Abstract
Background
The study purpose was to examine and compare the effect of the first 18 months anastrozole therapy on cognitive function in women with breast cancer.
Methods
This large, longitudinal cohort study was composed of postmenopausal women with early-stage breast cancer who receive chemotherapy-plus-anastrozole (n=114) or anastrozole alone (n=173) and a control group (n=110). Cognitive function was assessed before systemic therapy and at six, 12, and 18 months after therapy initiation and at comparable timepoints in controls.
Results
The chemotherapy-plus-anastrozole and anastrozole alone groups had poorer executive function than controls at nearly all timepoints (p<.0001 to p=.09). A pattern of deterioration in working memory and concentration was observed during the first six months of anastrozole therapy for the chemotherapy-plus-anastrozole (p<.0001; p<.0009 respectively) and anastrozole alone groups (p=.0008; p=.0002 respectively). This was followed by improved working memory and concentration from six to 12 months in both groups. The anastrozole alone group had a second decline in working memory and concentration from 12 to 18 months post-initiation of therapy (p<.0001; p=.02).
Conclusion
Women with breast cancer had poorer executive functioning from pre-therapy through the entire first 18 months of therapy. A pattern of decline in working memory and concentration with initial exposure to anastrozole was observed. Women receiving anastrozole alone had a second deterioration in working memory and concentration from 12 to 18 months post-therapy initiation. The longer term (> 18 months) effects of anastrozole on cognitive function remain to be determined.
Keywords: Breast cancer, cognitive function, endocrine therapy, chemotherapy, anastrozole
Despite the fact that over 70% of women with breast cancer receive adjuvant endocrine therapy (ET), few studies have examined the specific influence of ET on cognitive function in this population. Most research on ET-associated cognitive changes focused on selective estrogen receptor modulators, particularly tamoxifen.1,2 Few studies have examined cognitive function with aromatase inhibitors (AI), more commonly used in postmenopausal women. To date, study results have been inconsistent partly because of methodological differences.3-10 Among the few prospective studies,8,11 sample sizes were small and some participants had begun ET at the baseline assessment; thus, there was no true pre-treatment cognitive evaluation. Finally, to our knowledge, no studies have examined the potential contribution of chemotherapy to the influence of ET on cognitive function in women with breast cancer.
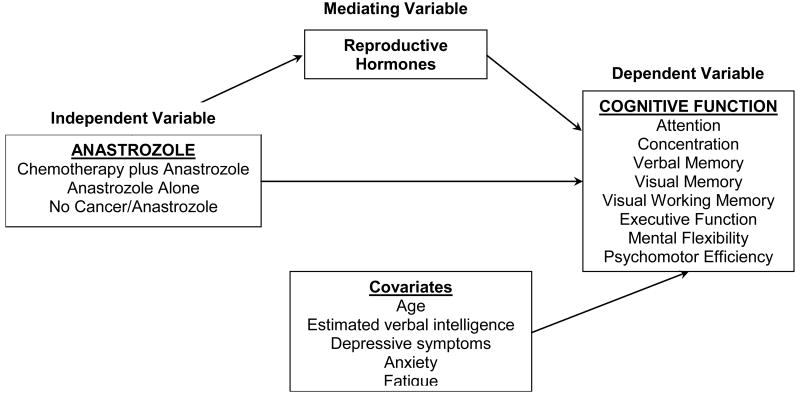
Multiple mechanisms likely underlie cognitive decline in women with breast cancer including changes in reproductive hormones. (Figure 1) AIs provide almost complete estradiol (E2) withdrawal by blocking the aromatase enzyme,12 and we found that lower E2 was associated with poorer psychomotor efficiency, attention and executive function with therapy13.
Figure 1. Hypothesized mechanism, the influence of anastrozole on cognitive function.
We also found poorer cognitive function with anastrozole compared to tamoxifen in a small, cross-sectional study.14 We now report the results of a large cohort study of postmenopausal women with early-stage breast cancer who receive chemotherapy-plus-anastrozole or anastrozole alone compared to a control group of women without breast cancer. The study purpose was to examine and compare the effect of anastrozole on cognitive function in these three cohorts before therapy and at six, 12, and 18 months after therapy commenced and at comparable time points in controls. We hypothesized that women with breast cancer would experience cognitive decline with anastrozole and that their cognitive function would be poorer than controls over time.
Methods
Women with breast cancer were recruited from the Comprehensive Breast Cancer Program of the University of Pittsburgh Cancer Institute between 2005 and 2012. Of the eligible women approached, 397 agreed to participate. Eligible women were newly diagnosed with stage I, II or IIIa breast cancer, scheduled to receive chemotherapy- plus-anastrozole (n=114) or anastrozole alone (n=173), postmenopausal, aged ≤75 years, and able to speak and read English with ≤8 years of education. Women were excluded with a history of neurological illness or cancer, reported hospitalization for psychiatric illness within 2 years, or evidence of metastases.
Age and education-matched controls without breast cancer (n=110) were recruited from the University Center for Social and Urban Research via random digit dialing, response to a local ad, or referral of a friend by breast cancer participants. Controls met the same participation criteria. All participants provided written informed consent; the study protocol was approved by the Institutional Review Board.
Design
Using a prospective, observational cohort, repeated-measure design, participants were evaluated following surgery but prior to beginning chemotherapy, if applicable, and anastrozole and at six month intervals up to 18-months after beginning anastrozole. (Table 1) The six month assessment in the chemotherapy-plus-anastrozole group occurred after chemotherapy and before anastrozole initiation. Controls were assessed at comparable timepoints. Demographic information was collected at baseline and treatment information was verified via the medical record.
Table 1.
Timepoints per group.
| Group | Pre-Chemotherapy | Pre-Anastrozole | 6 Months Post-Anastrozole Initiation | 12 Months Post-Anastrozole Initiation | 18 Months Post-Anastrozole Initiation |
|---|---|---|---|---|---|
| Chemotherapy-plus-Anastrozole | X | X | X | X | NA |
| Anastrozole Alone | NA | X | X | X | X |
| Controls | X | X | X | X | X |
NA, not applicable
Measures
Cognitive function was assessed with a standardized neuropsychological battery evaluating multiple cognitive domains. Cognitive tests were selected based on established sensitivity to cognitive changes in this population and the availability of alternate equivalent versions used with the controls' scores to mitigate practice effects at follow-up testing.14 The comprehensive battery was administered and scored by nurses trained by a licensed clinical neuropsychologist and was comprised of 13 measures, some yielding multiple scores. (Table 2) Because of the number of cognitive variables, we applied a data reduction technique to decrease the risk of type I error. Exploratory factor analysis with principal component extraction and orthogonal rotation was applied to the 29 scores derived from the measures to reduce dimensionality and cluster scores. Eight factors were derived, accounting for 71% of the total variance. Individual measures with the highest loadings were included in each factor. Measures had factor loadings >.400; factors and scores composing each factor are listed in Table 4. We reversed the direction of some scores (timed, errors) so that higher mean scores indicated better cognitive performance. Cognitive factors were derived as a mean of the individual measures Z-score transformed relative to the controls' baseline values.
Table 2. Summary of neuropsychological tests and outcome variables.
| Domain | Test | Outcomes | Range |
|---|---|---|---|
|
| |||
| Attention | Digit Vigilance47 | Time | 0+ |
| Errors | 0+ | ||
|
| |||
| CANTAB Rapid Visual Information Processing40 | Total hits | 0+ | |
| A prime | 0 to 1 | ||
| Mean latency | 0+ | ||
|
| |||
| Learning and memory | CANTAB Paired Associates Learning40 | Stages completed | 0 to 10 |
| Errors | 0+ | ||
|
| |||
| CANTAB Spatial Working Memory40 | Strategy | 8 to 56 | |
| Errors | 0+ | ||
|
| |||
| Rivermead Story Recall48 | Immediate | 0 to 21 | |
| Delayed | 0 to 21 | ||
|
| |||
| Rey Auditory Verbal Learning49 | Number correct (Total) | 0 to 15 | |
| Number correct (Delay) | 0 to 15 | ||
| Number correct (Trial 6) | 0 to 15 | ||
|
| |||
| Rey Complex Figure Immediate50 | Number accurate elements | 0 to 36 | |
| Rey Complex Figure Delayed50 | Number accurate elements | 0 to 36 | |
|
| |||
| Executive Function | CANTAB Stockings of Cambridge40 | Mean initial thinking time (5 moves) | 0+ |
| Mean subsequent thinking time (5 moves) | 0+ | ||
| Number problems solved | 0+ | ||
|
| |||
| D-KEFS Verbal Fluency51 | Number correct | 0+ | |
|
| |||
| Mental flexibility | Trail Making Test-B52 | Time | 0 to 240 |
|
| |||
| D-KEFS Color-Word Interference51 | Inhibition (scaled score) | 1 to 19 | |
| Inhibition/switching (scaled score) | 1 to 19 | ||
| Scaled score #1 + #2 | 2 to 38 | ||
| Composition scaled score | 1 to 19 | ||
|
| |||
| Psychomotor efficiency | Grooved Pegboard53 | Insertion time, dominant hand | 0+ |
| Insertion time, non-dominant hand | 0+ | ||
|
| |||
| Digit Symbol Substitution54 | Number correct | 0 to 133 | |
|
| |||
| Visuospatial ability | Rey Complex Figure Copy50 | Number of accurate elements | 0 to 36 |
CANTAB, Cambridge Neuropsychological Test Automated Battery; D-KEFS, Delis Kaplan Executive Function System.
Table 4. Differences in Factor and Individual Neuropsychological Scores Among Groups at Enrollment.
| Factors and Individual Tests | Chemotherapy + Anastrozole (1) (n = 114; 27.1%) |
Anastrozole Alone (2) (n = 173; 43.1%) |
Controls (0) (n = 110; 29.6%) |
Statistics and Post Hoc Comparisons |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Verbal Memory | −0.21 (0.69) | −0.30 (0.67) | −0.11 (0.75) | F(2,394)=2.5, p=.085 |
| Rey AVLT: total | 55.2 (8.14) | 52.9 (8.10) | 54.7 (9.52) | F(2,394)=2.9, p=.057 |
| Rey AVLT: interference | 11.3 (2.71) | 10.8 (2.82) | 10.8 (3.00) | F(2,394)=1.2, p=.307 |
| Rey AVLT: delay | 11.2 (2.80) | 10.7 (2.88) | 10.7 (3.05) | F(2,394)=0.9, p=.391 |
| Verbal Fluency Test: total | 39.5 (11.91) | 39.1 (11.48) | 39.6 (11.50) | F(2,392)=0.1, p=.934 |
| Rivermead Story: immediate recall | 7.2 (2.76) | 7.4 (2.37) | 8.4 (2.88) | F(2,226)=6.3, p=.002; 1,2<0 |
| Rivermead Story: delayed recall | 5.7 (2.81) | 5.8 (2.35) | 7.5 (2.82) | F(2,225)=16.0, p<.001; 1,2<0 |
|
| ||||
| Mental Flexibility | 0.16 (0.68) | 0.08 (0.84) | 20.1 (4.67) | F(2,394)=4.3, p=.015; 0<1 |
| Color Word Interference: 1+2-scaled score | 22.7 (3.51) | 21.8 (4.58) | 10.2 (2.39) | F(2,244)=10.7, p<.001; 0<1,2 |
| Color Word Interference: composition-scaled score | 11.6 (1.78) | 11.1 (2.33) | 11.3 (2.34) | F(2,244)=12.1, p<.001; 0<1,2 |
| Color Word Interference: inhibition/switching #4-norming method scaled score | 11.4 (2.25) | 11.2 (2.47) | 10.7 (2.35) | F(2,393)=0.2, p=.811 |
| Color Word Interference: inhibition #3-norming method scaled score | 10.8 (2.49) | 11.1 (2.52) | F(2,393)=1.4, p=.258 | |
|
| ||||
| Psychomotor Efficiency | −0.04 (0.85) | −0.22 (0.93) | −0.09 (0.86) | F(2,394)=1.7, p=.184 |
| Grooved Pegboard: non-dominant hand time | 91.0 (20.30) | 93.7 (23.91) | 91.8 (24.15) | F(2,382)=0.5, p=.597 |
| Grooved Pegboard: dominant hand time | 79.0 (17.46) | 83.9 (20.98) | 80.7 (16.86) | F(2,388)=2.4, p=.093 |
| Digit Symbol Substitution | 70.5 (14.03) | 68.7 (12.98) | 70.2 (12.85) | F(2,394)=0.8, p=.441 |
|
| ||||
| Attention | −0.23 (1.01) | −0.22 (1.01) | −0.06 (0.88) | F(2,388)=1.1, p=.320 |
| Rapid Visual Information Processing: total hits | 16.6 (4.53) | 16.9 (4.89) | 17.7 (4.59) | F(2,388)=1.5, p=.220 |
| Rapid Visual Information Processing: A' | 0.90 (0.048) | 0.90 (0.05) | 0.91 (0.05) | F(2,387)=1.6, p=.202 |
| Rapid Visual Information Processing: mean latency | 466.6 (125.53) | 472.1 (108.95) | 464.2 (93.37) | F(2,387)=0.2, p=.829 |
|
| ||||
| Visual Memory | 0.14 (0.50) | 0.01 (0.73) | −0.08 (0.91) | F(2,235)=3.0, p=.053 |
| CANTAB Paired Associate Learning: stages completed | 4.9 (0.30) | 4.9 (0.41) | 4.8 (0.56) | F(2,233)=2.2, p=.116 |
| CANTAB Paired Associate Learning: errors-adjusted | 19.8 (14.05) | 25.3 (21.90) | 23.1 (24.99) | F(2,238)=3.5, p=.032; no significant post hoc contrasts |
| Rey Complex Figure: copy | 32.6 (2.79) | 32.5 (3.10) | 31.8 (3.06) | F(2,394)=2.3, p=.097 |
|
| ||||
| Executive Function | −0.33 (0.67) | −0.47 (0.61) | −0.07 (0.71) | F(2,394)=12.7, p<.001; 1,2<0 |
| CANTAB Stockings of Cambridge: mean initial thinking time-5 moves | 9,899.5 (8,461.51) | 10,795.5 (8,254.06) | 15,322.7 (9,697.61) | F(2,393)=12.8, p<.001; 1,2<0 |
| CANTAB Stockings of Cambridge: problems solved, minimum moves | 7.8 (1.93) | 7.9 (1.76) | 8.6 (1.75) | F(2,394)=6.29, p=.002; 1,2<0 |
| CANTAB Spatial Working Memory: errors | 37.3 (17.91) | 43.4 (16.49) | 37.1 (17.96) | F(2,394)=6.2, p=.002; 2>0,1 |
| CANTAB Spatial Working Memory: strategy | 34.7 (5.83) | 36.7 (5.08) | 34.4 (5.67) | F(2,394)=7.6, p=.001; 2>0,1 |
|
| ||||
| Visual Working Memory | 0.06 (0.70) | −0.11 (0.85) | −0.08 (0.85) | F(2,394)=1.5, p=.222 |
| CANTAB Stockings of Cambridge: mean subsequent thinking time-5 moves | 1,857.6 (2,059.06) | 3,172.7 (5,290.80) | 2,749.1 (4,270.86) | F(2,230)=5.4, p=.005; 2>1 |
| Rey Complex Figure: delayed recall | 21.1 (6.19) | 20.6 (5.80) | 20.5 (6.49) | F(2,392)=0.4, p=.665 |
| Rey Complex Figure: immediate recall | 22.0 (6.40) | 21.5 (5.92) | 21.6 (6.54) | F(2,394)=0.2, p=.795 |
|
| ||||
| Concentration | −0.09 (0.80) | 0.01 (0.90) | −0.003 (0.87) | F(2,391)=0.5, p=.617 |
| Digit Vigilance: time | 177.9 (34.30) | 177.1 (35.60) | 174.3 (35.74) | F(2,391)=0.3, p=.712 |
| Digit Vigilance: errors | 3.9 (4.62) | 4.7 (5.09) | 4.2 (4.41) | F(2,391)=0.8, p=.445 |
We also examined potential covariates of cognitive function including age, and well-validated measures of estimated verbal intelligence (National Adult Reading Test-Revised15), depressive symptoms (Beck Depression Inventory-II16), anxiety (Profile of Mood States Tension/Anxiety subscale17) and fatigue (Profile of Mood States Fatigue/Inertia subscale17). Age and estimated verbal intelligence were assessed at baseline in all groups; depressive symptoms, anxiety and fatigue were assessed at all study timepoints.
Statistical Analysis
Descriptive statistics were generated to characterize the groups and identify any data anomalies that may have invalidated planned analyses. Groups were compared on categorical descriptors with chi-square tests and continuous characteristics using analysis of variance.
We performed mixed effects modeling adjusting for age and estimated verbal intelligence using z-scores, accommodating data that were missing at random. Where we found significant group, time, or group-by-time effects, we examined differences between groups and changes over time and calculated effect sizes for significant differences. To control for multiple comparisons, we established a conservative significance level at p<.01. Due to the potential influence of practice effects, we applied a standard regression-based approach where applicable; data from the controls were used to adjust for practice effects in the treatment groups.
Results
Table 3 shows the sample characteristics at enrollment. The anastrozole alone group was older (p<.001) and controls had higher estimated intelligence scores (p<.001). The chemotherapy-plus-anastrozole group had higher disease stage than the anastrozole alone group (p<.001) and greater anxiety compared to both groups (p<.001).
Table 3. Sample characteristics at enrollment (N=397).
| Characteristic | Chemotherapy-plus-Anastrozole (n = 114) |
Anastrozole Alone (n = 173) |
Controls (n = 110) |
P | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| M | SD | M | SD | M | SD | ||
|
|
|||||||
| Age | 59.2 | 5.5 | 61.8 | 6.5 | 58.6 | 6.1 | <.001 |
|
| |||||||
| Education | 14.8 | 2.9 | 14.9 | 2.8 | 14.9 | 2.9 | .950 |
|
| |||||||
| NART-R | 107.6 | 9.2 | 108.4 | 8.7 | 112.4 | 9.1 | <.001 |
|
| |||||||
| Race, n (%) | .041 | ||||||
| White | 107 | 93.9 | 169 | 97.7 | 100 | 90.9 | |
| Black | 7 | 6.1 | 4 | 2.3 | 10 | 9.1 | |
|
| |||||||
| Stage, n (%) | <.001 | ||||||
| I | 45 | 39.5 | 149 | 86.6 | n/a | n/a | |
| IIa | 38 | 33.3 | 19 | 11.0 | n/a | n/a | |
| IIb | 19 | 16.7 | 4 | 2.3 | n/a | n/a | |
| IIIa | 12 | 10.5 | 0 | 0.0 | n/a | n/a | |
|
| |||||||
| BDI-II | 6.6 | 6.83 | 5.2 | 5.89 | 5.6 | 6.33 | .192 |
|
| |||||||
| POMS tension-anxiety | 9.6 | 6.21 | 6.8 | 5.10 | 6.9 | 6.10 | <.001 |
|
| |||||||
| POMS fatigue-inertia | 5.7 | 5.33 | 5.5 | 6.08 | 5.6 | 5.66 | .783 |
M, mean; SD, standard deviation; NART-R, National Adult Reading–Revised; BDI-II, Beck Depression Inventory II; POMS, Profile of Mood States.
Differences at enrollment in factor z scores and individual neuropsychological test scores are shown in Table 4. Before therapy, women with breast cancer performed worse than controls on measures of mental flexibility (p<.01). In contrast, women who would receive chemotherapy-plus-anastrozole had better executive function than controls (p=.016). The groups did not differ at pre-therapy on the other cognitive factors.
Cognitive Function
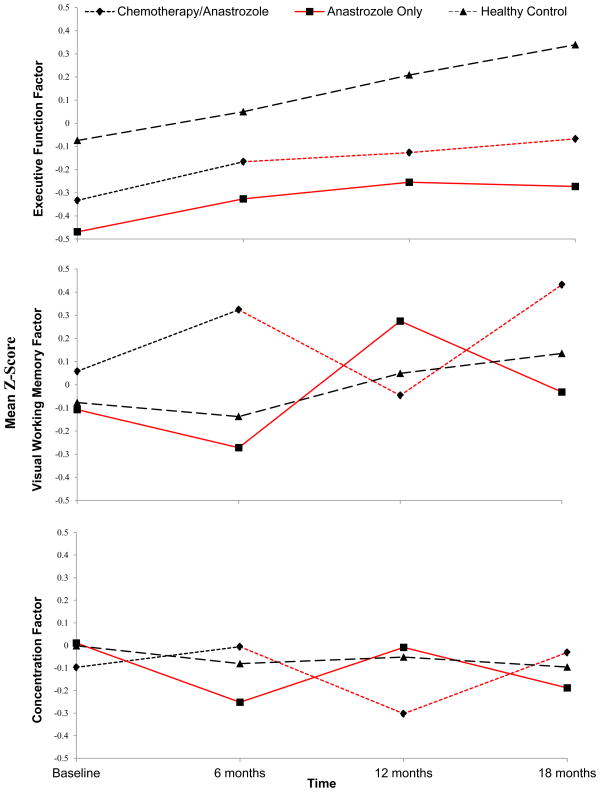
Controlling for age and estimated intelligence, we found that the controls had better executive function than the anastrozole alone group at pre-therapy (p=.001, d=.14); and six (p=.002, d=.12), 12 (p=.0001, d=.14) and 18 (p<.0001, d=.16) months post-therapy initiation (Figures 2a-2c). Similarly, there was a trend toward the controls performing better than the chemotherapy-plus-anastrozole groups at pre-chemotherapy (p=.04, d=.08), and six months (p=.09, d=.06), and controls performed significantly better at 12 (p=.005, d=.10) and 18 (p=.001, d=.11) months.
Figure 2.
a–2c Results for the anastrozole alone group were shifted for comparison due to the lack of a pre-chemotherapy assessment in that group. Red indicates exposure to anastrozole.
2a. Group response profile, executive function factor over 18 months.
2b Group response profile, visual working memory factor over 18 months.
2c. Group response profile, concentration factor over 18 months.
We also found significant group, (p=.004) time (p<.0001) and group-by-time (p<.0001) effects for visual working memory and group-by-time (p=.0005) effects for concentration. Both the anastrozole alone and chemotherapy-plus-anastrozole groups showed a pattern of decline during the first six months of anastrozole for these factors. We observed decline in visual working memory in the first six months of therapy (p=.0008; d=.15) in the anastrozole alone group; this was followed by improvement from six to 12 months (p<.0001; d=.45) and another decline from 12 to 18 months (p<.0001; d=.24). After initial improvement in visual working memory during chemotherapy, the chemotherapy-plus-anastrozole group also displayed a deterioration during the first six months of anastrozole (p<.0001; d=.26) followed by improvement in function from 12 to 18 months (p<.0001; d=.32). Performance of the controls improved from six to 12 months (p=.003). Similarly, we observed a deterioration in concentration from pre-therapy to six months post-therapy initiation in the anastrozole alone group (p=.0002; d=.17), an improvement from six to 12 months (p=.001; d=.15), and a trend toward a decline from 12 to 18 months (p=.02; d=.12). In the chemotherapy-plus-anastrozole group, we observed a deterioration in concentration during the first six months of anastrozole (p<.0009; d=.15) followed by improvement from 12 to 18 months (p=.008; d=.14). No change in concentration was observed in controls.
There were also group differences for visual memory (p=.002); the controls performed more poorly than the chemotherapy-plus-anastrozole groups at pre-therapy (p=.004) and the anastrozole alone and chemotherapy-plus-anastrozole groups at 18 months, (p=.001, p=.009 respectively) and controls were poorer than the chemotherapy-plus-anastrozole group at 12 months (p=.002). Similarly, there were group (p<.0001) and group-by-time (p=.00006) effects for mental flexibility with the controls performing more poorly than the chemotherapy-plus-anastrozole and anastrozole alone groups at pre-therapy (p<.0001; p=.0007 respectively). There was also improved performance in verbal memory and psychomotor efficiency for all groups, likely demonstrating practice effects.
Discussion
In this first large cohort study to comprehensively assess cognitive function over 18 months, we found that, compared to controls, women who received anastrozole alone or chemotherapy-plus-anastrozole had significantly poorer executive function from pre-therapy through the first 18 months of treatment. We also found a consistent pattern of changes in visual working memory and concentration with therapy.
Poorer Executive Function
Women in both breast cancer groups had poorer executive functioning before and during therapy that does not appear to be influenced by treatment. Multiple mechanisms may explain this persistently poorer executive functioning including changes in inflammatory cytokines, neurotransmitter dysregulation, stress and mood.18 We found depressive symptoms to be related to executive function over time but this relationship did not substantively change the pattern of results. Executive functioning is critical for planning, organizing and decision making and impairment of this domain can have a deleterious effect on one's ability to perform effectively at work and socially.
Pre-chemotherapy to Pre-Anastrozole
At pre-chemotherapy, women with breast cancer who would receive chemotherapy had had a trend toward better visual working memory compared to controls and their performance improved at the pre-anastrozole assessment suggesting practice effects. There was no change in the controls on this factor.
Pre-Anastrozole to Six Months Post-Anastrozole Initiation
There was a significant deterioration in visual working memory and concentration in both the chemotherapy-plus-anastrozole and anastrozole alone groups with the first six months of anastrozole. Compared to controls, women who received chemotherapy-plus-anastrozole had a trend toward poorer performance at six months post-anastrozole initiation. Controls had no change in performance in these factors. Reductions in reproductive hormones that occur with AIs may explain this initial decline in performance in both treatment groups.
Six to Twelve Months Post-Anastrozole Initiation
Paradoxically, the deterioration in visual working memory and concentration that occurred with the initial six months of anastrozole was followed by improved performance in these domains at 12 months. Compared to controls, women in the chemotherapy-plus-anastrozole and anastrozole alone groups performed better at 12 months post-anastrozole initiation. It is not clear why women with breast cancer have improved performance in these domains during this interval. Their reproductive hormone levels likely remain low with continued therapy. This may reflect compensation for the cognitive changes initially experienced.
We explored whether cognitive reserve contributed to this improvement. Cognitive reserve theory postulates that intelligence, education, mental activity and social engagement mitigate or compensate for cognitive deterioration.19,20 In our study, higher estimated verbal intelligence was highly significantly correlated with better cognitive function in all domains. Therefore, we explored whether cognitive reserve, assessed via estimated verbal intelligence (NART-R scores classified as IQ ≤ 110 or > 110) explained this pattern. We found that NART-R classification moderated the group-by-time effect for visual working memory (p=.05) but not concentration, such that performance of women receiving anastrozole alone with higher estimated intelligence had better working memory than those with lower estimated intelligence (p=.05). Therefore, greater cognitive reserve may partially explain the improvement observed with respect to visual working memory.
Twelve to Eighteen Months Post-Anastrozole Initiation
However, from 12 to 18 months, the anastrozole alone group again exhibited a decline in working memory and a trend toward a deterioration in concentration. If cognitive reserve theory provides a plausible explanation for the improvement in working memory and concentration observed from 6 to 12 months, the deterioration in working memory at 18 months suggests that the ability of variables such as intelligence and education to mitigate the effects of therapy on cognitive function diminish over time. Another plausible mechanism for these later cognitive declines may be the additive effect of chronic stress associated with the cancer diagnosis and treatment resulting in changes in the prefrontal regions.21 The domains affected suggest a central neurotoxicity with some specificity to the prefrontal cortices and hippocampus which are supported by imaging studies.22,23 Initial exposure to anastrozole and the secondary hypoestrogenism might reduce brain metabolism24,25 and synaptic connectivity,26,27 leading to cognitive decline.28 Hypocortisolemia from stress also might independently reduce brain metabolism and synaptic density. Thus, the combination of stress and hypoestrogenism may compromise cognitive function in domains such as working memory and concentration.29 Initially, the brain may have sufficient reserve to be able to generate new cognitive strategies, but with persistent hypoestrogenism, with or without stress, even alternative neural pathways may be compromised30.
To explore this possibility, we controlled for depression, anxiety and fatigue over time in the mixed effects modeling and found that higher anxiety was related to poorer visual working memory (p=.04). Based on this finding, we compared anxiety scores between groups and explored changes over time and found a group-by-time interaction for the chemotherapy-plus-anastrozole group, indicating that these women had significantly more anxiety at baseline, improved to show no differences compared to the other groups at six months, and then became more anxious than the other groups from 12 to 18 months. No differences in anxiety were found between the anastrozole-alone and control groups, with anxiety scores generally decreasing over 18 months. These results point to an association between anxiety and visual working memory for women who received chemotherapy-plus-anastrozole, but do not fully explain the trajectory of this cognitive factor. Neither depressive symptoms nor fatigue were consistently associated with the cognitive function factors at any timepoint. While these results lend some support to the relationship between chronic stress and the deterioration in cognitive function in women receiving adjuvant therapy, they do not fully explain our results. It is important to keep in mind that a measure of anxiety (POMS Tension/Anxiety subscale) may not be an optimal surrogate of chronic stress. Ultimately, these results point to a need for further exploration of this potential mechanism with more sensitive approaches to the assessment of stress including use of biomarkers and neuroimaging techniques.
Studies of cognitive function with ET in breast cancer have yielded conflicting results. Tamoxifen has been associated with deteriorations in visual and verbal memory, verbal ability, processing speed, and visuospatial ability.7,31-33 The evidence for cognitive changes with AIs is less clear, in part because few studies have examined cognitive function exclusively with AIs. Moreover, methodological concerns and differences hinder efforts to compare results across studies. Samples in some earlier studies were heterogeneous, combining pre and postmenopausal women3,10,11,32 and women who received AIs with women who received tamoxifen.8,10,33,34 Several studies had small samples3,31-33,35,36 and lacked control groups that are essential for comparison and isolation of the influence of practice effects.3,10,11,37.
Different approaches to cognitive assessment may explain the contradictory results. Some studies employed cognitive screening, providing information about global cognitive changes but failing to detect subtle changes more commonly experienced or to identify changes in specific cognitive domains.1 Other studies relied on self-report of cognitive problems6,38 or used measures that were initially developed to assess gross cognitive disorders in patients with stroke, neuro-trauma, or dementia.3,7,10,11,39 We included measures from the CANTAB,40 a computerized battery comprised of challenging cognitive tasks that may be more sensitive to these subtle changes. Importantly, several earlier studies employed a cross-sectional design4,31-33,36,37 and in some longitudinal studies no true “pre-therapy” assessment was made because many participants had already begun ET therapy at baseline3,8,11,41 or received chemotherapy before the initial cognitive assessment.10 With these designs, it is not possible to discern whether cognitive impairments existed before therapy or if there were cognitive changes with AI therapy. Our results indicate that women with breast cancer have poorer executive function before they begin therapy, demonstrating the importance of longitudinal designs that include assessments before initiation of any systemic therapy including chemotherapy.
Finally, conflicting results across longitudinal studies may reflect differences in the timing of follow-up assessments.7,10 Our study is the first to report assessments at six month intervals up to 18 months post-initiation of ET.
With the exception of the poorer executive function for the anastrozole alone group versus controls before the AI initiation (d=0.61), most effects sizes for differences between patients and controls were small to medium (i.e., d<0.4). Studies using objective neuropsychological tests have shown subtle cognitive declines during AI therapy. These effects may reflect the level of sensitivity of some study measures to subtle cognitive changes experienced by women with breast cancer.42,43 These subtle cognitive changes may decrease women's ability to perform in cognitively challenging situations.44
Although the cohorts differed in age, estimated intelligence and anxiety at pre-therapy, these differences are likely not clinically meaningful. Furthermore, we controlled for age and intelligence in our analysis, and the level of anxiety in the chemotherapy-plus-anastrozole group (mean=9.8) is within the normative value for adult women (mean=9.2).45
Strengths of this study include the longitudinal design, inclusion of a pre-therapy assessment and the ability to examine the potential additive influence of chemotherapy to the effect of AIs on cognitive function. The study is limited by a sample predominantly composed of white, well-educated women, limiting generalizability.
Additional research is needed to examine cognitive function across the entire trajectory of AI therapy and to determine whether cognitive function improves following treatment completion. Interventions to attenuate cognitive decline are also needed. Physical activity interventions may be of particular benefit because they are associated with improved working memory, executive function and psychomotor efficiency in older adults, the very cognitive domains that deteriorate with adjuvant therapy use in breast cancer46.
Acknowledgments
Research is supported by the National Cancer Institute (R01 CA 107408)
Footnotes
There are no potential conflicts of interest.
Contributor Information
John Merriman, University of Pittsburgh.
Amanda Gentry, University of Pittsburgh.
Gretchen Ahrendt, University of Pittsburgh Medical Center.
Sarah Berga, Wake Forest School of Medicine.
Adam Brufsky, University of Pittsburgh Medical Center.
Frances Casillo, University of Pittsburgh.
Meredith Dailey, University of Pittsburgh.
Kirk Erickson, University of Pittsburgh.
Frances Kratofil, University of Pittsburgh.
Priscilla McAullife, University of Pittsburgh Medical Center.
Margaret Rosenzweig, University of Pittsburgh.
Christopher Ryan, University of Pittsburgh.
Susan Sereika, University of Pittsburgh.
References
- 1.Legault C, Maki PM, Resnick SM, Coker L, Hogan P, Bevers TB, Shumaker SA. Effects of tamoxifena and ralixifene on memory and other cognitive abilities: Cognition in the study of tamoxifen and raloxifene. Journal of Clinical Oncology. 2009;31:5144–5152. doi: 10.1200/JCO.2008.21.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nystedt M, Berglund G, Bolund C, Fornander T, Rutqvist LE. Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: A prospective randomized study. Journal of Clinical Oncology. 2003;21(9):1836–1844. doi: 10.1200/JCO.2003.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients. Cancer. 2008;113(9):2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A Pilot Study. Psycho-Oncology. 2004;13(1):61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KA, Aldridge J, Ribi K, et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1-98 trial. Breast Cancer Research & Treatment. 2011;126(1):221–226. doi: 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurria A, Patel SK, Mortimer J, Luu T, Somlo G, Katheria V, Ramani R, Hansen K, Feng T, Chuang C, Geist CL, Silverman DHS. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clinical Breast Cancer. 2014;14(2):132–140. doi: 10.1016/j.clbc.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schilder CM, Seyaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Veldem CJ, van Dam FS, Schagen SB. Effects of tamoxifen and Exemestane on cognitive functioning of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of Clinical Oncology. 2010;28(8):1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 8.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psycho-Oncology. 2009;18:811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 9.Lejbak L, Vrbancic M, Crossley M. Endocrine therapy is associated with low performance on some estrogen-sensitive cognitive tasks in postmenopausal women with breast cancer. Journal of Clinical and Experimental Neuropsychology. 2010;32(8):836–846. doi: 10.1080/13803391003596389. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA, Petersen L, Castellon SA, et al. Cognitive Function After the Initiation of Adjuvant Endocrine Therapy in Early-Stage Breast Cancer: An Observational Cohort Study. J Clin Oncol. 2014 Sep 29; doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper-Wynne C, Ross G, Sacks N, et al. Effects of the aromatase inhibitor letrozole on normal breast epithelial cell proliferation and metabolic indices in postmenopausal women: a pilot study for breast cancer prevention. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(7):614–621. [PubMed] [Google Scholar]
- 13.Bender CM, Sereika SM, Ryan CM, Brufsky AM, Berga SL. Cognitive function and reproductive hormones in women receiving anastrozole. Presented at the 34th Annual San Antonio International Breast Cancer Symposium; 2011; San Antonio, TX. [Google Scholar]
- 14.Bender CM, Sereika S, Brufsky A, et al. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early stage breast cancer. Menopause. 2007;14(6):995–998. doi: 10.1097/gme.0b013e318148b28b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson H. Nelson Adult Reading Test (NART) manual. Windsor: NFER-Nelson; 1981. [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 17.McNair D, Lorr M, Droppleman LF. EdITS Manual for the Profile of Mood States. San Diego: EdITS/Educational and Industrial Testing Service; 1992. [Google Scholar]
- 18.Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer- and treatment-related cognitive changes. Semin Oncol Nurs. 2013 Nov;29(4):260–269. doi: 10.1016/j.soncn.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreotti C, Root JC, Ahles TA, McEwen BS, Compas BE. Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psychooncology. 2014 Oct 6; doi: 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurria A, Lachs M. Is cognitive dysfunction a complication of adjuvant chemotherapy in the older patient with breast cancer? Breast Cancer Research and Treatment. 2007;103(3):259–268. doi: 10.1007/s10549-006-9383-9. [DOI] [PubMed] [Google Scholar]
- 23.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behavior and Immunity. 2013 Mar 15;30:S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DH. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One. 2014;9(3):e89095. doi: 10.1371/journal.pone.0089095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman DHS, G CL, Kenna HA, Williams K, Wroolie T, Powers B, Brooks J, Rasgon NL. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36(4):502–513. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao J, Janssen WG, Tang Y, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. The Journal of comparative neurology. 2003 Oct 27;465(4):540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Janssen WGM, Hao J, Roberts JA, McKay H, Lasley B, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen Replacement Increases Spinophilin-immunoreactive Spine Number in the Prefrontal Cortex of Female Rhesus Monkeys. Cereb Cortex. 2004;14(2):215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 28.Weber M, R L, Maki P. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20(5):511–517. doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010 Jan 28;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 30.Luine VN. Estradiol and cognitive function: past, present and future. Hormones and behavior. 2014 Sep;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of Clinical & Experimental Neuropsychology. 2004;26(7):955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 32.Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. Journal of Cancer Survivors. 2008;2(4):275–282. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- 33.Schilder CM, Eggens SPC, Seyaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, Van Dam FS, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: Cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncologica. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 34.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008 Jun;16(6):577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 35.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psycho-Oncology. 2009;18(8):811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 36.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. NeuroImage. 2004;21(1):364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Research & Treatment. 2000;64(2):165–176. doi: 10.1023/a:1006426132338. [DOI] [PubMed] [Google Scholar]
- 38.Ribi K, Aldridge J, Phillips KA, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AA, Goldhirsch A, Price KN, Gelber RD, Bernhard J, the the BIG I-98 Collaborative Group and the International Breast Cancer Study Group (IBCSG) Subjective cognitive complaints one year after ceasing adjuvant endocrine treatment for early-stage breast cancer. British Journal of Cancer. 2012;106:1618–1625. doi: 10.1038/bjc.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins VA, Ambrosine LM, Atkins L, Cuzick J, Howell A, Fallowfield LJ. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II) Lancet Oncology. 2008;9(10):953–961. doi: 10.1016/S1470-2045(08)70207-9. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TW, James M, Owen A, Sahakian BJ, McInnes L, Rabbitt PM. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 41.Mar Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. Journal of Clinical Oncology. 2005;23(31):8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 42.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011 Jul;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 43.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006 May;15(5):422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 44.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 45.McNair D, Lorr M, Droppleman LF. EdITS Manual for the Profile of Mood States. San Diego: EdITS/Educational and Industrial Testing Service; 1992. [Google Scholar]
- 46.Erickson KI. Therapeutic effects of exercise on cognitive function. J Am Geriatr Soc. 2013 Nov;61(11):2038–2039. doi: 10.1111/jgs.12529. [DOI] [PubMed] [Google Scholar]
- 47.Lafayette clinical repeatable neuropsychological test battery. Sagamore: Lafayette Clinical Instrument Company; 1989. [Google Scholar]
- 48.Cockburn J, Smith PT. Correlates of everyday memory among residents of Part III homes. British Journal of Clinical Psychology. 1993;32(Pt 1):75–77. doi: 10.1111/j.2044-8260.1993.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 49.Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Archives de Psychologie. 1964;122:382–340. [Google Scholar]
- 50.Osterrieth PA. Test of copying a complex figure; contribution to the study of perception and memory. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- 51.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan (D-KEFS) Executive Function System, Examiners Manual. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 52.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills. 1958;8:271–276. [Google Scholar]
- 53.Klove H. Clinical neuropsychology. In: Forster FM, editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- 54.Wechsler D. The Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]