Abstract
Background
The lymph node ratio (LNR) has been shown to be an important prognostic factor in patients with gastric, breast, pancreatic and colorectal cancer. We investigated the prognostic impact of the LNR in addition to TNM classification in patients with locally advanced rectal cancer.
Methods
We retrospectively analyzed patients who underwent curative resection for locally advanced rectal cancer between July 2005 and December 2010. We determined the LNR cutoff value using a receiver operating characteristic curve. The Kaplan–Meier method was used to estimate survival curves, while Cox regression analyses were used to evaluate the relationship between LNR and survival.
Results
We included 180 patients aged 28–83 years with median follow-up of 41.8 months. The median number of lymph nodes examined and lymph nodes involved were 11.5 and 4, respectively, and the median LNR was 0.366. An LNR of 0.19 (19%) was the cutoff point to separate patients with regard to median overall survival. Median overall survival was 64.2 months for patients with an LNR of 0, 59.1 for an LNR of 0.19 or less and 37.6 for an LNR greater than 0.19 (p = 0.004). The median disease-free survival was 32.9 months for patients with an LNR of 0, 30.4 for an LNR of 0.19 or less and 17.8 for an LNR greater than 0.19 (p = 0.002).
Conclusion
Our results suggest that LNR should be considered an additional prognostic factor in patients with locally advanced rectal cancer.
Abstract
Contexte
Il a été démontré que le ratio de ganglions lymphatiques positifs est un important facteur pronostique chez les patients atteints de cancer de l’estomac, de cancer du sein, de cancer du pancréas et de cancer colorectal. Nous avons étudié l’incidence pronostique de l’utilisation de ce ratio en plus de la classification TNM chez les patients présentant un cancer du rectum localement avancé.
Méthodes
Nous avons analysé rétrospectivement des patients ayant subi une résection curative visant à traiter un cancer du rectum localement avancé entre juillet 2005 et décembre 2010. Nous avons déterminé la valeur seuil du ratio de ganglions lymphatiques positifs à l’aide d’une courbe caractéristique de la performance. La méthode de Kaplan-Meyer a été utilisée pour estimer les courbes de survie, tandis que le modèle de régression des hasards proportionnels de Cox a servi à évaluer la corrélation entre le ratio à l’étude et la survie.
Résultats
Notre étude a porté sur 180 patients de 28 à 83 ans dont la durée médiane du suivi était de 41,8 mois. Les nombres médians de ganglions lymphatiques examinés et de ganglions lymphatiques positifs étaient de 11,5 et 4, respectivement, et le ratio médian de ganglions lymphatiques positifs était de 0,366. Nous avons utilisé une valeur seuil de 0,19 (19 %) pour séparer les patients en ce qui a trait à la survie globale médiane. Cette mesure était de 64,2 mois pour les patients présentant un ratio de 0, de 59,1 mois pour ceux présentant un ratio de 0,19 ou moins, et de 37,6 mois pour ceux dont le ratio était supérieur à 0,19 (p = 0,004). La survie sans récidive médiane était de 32,9 mois pour les patients présentant un ratio de 0, de 30,4 mois pour ceux présentant un ratio de 0,19 ou moins, et de 17,8 mois pour ceux dont le ratio était supérieur à 0,19 (p = 0,002).
Conclusion
Nos résultats indiquent que le ratio de ganglions lymphatiques positifs devrait être envisagé comme facteur pronostique supplémentaire pour les patients atteints d’un cancer du rectum localement avancé.
Colorectal cancer (CRC) is 1 of the 3 most commonly diagnosed malignant tumours worldwide. Its incidence in China has shown an increasing trend in recent years, especially in Shanghai. Epidemiological statistics in 20121 showed that the number of new cases increased from the sixth most numerous to the second most numerous since the 1970s. The morbidity of CRC increased from 12 per 100 000 to 56 per 100 000, and the average annual growth rate was greater than 4%. More than half of the patients had locally advanced CRC at the time of diagnosis.
Currently, the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system is considered the most robust tool for predicting prognosis. The lymph nodes (LN) classification of metastasis (pN) is established on the basis of the number of LNs involved (Box 1). A population-based large-scale study revealed that node-negative patients with rectal cancer in whom 7 or fewer LNs were examined had a lower recurrence-free interval than patients in whom at least 8 LNs were examined (17.0% v. 10.7%, p = 0.016).2 The National Cancer Institute guidelines recommended a minimum of 12 LNs to stage LN-negative CRC. The greater number of LNs retrieved, the greater the chance that metastatic LNs can be found. This results in more accurate disease staging, which would allow more appropriate adjuvant treatment planning and better calculation of a patient’s long-term prognosis.
Box 1. Lymph nodes (LN) classification of metastasis (pN).
| Classification | Description |
|---|---|
| pN1a | Metastases in 1 regional LN |
| pN1b | Metastases in 2–3 regional LNs |
| pN1c | Tumour deposits in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis |
| pN2a | Metastases in 4–6 regional LNs |
| pN2b | Metastases in 7 or more regional LNs |
Berger and colleagues3 were the first to analyze the LN ratios (LNRs) of patients enrolled in a large adjuvant chemotherapy trial following complete colon cancer (stages II and III) resection using LNR groups based on quartiles. Outcomes included overall survival (OS), cancer-specific survival (CSS) and disease-free survival (DFS). Survival decreased significantly as LNR increased for all 3 outcomes. When a subgroup analysis was performed of the number of positive LNs, this variable was significant only in predicting survival in those who had fewer than 10 LNs in their pathological sample; for those with an LN count of 10–15 or greater than 15, the LNR was once again the most significant predictor of survival.
The LNR has also been shown to be an independent prognosticator in patients with rectal cancer.4–8 Peng and colleagues8 were the first to demonstrate the association between LNR and survival rate in patients with rectal cancer. The mean LNR was 0.34, and they reported that LNR was an independent risk factor for local recurrence, DFS and OS. In these studies,4–8 the LNR cutoff values were 0.01–0.61. Some studies used LNR quartiles, while others used median LNR. This may be related to cancer stage, patient race, sample size and other factors.
It is well known that the survival rate for locally advanced rectal cancer, especially stage III, varies widely.9 According to the seventh edition of the TNM classification, patients with stage III cancer are classified based on the number of positive nodes. Intuitively, it seems safe to believe that the prognostic significance of 5 positive nodes out of a total of 5 will be completely different from 5 positive nodes out of a total of 30. The LNR has been shown to be an important prognostic factor in gastric, breast, pancreatic and CRC.10,11 In this study, we sought to evaluate the prognostic impact of the LNR in patients with locally advanced rectal cancer.
Methods
Patients and pretreatment evaluation
We retrospectively analyzed patients who underwent curative resection for locally advanced rectal cancer between July 2005 and December 2010 at Renji Hospital, Shanghai Jiaotong University of Medicine. We excluded patients who underwent local excision. All of the participants underwent a digital rectal examination, colonoscopy with biopsy, abdominal and pelvic computed tomography (CT) and chest radiography.
Treatment
All patients underwent radical resection. Abdominoperineal resection or low anterior resection was performed according to the surgeon’s preference. Adjuvant chemoradiotherapy (CRT) was scheduled for 4–8 weeks after surgery. Postoperative radiotherapy consisted of a total dose of 45 Gy delivered to the pelvis in 25 fractions or 46 Gy delivered to the pelvis in 23 fractions. The clinical target volume was demarcated as follows: the superior border was located 1.5 cm above the sacral promontory (L5 level), the inferior border was located below the perineal scar, the lateral border was located 1.5 cm lateral to the bony pelvis, the anterior border included one-quarter to one-third of the posterior wall of the bladder, and the posterior border was located 0.5 cm posterior to the sacral surface. Chemotherapy included a bolus injection of fluorouracil (5-FU) and leucovorin (LV) for the first and last week of radiotherapy or capecitabine administered daily during radiotherapy.
Follow-up
All of the patients who were registered in the prospective rectal database attended postoperative follow-up visits every 3 months for 2 years. Physical examinations, serum carcinoembryonic antigen level measurements, chest radiography and abdominal and pelvic CT were performed at each follow-up visit. Bone scintigraphy and colonoscopy procedures were performed annually. After 2 years, follow-up visits occurred every 6 months. Follow-up lasted until the cutoff date (Dec. 31, 2013) or until the patient died.
Response evaluation
Treatment outcomes were evaluated as follows. Local failure was defined as any recurrence in the pelvic radiation field, and distant metastasis was defined as recurrence outside the radiation field. Recurrence, whether locoregional or distant, was confirmed histologically or clinically (i.e., tumour that may be associated with clinical deterioration identified on imaging studies and verified with increases in serum carcinoembryonic antigen level). Disease-free survival was defined as the duration from the end of treatment to the time of recurrence, and OS was defined as the duration from the end of treatment to the time of death or to the end of the follow-up period.
Statistical analysis
We analyzed the LNR cutoff value using a receiver operating characteristic (ROC) curve. Survival curves were generated using the Kaplan–Meier method, and differences between the curves were analyzed by the log-rank test. We used the Cox regression model for the multivariate analysis of risk factors for survival outcomes in patients with locally advanced rectal cancer. All statistical tests were 2-sided, and we considered results to be significant at p < 0.05. We analyzed data using the Statistical Package for the Social Sciences for Windows version 19.0.
Results
Patients
During the study period, a total of 197 patients with locally advanced rectal cancer underwent curative resection at our hospital. We excluded 17 patients (9 had local excision and 8 were lost to follow-up). The remaining 180 patients were included in our analysis. Patient demographic characteristics and pathological features are summarized in Table 1. The study cohort consisted of 111 men and 69 women with an average age of 59 (range 28–83) years. The median numbers of harvested and metastatic LNs were 11.4 (range 3–46) and 4 (range 0–36), respectively. More than 12 LNs were harvested in 97 (53.9%) patients, whereas fewer than 7 were harvested in 32 (17.8%) patients. Most patients received postoperative chemotherapy, and half received postoperative CRT.
Table 1.
Patient characteristics
| Category | No. (%) of patients |
|---|---|
| Sex | |
| Male | 111 (61.7) |
| Female | 69 (38.3) |
| Age, yr | |
| < 65 | 117 (65.0) |
| ≥ 65 | 63 (35.0) |
| Distance from anal verge, cm | |
| < 7 | 111 (61.7) |
| ≥ 7 | 69 (38.3) |
| Type of surgery | |
| APR | 69 (38.3) |
| LAR | 111 (61.7) |
| pT stage | |
| T1–2 | 14 (7.8) |
| T3 | 62 (34.4) |
| T4a | 83 (46.1) |
| T4b | 21 (11.7) |
| pN stage | |
| N1a | 10 (5.6) |
| N1b | 36 (20.0) |
| N1c | 12 (6.7) |
| N2a | 35 (19.4) |
| N2b | 41 (22.8) |
| TNM stage | |
| IIB/IIC | 46 (25.6) |
| IIIA | 6 (3.3) |
| IIIB | 66 (36.7) |
| IIIC | 62 (34.4) |
| Margin status | |
| Positive | 4 (2.2) |
| Negative | 176 (97.8) |
| LNs examined | |
| < 12 | 83 (46.1) |
| ≥ 12 | 97 (53.9) |
| Adjuvant treatment | |
| Chemotherapy | 67 (37.2) |
| Radiotherapy | 9 (5.0) |
| Chemoradiation | 92 (51.1) |
| None | 12 (6.7) |
APR = abdominoperitoneal resection; LAR = low anterior resection; pN = metastasis classification of lymph nodes; pT = primary tumour classification; TNM = tumour-node-metastasis.
LNR
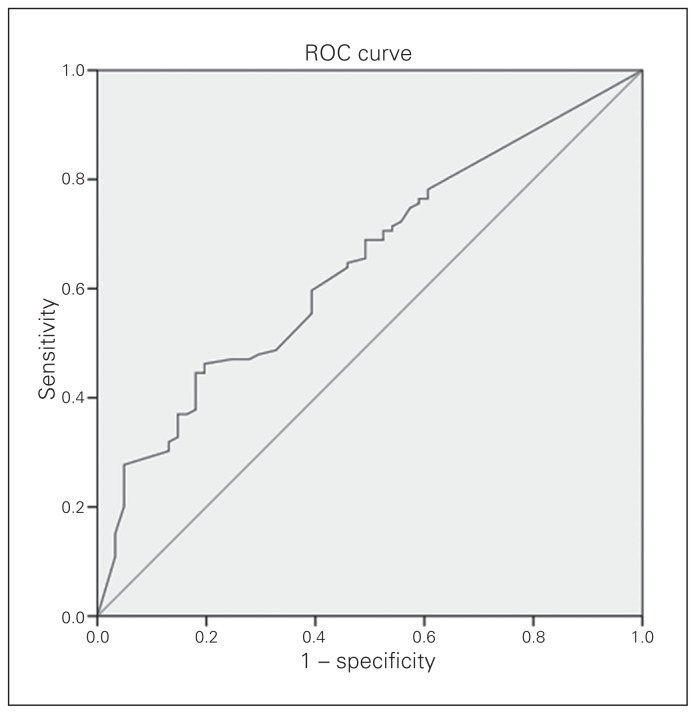
The metastatic LNR is the ratio of pathologically involved LNs to total number of resected LNs. The median LNR was 0.366. We used ROC curves to analyze the predictive value of the LNR (Fig. 1). The cutoff value of LNR was 0.19, at which we observed the most significant difference in OS. Its sensitivity was 51.9% and specificity was 67.3%. The patients were divided into 3 groups based on LNR: LNR = 0 (n = 50), LNR ≤ 0.19 (n = 15) and LNR > 0.19 (n = 115).
Fig. 1.
Receiver operating characteristic (ROC) curve.
Survival analysis
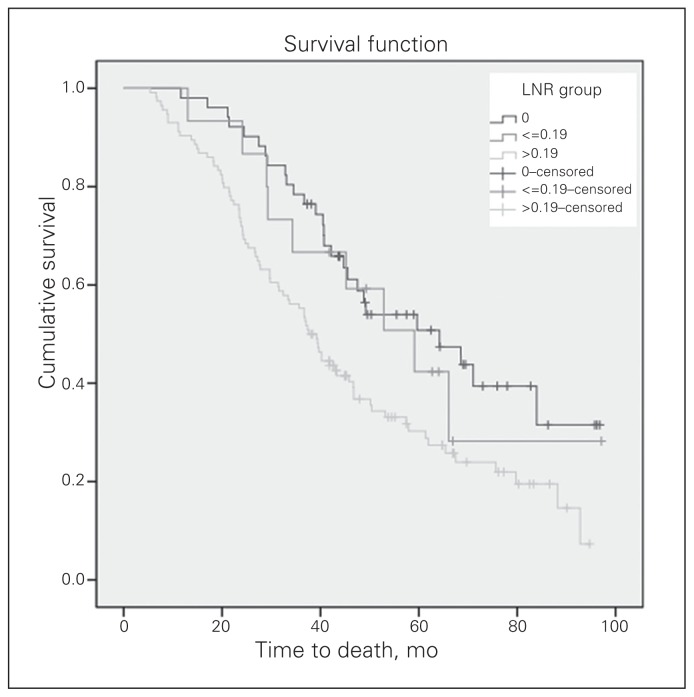
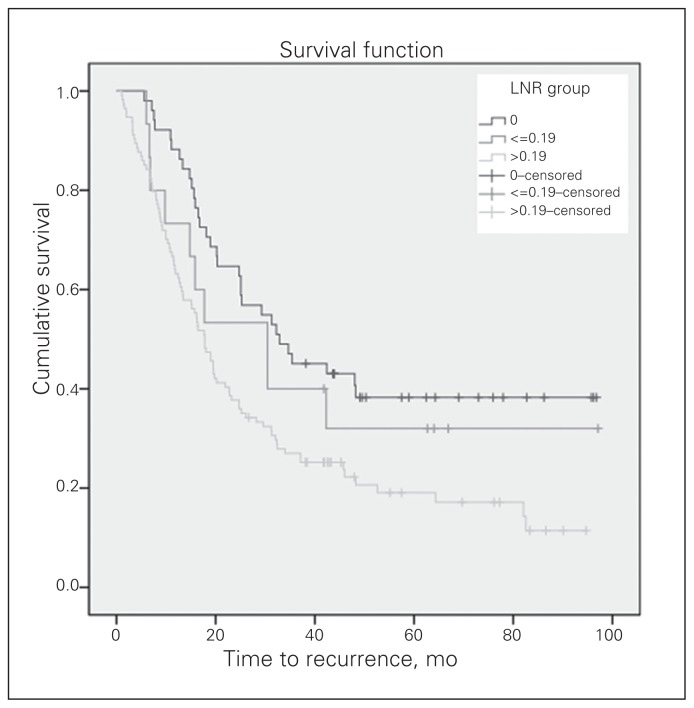
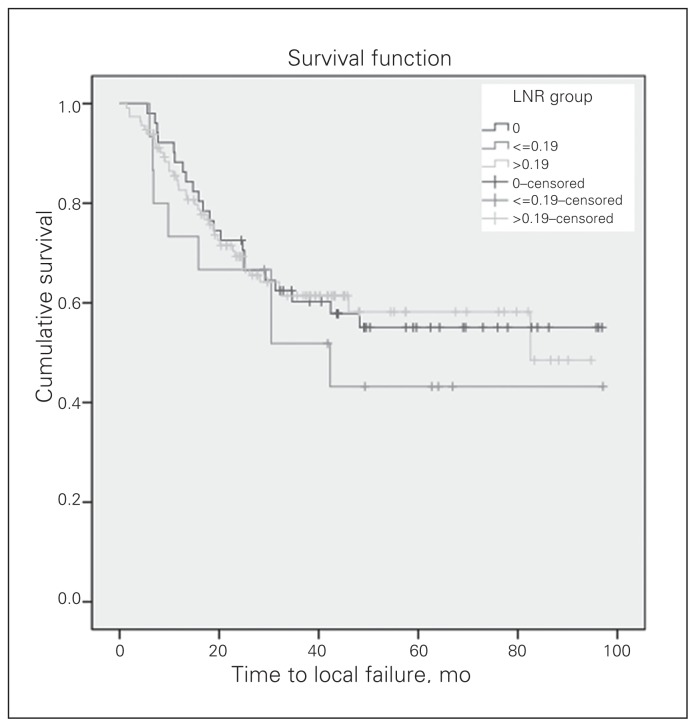
The median duration of follow-up was 41.8 (range 5.4–97.1) months. The 3-year OS and DFS for the 180 patients with locally advanced rectal cancer were 63% and 33%, respectively. The 3-year local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) were 60% and 53%, respectively. The OS curves of the 3 LNR groups differed significantly (Fig. 2). The median OS was 64.2 months for patients with an LNR of 0, 59.1 months for an LNR of 0.19 or less and 37.6 months for an LNR greater than 0.19 (p = 0.004). In addition, the DFS curves of the 3 LNR groups differed significantly (Fig. 3). The median DFS was 32.9 months for patients with an LNR of 0, 30.4 months for an LNR of 0.19 or less and 17.8 months for an LNR greater than 0.19 (p = 0.002). There was no significant difference in LRFS among the 3 groups (Fig. 4). The median DMFS was 30.4 months for patients with an LNR of 0.19 or less and 31.3 months for patients with an LNR greater than 0.19 (p = 0.006; Fig. 5).
Fig. 2.
The overall survival curve according to the groups by lymph note ratio (LNR). The median overall survival was 64.2 months for patients with an LNR of 0, 59.1 months for an LNR of 0.19 or less and 37.6 months for an LNR greater than 0.19 (p = 0.004).
Fig. 3.
The disease-free survival (DFS) curve according to the groups by lymph note ratio (LNR). The median DFS was 32.9 months for patients with an LNR of 0, 30.4 months for an LNR of 0.19 or less and 17.8 months for an LNR greater than 0.19 (p = 0.002).
Fig. 4.
The local recurrence-free survival curve according to the groups by lymph node ratio (LNR). There was no significant difference among the 3 groups (p = 0.64).
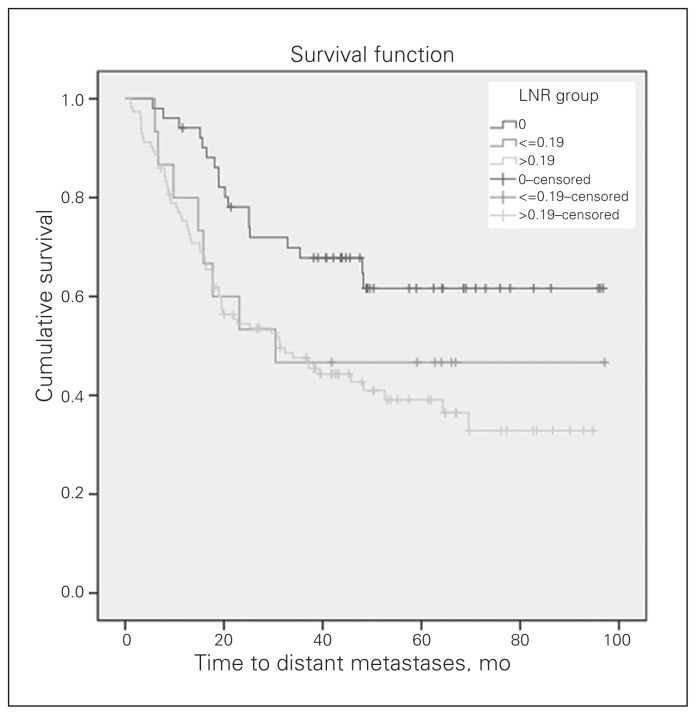
Fig. 5.
The distant metastasis-free survival (DMFS) curve according to the groups by lymph node ratio (LNR). The median DMFS was 30.4 months for patients with an LNR of 0.19 or less and 31.3 months for patients with an LNR greater than 0.19 (p = 0.006).
Univariate analysis showed that sex, age, tumour location and postoperative chemotherapy were not associated with improved OS (Table 2). However, the log-rank test showed that pathology, tumour differentiation, number of harvested LNs, LNR, N stages, TNM stage and postoperative radiotherapy had significant prognostic value in OS and DFS. Mucinous adenoma, poorly differentiated tumours, inadequate LN dissection (< 7 harvested LNs), higher LNR, higher N stage, higher TNM stage and no postoperative radiotherapy were associated with significantly decreased OS and DFS. The Cox regression analysis for OS showed that tumour differentiation (p = 0.026), LN examined (p = 0.030), LNR (p = 0.017) and postoperative radiotherapy (p < 0.001) were independent prognostic factors (Table 3).
Table 2.
Univariate analysis according to clinicopathological factors in advanced rectal cancer patients.
| Factor | No. of patients | Median OS, mo | p value | Median DFS, mo | p value |
|---|---|---|---|---|---|
| Sex | 0.22 | 0.08 | |||
| Male | 111 | 46.7 | 20.3 | ||
| Female | 69 | 40.2 | 20.2 | ||
| Age, yr | 0.60 | 0.35 | |||
| < 65 | 117 | 43.0 | 17.8 | ||
| ≥ 65 | 63 | 57.9 | 29.6 | ||
| Distance from anal verge, cm | 0.98 | 0.98 | |||
| < 7 | 111 | 44.7 | 21.9 | ||
| ≥ 7 | 69 | 42.2 | 19.5 | ||
| Pathological type | 0.042 | 0.024 | |||
| Canalicular adenoma | 157 | 45.5 | 23.2 | ||
| Mucinous adenoma | 23 | 34.5 | 16.4 | ||
| Differentiation | 0.007 | 0.022 | |||
| Well | 28 | 67.5 | 11.2 | ||
| Moderate | 138 | 46.7 | 23.2 | ||
| Poor | 14 | 33.4 | 17.7 | ||
| LNs examined | 0.001 | < 0.001 | |||
| < 12 | 83 | 39.4 | 15.8 | ||
| ≥12 | 97 | 59.1 | 32.2 | ||
| LNR | 0.004 | 0.002 | |||
| 0 | 51 | 64.2 | 32.9 | ||
| ≤ 0.19 | 15 | 59.1 | 30.4 | ||
| > 0.19 | 114 | 37.6 | 17.8 | ||
| pN stage | < 0.001 | < 0.001 | |||
| N0 | 46 | 68.5 | 35.4 | ||
| N1a | 10 | 52.9 | 15.8 | ||
| N1b | 36 | 59.1 | 16.4 | ||
| N1c | 12 | 26.7 | 12.7 | ||
| N2a | 35 | 46.7 | 34.0 | ||
| N2b | 41 | 27.2 | 20.2 | ||
| TNM stage | < 0.001 | 0.001 | |||
| II | 46 | 68.5 | 35.4 | ||
| IIIA | 6 | 75.7 | 24.7 | ||
| IIIB | 66 | 45.6 | 17.7 | ||
| IIIC | 62 | 29.7 | 15.8 | ||
| Postoperative chemotherapy | 0.48 | 0.22 | |||
| Yes | 168 | 45.2 | 20.2 | ||
| No | 12 | 19.7 | |||
| Postoperative radiotherapy | < 0.001 | < 0.001 | |||
| Yes | 101 | 59.6 | 34.0 | ||
| No | 79 | 37.6 | 15.1 |
DFS = disease-free survival; LNs = lymph nodes; LNR = lymph node ratio; OS = overall survival; pN = metastasis classification of lymph nodes; TNM = tumour-node-metastasis.
Table 3.
Multivariate analysis of clinicopathological factors associated with overall survival in patients with advanced rectal cancer
| Factor | β | SE | Wald | df | p value | HR (95%CI) |
|---|---|---|---|---|---|---|
| Pathology | −0.094 | 0.310 | 0.091 | 1 | 0.76 | 0.911 (0.496–1.672) |
| Differentiation | −0.546 | 0.245 | 4.968 | 1 | 0.026 | 0.579 (0.358–0.936) |
| LNs examined | 0.439 | 0.203 | 4.684 | 1 | 0.030 | 1.551 (1.042–2.307) |
| LNR | −0.535 | 0.224 | 5.732 | 1 | 0.017 | 0.585 (0.378–0.907) |
| pN stage | 0.202 | 0.104 | 3.785 | 1 | 0.05 | 1.224 (0.998–1.501) |
| TNM stage | 0.239 | 0.155 | 2.390 | 1 | 0.12 | 1.270 (0.938–1.721) |
| Postoperative radiotherapy | −0.869 | 0.194 | 20.067 | 1 | < 0.001 | 0.419 (0.287–0.613) |
CI = confidence interval; df = degrees of freedom; HR = hazard ratio; LN = lymph node; LNR = lymph node ratio; pN = metastasis classification of lymph nodes; SE = standard error; TNM = tumour-node-metastasis.
Discussion
Lymph node involvement is one of the most important prognostic factors in rectal cancer. The N stage is established according the number of involved regional nodes based on AJCC/UICC criteria. There is increasing evidence that the number of LNs alone may not enable adequate rectal cancer staging.2 Several factors may influence the total LN status, including surgeon skill in achieving total mesenteric excision and the quality assessment of their standard operating procedure, especially for preoperative neoadjuvant therapy, which might downgrade or upgrade pathological stage.10,11 Attention has now turned to more accurate pathological markers to help determine prognosis following rectal cancer resection. The metastatic LNR is the ratio of pathologically involved LNs to total resected LNs. The LNR has been shown to be an important prognostic factor in gastric, breast, pancreatic and CRC,12,13 but the value of LNR in different studies varies. The purpose of our study was to assess the impact of metastatic LNR on survival in patients with locally advanced rectal cancer, especially on OS, DFS, local failure and distant metastasis.
Huh and colleagues14 analyzed the data from a total of 514 patients who underwent curative surgery for CRC with proven LN metastases. Patients were categorized into 4 groups on the basis of quartiles: LNR1 (< 0.09), LNR2 (0.09–0.18), LNR3 (> 0.18 but < 0.34), and LNR4 (≥ 0.34). With a median follow-up of 48.5 months, the 5-year OS rates of patients with LNR1, LNR2, LNR3 and LNR4 were 79%, 72%, 62% and 55%, respectively (p < 0.001), while the 5-year DFS rates were 73%, 67%, 54% and 42%, respectively (p < 0.001). In the multivariate analysis, the LNR was an independent prognostic factor for both OS (p = 0.012) and DFS (p = 0.009), as were pT and pN. The LNR remained significant in patients with fewer than 12 or 12 or more retrieved LNs. Similarly, Lee and colleagues15 evaluated the prognostic effect of LNR in 154 patients with node-positive rectal cancer and found a prognostic impact of LNR (≤ 0.15, 0.16–0.3 and > 0.3) on 5-year OS (90.3%, 75.1%, and 45.1%, p < 0.001) and DFS (66.7%, 55.8%, and 21.9%, p < 0.001) in patients with fewer than 12 or 12 or more harvested LNs. In a study of 180 patients with stage III CRC, Xue and colleagues16 selected an LNR cutoff point of 0.17 because there was significant distant metastasis difference at that LNR. The LNR correlated independently with distant organ metastasis of CRC and serves as an important predicative factor for estimating prognosis.
In our study, the LNR was once again shown to be an independent predictor of survival in patients with locally advanced rectal cancer following multivariate analysis. Here we focused only on locally advanced rectal cancer (T3/4 or N+), used ROC curves to analyze the predictive value of the LNR, and determined a cutoff value of 0.19. We found that the OS and DFS curves of the 3 LNR groups differed significantly. The median OS was 64.2 months for patients with an LNR of 0, 59.1 months for an LNR of 0.19 or less and 37.6 months for an LNR greater than 0.19 (p = 0.004). In addition, the median DFS was 32.9 months for patients with an LNR of 0, 30.4 months for an LNR of 0.19 or less and 17.8 months for an LNR greater than 0.19 (p = 0.002). Most of the patients enrolled in our study were stage T3 or T4; in contrast, those who were stage T1 or T2 accounted for only 7.8% of the cohort. Perhaps this selection bias was the reason for there being no significant difference between the 2 groups in LRFS. However, we still found a difference between the 2 groups in DMFS. We guessed that T stage could have a more important impact on local recurrence than N stage. Moreover, we demonstrated that the cutoff of 12 LNs proposed by the AJCC/UICC as a prognostic threshold for correct nodal staging and stratification influences the OS and DFS. The LNR also significantly influenced the OS and DFS, as shown by both univariate and multivariate analysis. The LNR might be more accurate in predicting survival than pathology type and pN stage.
When stratified by LNR, such significant differences in survival for patients with similar pathological staging suggest marked heterogeneity of patients at each stage. Therefore, the LNR could be used to identify high-risk patients who are likely to benefit the most from adjuvant therapy. Following a retrospective analysis of 1098 patients who underwent CRC resection, Thomas and colleagues17 found that 41% were staged as Dukes C. Sixty-four percent of their patients received chemotherapy. Of the patients who received chemotherapy, 5-year survival was 69.3% for those with an LNR of 0.1 or less and 23.6% for those with an LNR of 0.61 or more. When no chemotherapy was given, the 5-year survival was 43.1% for those with an LNR of 0.1 or less and 8.7% for those with an LNR of 0.61 or more.
According to the current TNM staging system, at least 12 LNs are needed for accurate nodal staging. Neoadjuvant CRT with total mesorectal excision is the standard treatment for T3/4 and/or N1/2 rectal cancer, especially for low rectal cancer. Recent studies2,11,18–21 have demonstrated fibrosis and lymphocyte depletion were caused by radiotherapy and/or chemotherapy, probably because of LN atrophy,22 and the number of harvested LNs was frequently less than 12. Lee and colleagues15 found that fewer than 12 LNs were harvested in 30.5% of patients after preoperative CRT. A study based on the Surveillance, Epidemiology, and End Result registry showed that only 19% of patients with stage III rectal cancer had at least 12 retrieved LNs after preoperative CRT.23 The decreased LN yield in rectal carcinoma specimens after neoadjuvant radiochemotherapy has no prognostic relevance. In a study by Kang and colleagues24 involving 75 node-positive patients who underwent preoperative CRT followed by curative resection, patients were categorized into 2 groups based on a median LNR of 0.143. Patients with lower LNR had better OS. There was no difference in the survival rates of patients with higher LNR and those with N2 stage. We wonder if it is necessary to administer postoperative chemotherapy to those patients who achieve complete pathological response after neoadjuvant CRT. The LNR may help us select more suitable treatment for those patients, and these issues are additional future topics to be validated.
Limitations
Our study had several important limitations, including its relatively small sample size and retrospective design. However, there have been few reports on the prognostic value of LNR in patients with locally advanced disease. Moreover, LNR showed prognostic significance on multivariate analysis, and there were noticeable disparities among the LNR groups in the OS and DFS curves. In contrast to postoperative CRT, preoperative treatment has been shown in several studies to decrease LN yield, whereas other studies reported that preoperative treatment had no effect on LN yield. These results need further validation through a large-scale prospective study.
Conclusion
We have shown the LNR to be an important prognostic factor for both the OS and DFS of patients with rectal cancer. We also demonstrated that a ratio of 19% represents the LNR cutoff point for predicting the prognosis of patients with rectal cancer. The LNR can be used with pathological differentiation and pN stage to identify high-risk patients for postoperative treatment.
Acknowledgements
The authors thank the Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning.
Footnotes
Competing interests: None declared.
Contributors: D. Zhou and M. Ye designed the study. D. Zhou and L. Rong acquired the data, which D. Zhou, Y. Bai, L. Rong and Y. Hou analyzed. D. Zhou and M. Ye wrote the article, which all authors reviewed and approved for publication.
References
- 1.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. [accessed 2015 May 4]. http://seer.cancer.gov/csr/1975_2011/browse_csr.php?sectionSEL=6&pageSEL=sect_06_table.04.html.
- 2.Mekenkamp LJ, van Krieken JH, Marijnen CA, et al. Lymph node retrieval in rectal cancer is dependent on many factors–the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547–53. doi: 10.1097/PAS.0b013e3181b2e01f. [DOI] [PubMed] [Google Scholar]
- 3.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 4.Klos CL, Bordeianou LG, Sylla P, et al. The prognostic value of lymph node ratio after neoadjuvant chemoradiation and rectal cancer surgery. Dis Colon Rectum. 2011;54:171–5. doi: 10.1007/DCR.0b013e3181fd677d. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JW, Peeters KC, Putter H, et al. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM classification. Eur J Surg Oncol. 2010;36:1180–1186. doi: 10.1016/j.ejso.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Kim JH, Yoon SM, et al. Lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:796–802. doi: 10.1016/j.ijrobp.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 7.Peschaud F, Benoist S, Julie C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–73. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 8.Peng J, Xu Y, Guan Z, et al. Prognostic significance of the metastatic lymph node ratio in node-positive rectal cancer. Ann Surg Oncol. 2008;15:3118–23. doi: 10.1245/s10434-008-0123-8. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–63. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beresford M, Glynne-Jones R, Richman P, et al. The reliability of lymph-node staging in rectal cancer after preoperative chemoradiotherapy. Clin Oncol (R Coll Radiol) 2005;17:448–55. doi: 10.1016/j.clon.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Wichmann MW, Muller C, Meyer G, et al. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137:206–10. doi: 10.1001/archsurg.137.2.206. [DOI] [PubMed] [Google Scholar]
- 12.Danko ME, Bennett KM, Zhai J, et al. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1,788 patients with long-term follow-up. J Am Coll Surg. 2010;210:797–805 e791. doi: 10.1016/j.jamcollsurg.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Bhatti I, Peacock O, Awan AK, et al. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg. 2010;34:768–75. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 14.Huh JW, Kim YJ, Kim HR. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann Surg Oncol. 2010;17:2640–6. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee SD, Kim TH, Kim DY, et al. Lymph node ratio is an independent prognostic factor in patients with rectal cancer treated with preoperative chemoradiotherapy and curative resection. Eur J Surg Oncol. 2012;38:478–83. doi: 10.1016/j.ejso.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Xue H, Du X, Xiao C, et al. Predictive value of lymph node ratio for postoperative distant metastasis of stage III colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:458–62. [PubMed] [Google Scholar]
- 17.Thomas M, Biswas S, Mohamed F, et al. Dukes C colorectal cancer: Is the metastatic lymph node ratio important? Int J Colorectal Dis. 2012;27:309–17. doi: 10.1007/s00384-011-1340-3. [DOI] [PubMed] [Google Scholar]
- 18.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–34. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Baxter NN, Morris AM, Rothenberger DA, et al. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426–31. doi: 10.1016/j.ijrobp.2004.06.259. [DOI] [PubMed] [Google Scholar]
- 20.Sermier A, Gervaz P, Egger JF, et al. Lymph node retrieval in abdominoperineal surgical specimen is radiation time-dependent. World J Surg Oncol. 2006;4:29. doi: 10.1186/1477-7819-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rullier A, Laurent C, Capdepont M, et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 22.Marks JH, Valsdottir EB, Rather AA, et al. Fewer than 12 lymph nodes can be expected in a surgical specimen after high-dose chemoradiation therapy for rectal cancer. Dis Colon Rectum. 2010;53:1023–9. doi: 10.1007/DCR.0b013e3181dadeb4. [DOI] [PubMed] [Google Scholar]
- 23.Doll D, Gertler R, Maak M, et al. Reduced lymph node yield in rectal carcinoma specimen after neoadjuvant radiochemotherapy has no prognostic relevance. World J Surg. 2009;33:340–7. doi: 10.1007/s00268-008-9838-8. [DOI] [PubMed] [Google Scholar]
- 24.Kang J, Hur H, Min BS, et al. Prognostic impact of the lymph node ratio in rectal cancer patients who underwent preoperative chemoradiation. J Surg Oncol. 2011;104:53–8. doi: 10.1002/jso.21913. [DOI] [PubMed] [Google Scholar]