 reported an overall prevalence of malignancy in CT-scan diagnosed diverticulitis of 1.6%, but complicated diverticulitis with pericolic or pelvic abscess was associated with a higher rate of invasive malignancy: 5.4%. Although our study did not distinguish between complicated and uncomplicated diverticulitis by examining specific radiological features, our results are in line with those of the aforementioned study. It is possible that in our study, patients with more serious clinical presentation/course were followed more closely and thus were more likely to be assessed endoscopically after the CT scan. This may explain why all the colon cancers were found in the group that underwent endoscopic examination and would, in fact, support that selective follow-up endoscopy suffices to exclude malignancy if high-resolution CT scan findings clearly favour uncomplicated diverticulitis.
The fact that none of the patients with malignancy had any previous endoscopic CRC screening suggests the importance of follow-up after CT-diagnosed diverticulitis in endoscopy-naive patients, who represent a substantial proportion of the population despite CRC screening programs.24,25 In addition, similar to the findings of a previous report,26 suspected mass lesion with obstruction was a common high-resolution CT scan feature shared in all 4 patients with malignancy; this feature could be used in the strategy to identify appropriate patients for follow-up endoscopy.
Taken together, the results from our study suggest that patients with diverticulitis diagnosed on high-resolution CT scan are not at increased risk for colon cancer, and thus routine endoscopic evaluation following the acute episode of diverticulitis to exclude malignancy may not be necessary for all patients. Instead, a more selective approach in which only patients with complicated, severe or recurrent cases of diverticulitis would undergo follow-up colonoscopy seems more appropriate. This approach would allow a more efficient use of limited resources for CRC screening given the current long wait times for colonoscopy in Canada.27 Using a selective approach, colonoscopy would be offered to those 50 years of age or older who had not undergone previous CRC screening, those with a protracted course or recurrence of diverticulitis despite medical therapy28 and those with suspicious CT findings, such as a mass lesion with obstruction.26
Limitations
Limitations of our study include the retrospective and single-centre design and the relatively low rate of follow-up colonoscopy, which is likely secondary to multiple contributing factors. As our institution is a tertiary care centre providing care to patients from across the province, the patients may have had follow-up evaluation at a local facility closer to home. The low rate is further complicated by the referral-based system and wait time for elective outpatient procedures, resulting in delayed investigation. We chose 1 year as the cut-off because it is felt endoscopic findings past that period of time may not accurately represent the colonic state at the time of diverticulitis diagnosis. However, no additional malignancy was identified in 22 patients who underwent endoscopic evaluation more than 12 months after the diagnosis of diverticulitis. It is also possible that a repeat endoscopic procedure was deemed unnecessary if there had been an endoscopic evaluation within 1 year before the CT scan, as was the case for 20 patients. Ultimately, to address the issue of missing or late colonic evaluations, we queried the provincial cancer registry (which captures more than 99% of all cancers in the province) to identify all patients with a tissue diagnosis of colon cancer. The group of patients at risk of being missed by this method would be those who were not referred to the cancer agency in favour of conservative palliative approach. It should also be noted that the registry did not provide information on colonic polyps.
Conclusion
The present study lends further support to a selective approach to determining who should undergo follow-up colonoscopy after resolution of acute diverticulitis diagnosed on CT scan. By performing colonoscopy selectively in patients who have not undergone screening colonoscopy, patients with recurrent/protracted diverticulitis and patients with high-resolution CT findings suggestive of mass lesion with obstruction, we can potentially reduce the number of unnecessary procedures and optimize the utilization of limited resources. Additional prospective research to further define the impact of such a selective approach is warranted.
Footnotes
Presented at Canadian Digestive Diseases Week 2012, Montréal, Que., and Digestive Disease Week 2013, Orlando, Fla.
Competing interests: None declared.
Contributors: G. Rosenfeld, J. Brown, N. Chan and B. Bressler designed the study. G. Ou, J. Brown, N. Chan, T. Hong and H. Lim acquired the data, which G. Ou, G. Rosenfeld, N. Chan and B. Bressler analyzed. G. Ou, G. Rosenfeld and B. Bressler wrote the article, which all authors reviewed and approved for publication.
References
- 1.Szojda MM, Cuesta MA, Mulder CM, et al. Review article: management of diverticulitis. Aliment Pharmacol Ther. 2007;26:67–76. doi: 10.1111/j.1365-2036.2007.03491.x. [DOI] [PubMed] [Google Scholar]
- 2.Sheth AA, Longo W, Floch MH. Diverticular disease and diverticulitis. Am J Gastroenterol. 2008;103:1550–6. doi: 10.1111/j.1572-0241.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 3.Hulnick DH, Megibow AJ, Balthazar EJ, et al. Computed tomography in the evaluation of diverticulitis. Radiology. 1984;152:491–5. doi: 10.1148/radiology.152.2.6739821. [DOI] [PubMed] [Google Scholar]
- 4.Doringer E. Computerized tomography of colonic diverticulitis. Crit Rev Diagn Imaging. 1992;33:421–35. [PubMed] [Google Scholar]
- 5.Ambrosetti P, Jenny A, Becker C, et al. Acute left colonic diverticulitis-compared performance of computed tomography and water-soluble contrast enema: prospective evaluation of 420 patients. Dis Colon Rectum. 2000;43:1363–7. doi: 10.1007/BF02236631. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosetti P, Grossholz M, Becker C, et al. Computed tomography in acute left colonic diverticulitis. Br J Surg. 1997;84:532–4. doi: 10.1046/j.1365-2168.1997.02576.x. [DOI] [PubMed] [Google Scholar]
- 7.Padidar AM, Jeffrey RB, Jr, Mindelzun RE, et al. Differentiating sigmoid diverticulitis from carcinoma on CT scans: mesenteric inflammation suggests diverticulitis. AJR Am J Roentgenol. 1994;163:81–3. doi: 10.2214/ajr.163.1.8010253. [DOI] [PubMed] [Google Scholar]
- 8.Kircher MF, Rhea JT, Kihiczak D, et al. Frequency, sensitivity, and specificity of individual signs of diverticulitis on thin-section helical CT with colonic contrast material: experience with 312 cases. AJR Am J Roentgenol. 2002;178:1313–8. doi: 10.2214/ajr.178.6.1781313. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs DO. Diverticulitis. N Engl J Med. 2007;357:2057–66. doi: 10.1056/NEJMcp073228. [DOI] [PubMed] [Google Scholar]
- 10.Balthazar EJ, Megibow A, Schinella RA, et al. Limitations in the CT diagnosis of acute diverticulitis: comparison of CT, contrast enema, and pathologic findings in 16 patients. AJR Am J Roentgenol. 1990;154:281–5. doi: 10.2214/ajr.154.2.2105015. [DOI] [PubMed] [Google Scholar]
- 11.Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284–94. doi: 10.1097/DCR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 12.Werner A, Diehl SJ, Farag-Soliman M, et al. Multi-slice spiral CT in routine diagnosis of suspected acute left-sided colonic diverticulitis: a prospective study of 120 patients. Eur Radiol. 2003;13:2596–603. doi: 10.1007/s00330-003-1887-7. [DOI] [PubMed] [Google Scholar]
- 13.DeStigter KK, Keating D. Imaging update: acute colonic diverticulitis. Clin Colon Rectal Surg. 2009;22:147–55. doi: 10.1055/s-0029-1236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morini S, Zullo A, Hassan C, et al. Diverticulosis and colorectal cancer: between lights and shadows. J Clin Gastroenterol. 2008;42:763–70. doi: 10.1097/MCG.0b013e31816200fb. [DOI] [PubMed] [Google Scholar]
- 15.Meurs-Szojda MM, Terhaar sive Droste JS, Kuik DJ, et al. Diverticulosis and diverticulitis form no risk for polyps and colorectal neoplasia in 4,241 colonoscopies. Int J Colorectal Dis. 2008;23:979–84. doi: 10.1007/s00384-008-0510-4. [DOI] [PubMed] [Google Scholar]
- 16.Niv Y, Hazazi R, Levi Z, et al. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci. 2008;53:3049–54. doi: 10.1007/s10620-008-0286-y. [DOI] [PubMed] [Google Scholar]
- 17.Hjern F, Jonas E, Holmström B, et al. CT colonography versus colonoscopy in the follow-up of patients after diverticulitis — a prospective, comparative study. Clin Radiol. 2007;62:645–50. doi: 10.1016/j.crad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Daniels L, Unlü C, de Wijkerslooth TR, et al. Routine colonoscopy after left-sided acute uncomplicated diverticulitis: a systematic review. Gastrointest Endosc. 2014;79:378–89. doi: 10.1016/j.gie.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 19.De Vries HS, Boerma D, Timmer R, et al. Routine colonoscopy is not required in uncomplicated diverticulitis: a systematic review. Surg Endosc. 2014;28:2039–47. doi: 10.1007/s00464-014-3447-4. [DOI] [PubMed] [Google Scholar]
- 20.Sai VF, Velayos F, Neuhaus J, et al. Colonoscopy after CT diagnosis of diverticulitis to exclude colon cancer: a systematic literature review. Radiology. 2012;263:383–90. doi: 10.1148/radiol.12111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma PV, Eglinton T, Hider P, et al. Systematic review and meta-analysis of the role of routine colonic evaluation after radiologically confirmed acute diverticulitis. Ann Surg. 2014;259:263–72. doi: 10.1097/SLA.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 22.Laméris W, Randen A, Bipat S, et al. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18:2498–511. doi: 10.1007/s00330-008-1018-6. [DOI] [PubMed] [Google Scholar]
- 23.Brar MS, Roxin G, Yaffe PB, et al. Colonoscopy following nonoperative management of uncomplicated diverticulitis may not be warranted. Dis Colon Rectum. 2013;56:1259–64. doi: 10.1097/DCR.0b013e3182a26bfd. [DOI] [PubMed] [Google Scholar]
- 24.Ipsos. More Canadians getting screened for colon cancer. [accessed 2014 Dec. 24]. Available: www.ipsos-na.com/news-polls/pressrelease.aspx?id=5505.
- 25.American Cancer Society. Colorectal cancer facts & figures 2011–2013. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 26.Elmi A, Hedgire SS, Pargaonkar V, et al. Is early colonoscopy beneficial in patients with CT-diagnosed diverticulitis? AJR Am J Roentgenol. 2013;200:1269–74. doi: 10.2214/AJR.12.9539. [DOI] [PubMed] [Google Scholar]
- 27.Lau A, Gregor JC. Resource implications for a population-based colorectal cancer screening program in Canada: a study of the impact on colonoscopy capacity and costs in London, Ontario. Can J Gastroenterol. 2007;21:371–7. doi: 10.1155/2007/810941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Wall BJM, Reuling EMBP, Consten ECJ, et al. Endoscopic evaluation of the colon after an episode of diverticulitis: a call for a more selective approach. Int J Colorectal Dis. 2012;27:1145–50. doi: 10.1007/s00384-012-1448-0. [DOI] [PubMed] [Google Scholar]