Abstract
We report herein discrete triplex hybridization of DNA and RNA with polyacrylates. Length-monodisperse triazine-derivatized polymers were prepared on gram-scale by reversible addition–fragmentation chain-transfer polymerization. Despite stereoregio backbone heterogeneity, the triazine polymers bind T/U-rich DNA or RNA with nanomolar affinity upon mixing in a 1:1 ratio, as judged by thermal melts, circular dichroism, gel-shift assays, and fluorescence quenching. We call these polyacrylates “bifacial polymer nucleic acids” (bPoNAs). Nucleic acid hybridization with bPoNA enables DNA loading onto polymer nanoparticles, siRNA silencing delivery, and can further serve as an allosteric trigger of RNA aptamer function. Thus, bPoNAs can serve as tools for both non-covalent bioconjugation and structure–function nucleation. It is anticipated that bPoNAs will have utility in both bio- and nanotechnology.
The growing importance of nucleic acids in biotechnology1 and materials2 presents a need for well-defined methods to bridge native and artificial architectures. One conceptual approach to this goal involves the synthesis of polymers capable of biomimetic molecular recognition of nucleic acids. Polyacrylate analogues of nucleic acids were first reported in 1966 by Jones,3,4 followed by many other alternate backbones,5 including polyester, polyvinyl,6 and polyamide,7 presaging peptide nucleic acid (PNA)8 and other nucleic acid backbone replacement studies,9 although the hybridization was poorly defined and inefficient.10 These and other11 polymer nucleic acid analogues require several days of incubation with DNA to yield a hypochromic shift and exhibit a thermal transition. Recent studies using controlled living radical polymerization to produce nucleic acid mimics12 and hydrogen-bonding polymers have focused on fully artificial assemblies.13 Notably, nucleic acid hybridization with length-monodisperse polymers presenting non-native bases has not been studied. We describe herein “bifacial polymer nucleic acids” (bPoNAs), a family of low-polydispersity polyacrylates that engage T/U oligonucleotide tracts with nanomolar affinity via a synthetic triazine14,15 base-triple interface. In contrast to prior efforts to bind polymers to DNA via Watson–Crick base pairing, we find that biomimetic high-affinity, well-defined bPoNA–DNA triplex hybridization occurs upon mixing, enabling nonelectrostatic polymer nanoparticle loading, RNA silencing delivery, and RNA aptamer turn-on, thus demonstrating the possible applications and functionality of these constructs.
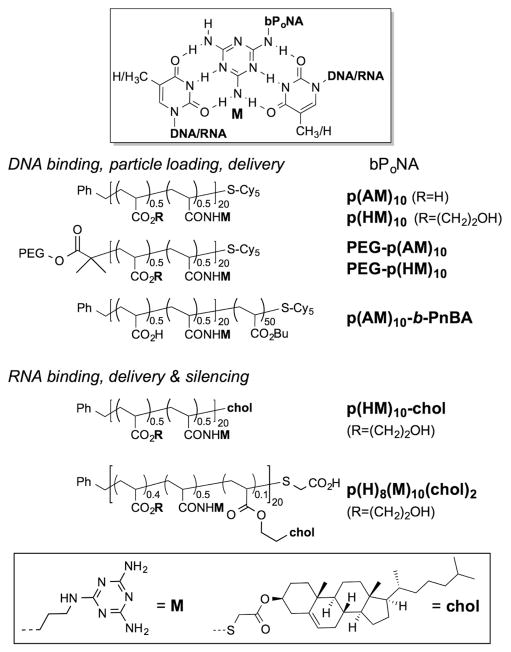
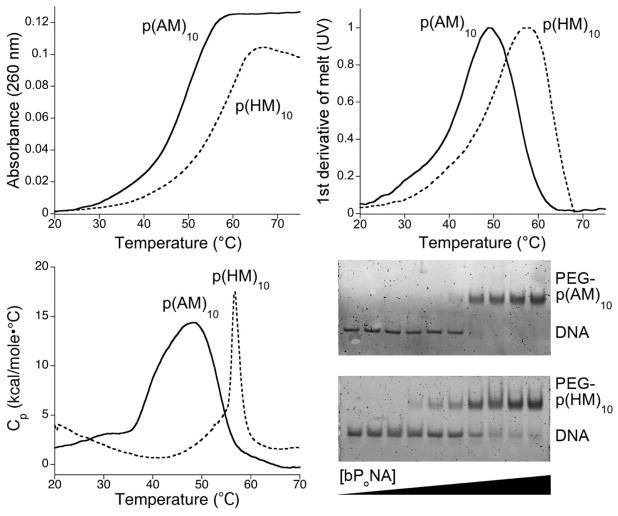
Triaminotriazine (melamine) can recognize thymine/uracil hydrogen-bonding patterns16–19 in both organic20,21 and aqueous milieu13,22–29 and facilitates functional binding to DNA and RNA on a peptide30–34 backbone. We hypothesized that polymer-displayed melamine could drive discrete polyacrylate–DNA triplex hybridization (Figure 1) despite regio-and stereochemical heterogeneity in the carbon backbone. Polyacrylates from tert-butyl and N-hydroxysuccinimidyl (NHS) ester monomers were prepared using reversible addition–fragmentation transfer (RAFT) polymerization.35 Amidation of NHS sites with aminoalkyl melamines (M), tert-butyl ester cleavage to give the acid (A), and fluorescent end labeling with Cy535,36 produced anionic p(AM)10 (Figure 1). Complexation of p(AM)10 to T-rich DNA (dT10C10T10) was reflected in a strong hypochromic shift of the DNA UV absorbance upon mixing. This polymer–DNA complex melted cooperatively at 49 °C (Figure 2). Electrostatic repulsion with DNA was reduced by replacement of the anionic carboxylate (A) with the neutral 2-hydroxyethyl (H) side chain, yielding bPoNA p(HM)10. Though the p(HM)10–DNA complex is more thermally stable (Tm = 59 °C), differential scanning calorimetry (DSC) analysis revealed that binding of DNA to p(AM)10 was more exothermic (−228 kcal/mol) than to p(HM)10 (−62 kcal/mol). The limited solubility of p(HM)10 complicates deeper analysis. Strongly exothermic assembly37 is consistent with melamine–thymine triplex base stacking.30,31 Indeed, loss of three M sites decreased the thermal stability of the polymer–DNA complex by ~10 °C, while a loss of seven M sites completely abolished DNA binding. Similarly, loss of thymine content via T → C substitutions in DNA rapidly degraded polymer complexation (Figures S2–S5 in the Supporting Information). Steric sensitivity was also observed: shortening of the dC10 linker by six nucleotides (dT10C4T10) resulted in total loss of DNA binding. Furthermore, shortening or lengthening of the polymer side chain by just one CH2 unit led to an ~8 °C decrease in the thermal stability of the bPoNA–DNA complexes (Figure S1). In view of the heterogeneity of the bPoNA backbone, the sensitivity of polyacrylate–DNA complexation to subtle structural perturbations is remarkable and reflects a well-defined molecular interaction governed primarily by the side-chain environment.
Figure 1.
(top) Melamine (M)-driven triplex hybridization of bifacial polymer nucleic acid (bPoNA) with T/U tracts in DNA and RNA. (bottom) Structures of bPoNA studied as DNA and RNA folding and delivery agents. PEG = 5 kDa.
Figure 2.
Cooperative and discrete exothermic assembly of bPoNAs p(AM)10 and p(HM)10 with dT10C10T10 DNA, as indicated by (top left) thermal denaturation curves, (top right) their normalized first derivative curves (UV), and (bottom left) DSC. (bottom right) Native PAGE of dT10C10T10 at a constant concentration with increasing levels of PEG diblock bPoNA.
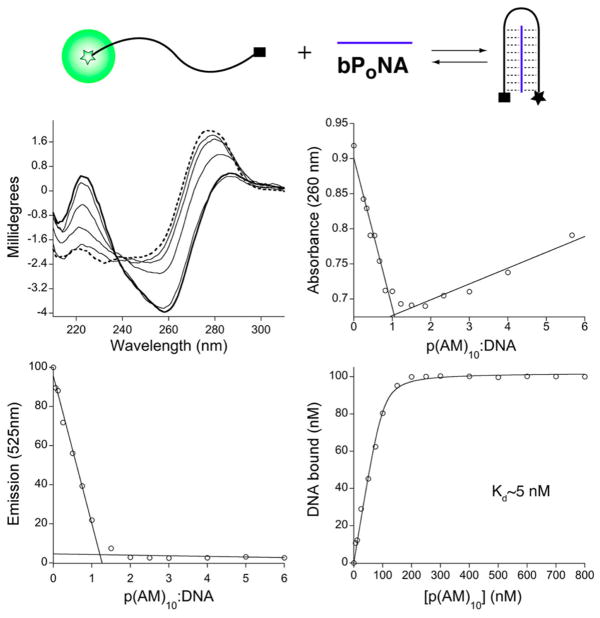
The (AM)10 and (HM)10 DNA complexes did not survive native polyacrylamide gel electrophoresis (PAGE), possibly because of nonspecific interactions with the gel. Sterically protected43 polymers (Figure 1) were prepared using a poly(ethylene glycol) (PEG)-derivatized chain-transfer agent.44 The resulting [PEG-bPoNA]–DNA complexes could be observed on gel, reflective of defined polymer–DNA hybridization (Figure 2). Discrete two-state complexation was further indicated by an isodichroic point (237 nm) in the circular dichroism (CD) spectra of dT10C10T10 upon titration with p(AM)10, amid inversion of a positive CD band at 277 nm into a negative signal at 260 nm and a positive absorption at 220 nm (Figure 3). The polymer itself exhibits negligible CD under these conditions. Binding of p(AM)10 with a dT10C10T10 DNA that was 3′- and 5′-derivatized with dabcyl and fluorescein resulted in full fluorescein quenching at a 1:1 ratio, thus providing support for the triplex hairpin binding model. This binding ratio was corroborated by a UV Job plot. Furthermore, fluorescence quenching and anisotropy curves fit well to a 1:1 binding model, yielding Kd = 2–5 nM (Figure 3). These data, together with the DSC results, indicate high-affinity enthalpically driven bPoNA–DNA triplex hybridization.
Figure 3.
Titration of p(AM)10 with dT10C10T10. (middle left) Change in free DNA CD (---) upon treatment with increasing p(AM)10 (—). (middle right) Job plot from UV absorbance. (bottom left) Job plot from fluorescence quenching using DNA with terminal fluorophore and quencher, as illustrated at in the scheme at the top. (bottom right) Fluorescence quenching isotherm fit to a 1:1 binding model (—).
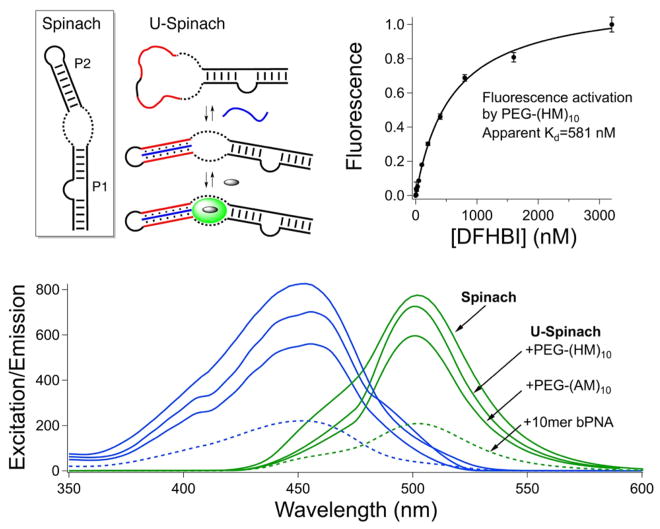
The functional compatibility of polymer hybrid triplex stems with aptamer folds was studied using a mutant of the RNA aptamer Spinach,38 which can capture a fluorogenic small molecule, 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), within a G-quadruplex binding pocket,39,40 eliciting green emission. Replacement of stem P2 with an unstructured U10CACAU10 loop, as in U-Spinach, ablates DFHBI binding and fluorescence; bifacial PNA (bPNA) can fold the U-loop into a triplex hybrid P2 stem and restore ~30% of the DFHBI emission intensity in the peptide–RNA hybrid complex.33 Strikingly, the polymer analogues p(AM)10, PEG-p(AM)10, and PEG-p(HM)10 can rescue aptamer function in U-Spinach with greater efficiency than the peptide (Figure 4), with excitation and emission intensities closer to those of the binary Spinach–DFHBI complex. Furthermore, the polymer–RNA hybrids activate DFHBI fluorescence with apparent Kd values of ~0.5 μM, similar to Spinach. The neutral PEG-(HM)10 is the most efficient fluorescence trigger, reflective of enhanced affinity over the negatively charged p(AM)10 polymers. The functional compatibility of PEG diblock bPoNA hybrid stems with folded RNA elements is likely to be general;33 bPoNA could thus be useful for non-covalent conjugation of aptamer modules with polymer carrier platforms for targeted delivery.41,42
Figure 4.
Aptamer turn-on by bifacial polymer nucleic acid. (top left) Spinach aptamer fold, with the fluorogen binding site shown as a dashed line; refolding of U-Spinach by bPoNA (blue) and DFHBI binding, with U tracts shown in red. For clarity, the PEG block is not indicated. (top right) Fluorescence activation of DFHBI by the bPoNA–U-Spinach binary complex, fit to a 1:1 binding model. (bottom) Excitation (blue) and emission (green) spectra for the indicated DFHBI complexes. The excitation and emission intensities follow the same trend.
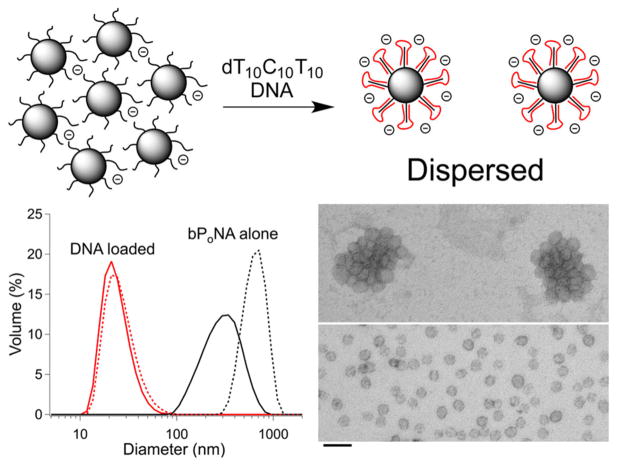
We set out to test the extent to which bPoNA could be used to connect nucleic acids to other synthetic architectures useful for delivery, such as polymer nanoparticles and lipids. The diblock polyacrylate amphiphile p(AM)10-b-PnBA (Figure 1) was constructed and found to form ~300 nm particles in PBS. Addition of dT10C10T10 spontaneously dispersed the polymer assembly into ~20 nm particles that remained stable over days in buffer, as judged by dynamic light scattering (DLS) and ambient transmission electron microscopy (TEM) (Figure 5). These data support binding of DNA to bPoNA strands on the particle surface, leading to dispersion through increased electrostatic repulsion. Sedimentation studies revealed that up to 62% of solution DNA could be cosedimented with polymer depending on the DNA thymine content (Table S3). These DNA-loaded particles were readily taken up by HEK-293 and MCF-7 cells in culture (Figure S14), indicating robust conjugation of DNA to diblock bPoNA nanoparticles. We probed the utility of bPoNA nanoparticles as vehicles for siRNA knockdown in HeLa cells stably expressing firefly and renilla luciferase.45 However, although an optimized46 sense/antisense RNA duplex targeted to firefly luciferase could be loaded onto diblock bPoNA nanoparticles via 3′- and 5′-terminal rU10 tracts, no silencing activity was observed.
Figure 5.
(top) Aggregated bPoNA nanoparticles disperse upon binding of DNA (red). (bottom left) DLS of p(AM)10-b-PnBA nanoparticles with (red) and without (black) DNA loading after 6 h (—) and 72 h (---). (bottom right) TEM images of uranyl acetate-stained particles clustered without DNA and dispersed after DNA binding. The scale bar is 100 nm.
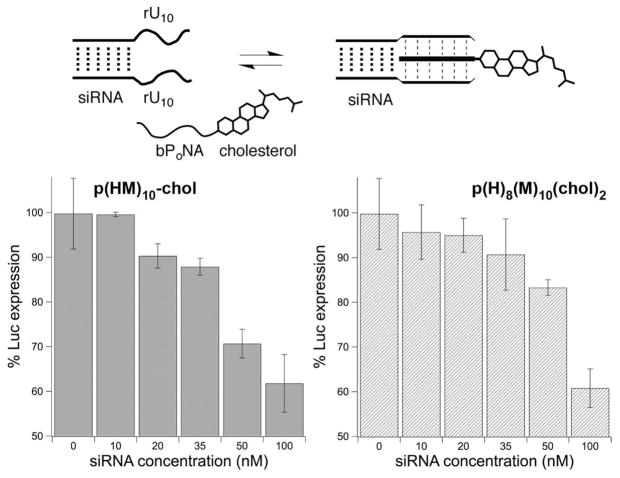
Binding to the siRNA duplex by sterol-derivatized bPoNA was confirmed by the emergence of a new melting transition at ~65 °C, representing the triplex hybrid domain (Figure S7). We therefore speculated that inefficient RNA release from the nanoparticle was preventing silencing. We prepared instead bPoNA hybrids for non-covalent lipidation of U-tract siRNA duplexes, which was expected to facilitate silencing.47 The cholesterol-modified bPoNAs p(HM)10-chol and p-(H)8(M)10(chol)2 (Figure 1) were synthesized to achieve duplex lipidation either via polymer end (p(HM)10-chol) or brush (p(H)8(M)10(chol)2) functionalization. Gratifyingly, dose-dependent knockdown of up to 40% luciferase silencing was observed using both of these charge-neutral carriers, similar to that observed47 with covalent siRNA lipidation (Figure 6). Notably, the same U-tract siRNA duplex can be functionalized with different bPoNA carriers, enabling facile evaluation of lipid polymers without preparation of new RNA derivatives. Thus, nucleic acid loading and delivery functions can be integrated into a single (neutral) polymer synthesis without covalent nucleic acid modification48,49 or the use of cationic components. This affords greater control over charge tuning in nucleic acid delivery systems.50,51
Figure 6.
(top) Concept of non-covalent siRNA lipidation with bPoNA-chol. (bottom) Luciferase silencing in HeLa-Luc cells upon delivery of siRNA at optimized polymer:RNA ratios of 5:1 and 10:1 using (left) p(HM)10-chol and (right) p(H)8(M)10(chol)2 as polymer carriers.
Taken together, these studies describe discrete, well-defined, and predictable triplex hybridization of T/U-rich DNA and RNA with bifacial polymer nucleic acids (bPoNAs). Unlike peptide synthesis or biological expression, the products of radical copolymerization are structurally diverse as a result of uncontrolled stereochemistry and monomer distribution. Despite this heterogeneity, triazine–thymine docking decisively drives assembly, eliciting recognition properties reminiscent of rigorously defined chemical entities. Rapid, biomimetic DNA/RNA docking on the abiotic triazine base-triple interface sets bPoNA apart from prior studies on polymer-displayed native nucleobases. bPoNAs thus provide access to discrete molecular binding motifs of T/U-rich DNA and RNA using polymer synthesis from cheaply available starting monomers. This scalable and well-defined assembly strategy enables seamless integration of polymer architectures with DNA and RNA and their use in aptamer turn-on, delivery, and siRNA silencing. We anticipate that bPoNAs may be tuned at the monomer level to accommodate a wide range of bio- and nanotechnology applications.
Supplementary Material
Acknowledgments
This research was supported in part by NSF-DMR and NIH. We are grateful to Alnylam Pharmaceuticals for sharing the dual-luciferase HeLa cell line.
Footnotes
Notes
The authors declare no competing financial interest.
Detailed experimental procedures and additional data. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b05481.
References
- 1.Burnett JC, Rossi JJ. Chem Biol. 2012;19:60. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roh YH, Ruiz RCH, Peng S, Lee JB, Luo D. Chem Soc Rev. 2011;40(12):5730. doi: 10.1039/c1cs15162b. [DOI] [PubMed] [Google Scholar]
- 3.Jones AS, Taylor N. Nature. 1967;215:505. doi: 10.1038/215505a0. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy F, Jones AS. Eur Polym J. 1966;2:319. [Google Scholar]
- 5.Jones AS. Int J Biol Macromol. 1979;1:194. [Google Scholar]
- 6.Boguslawski S, Olson PE, Mertes MP. Biochemistry. 1976;15:3536. doi: 10.1021/bi00661a022. [DOI] [PubMed] [Google Scholar]
- 7.Gait MJ, Jones AS, Jones MD, Shepherd MJ, Walker RT. J Chem Soc, Perkin Trans. 1979;1:1389. [Google Scholar]
- 8.Nielsen PE. Acc Chem Res. 1999;32:624. [Google Scholar]
- 9.Eschenmoser A. Science. 1999;284:2118. doi: 10.1126/science.284.5423.2118. [DOI] [PubMed] [Google Scholar]
- 10.Kaye H. J Am Chem Soc. 1970;92:5777. doi: 10.1021/ja00722a062. [DOI] [PubMed] [Google Scholar]
- 11.Lo PK, Sleiman HF. Macromolecules. 2008;41:5590. [Google Scholar]
- 12.McHale R, O’Reilly RK. Macromolecules. 2012;45:7665. [Google Scholar]
- 13.De Greef TFA, Smulders MMJ, Wolffs M, Schenning A, Sijbesma RP, Meijer EW. Chem Rev. 2009;109:5687. doi: 10.1021/cr900181u. [DOI] [PubMed] [Google Scholar]
- 14.Mittapalli GK, Reddy KR, Xiong H, Munoz O, Han B, De Riccardis F, Krishnamurthy R, Eschenmoser A. Angew Chem, Int Ed. 2007;46:2470. doi: 10.1002/anie.200603207. [DOI] [PubMed] [Google Scholar]
- 15.Vysabhattar R, Ganesh KN. Tetrahedron Lett. 2008;49:1314. [Google Scholar]
- 16.Lange RFM, Beijer FH, Sijbesma RP, Hooft RWW, Kooijman H, Spek AL, Kroon J, Meijer EW. Angew Chem, Int Ed Engl. 1997;36:969. [Google Scholar]
- 17.Zhou Z, Bong D. Langmuir. 2013;29:144. doi: 10.1021/la304457y. [DOI] [PubMed] [Google Scholar]
- 18.Thomas R, Kulkarni GU. Beilstein J Org Chem. 2007;3:17. doi: 10.1186/1860-5397-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arambula JF, Ramisetty SR, Baranger AM, Zimmerman SC. Proc Natl Acad Sci U S A. 2009;106:16068. doi: 10.1073/pnas.0901824106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitesides GM, Simanek EE, Mathias JP, Seto CT, Chin D, Mammen M, Gordon DM. Acc Chem Res. 1995;28:37. [Google Scholar]
- 21.Prins LJ, Reinhoudt DN, Timmerman P. Angew Chem, Int Ed. 2001;40:2382. doi: 10.1002/1521-3773(20010702)40:13<2382::aid-anie2382>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki T, Tokuhiro M, Kimizuka N, Kunitake T. J Am Chem Soc. 2001;123:6792. doi: 10.1021/ja010035e. [DOI] [PubMed] [Google Scholar]
- 23.Ariga K, Kunitake T. Acc Chem Res. 1998;31:371. [Google Scholar]
- 24.Ma M, Paredes A, Bong D. J Am Chem Soc. 2008;130:14456. doi: 10.1021/ja806954u. [DOI] [PubMed] [Google Scholar]
- 25.Ma M, Gong Y, Bong D. J Am Chem Soc. 2009;131:16919. doi: 10.1021/ja9072657. [DOI] [PubMed] [Google Scholar]
- 26.Ma M, Bong D. Acc Chem Res. 2013;46:2988. doi: 10.1021/ar400065m. [DOI] [PubMed] [Google Scholar]
- 27.Ma M, Bong D. Org Biomol Chem. 2011;9:7296. doi: 10.1039/c1ob05998j. [DOI] [PubMed] [Google Scholar]
- 28.Ma M, Bong D. Langmuir. 2011;27:8841. doi: 10.1021/la201415d. [DOI] [PubMed] [Google Scholar]
- 29.Ma M, Bong D. Langmuir. 2011;27:1480. doi: 10.1021/la104405r. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Y, Pratumyot Y, Piao X, Bong D. J Am Chem Soc. 2012;134:832. doi: 10.1021/ja2099326. [DOI] [PubMed] [Google Scholar]
- 31.Piao X, Xia X, Bong D. Biochemistry. 2013;52:6313. doi: 10.1021/bi4008963. [DOI] [PubMed] [Google Scholar]
- 32.Xia X, Piao X, Fredrick K, Bong D. Chem Bio Chem. 2014;15:31. doi: 10.1002/cbic.201300536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia X, Piao X, Bong D. J Am Chem Soc. 2014;136:7265. doi: 10.1021/ja5032584. [DOI] [PubMed] [Google Scholar]
- 34.Piao X, Xia X, Mao J, Bong D. J Am Chem Soc. 2015;137:3751. doi: 10.1021/jacs.5b00236. [DOI] [PubMed] [Google Scholar]
- 35.Keddie DJ, Moad G, Rizzardo E, Thang SH. Macromolecules. 2012;45:5321. [Google Scholar]
- 36.Bandyopadhyay S, Xia X, Maiseiyeu A, Mihai G, Rajagopalan S, Bong D. Macromolecules. 2012;45:6766. doi: 10.1021/ma301536f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SantaLucia J, Jr, Hicks D. Annu Rev Biophys Biomol Struct. 2004;33:415. doi: 10.1146/annurev.biophys.32.110601.141800. [DOI] [PubMed] [Google Scholar]
- 38.Paige JS, Wu KY, Jaffrey SR. Science. 2011;333:642. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang H, Suslov NB, Li NS, Shelke SA, Evans ME, Koldobskaya Y, Rice PA, Piccirilli JA. Nat Chem Biol. 2014;10:686. doi: 10.1038/nchembio.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner KD, Chen MC, Song W, Strack RL, Thorn A, Jaffrey SR, Ferré-D’Amaré AR. Nat Struct Mol Biol. 2014;21:658. doi: 10.1038/nsmb.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh SS, Lee BF, Leibfarth FA, Eisenstein M, Robb MJ, Lynd NA, Hawker CJ, Soh HT. J Am Chem Soc. 2014;136:15010. doi: 10.1021/ja5079464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farokhzad OC, Karp JM, Langer R. Expert Opin Drug Delivery. 2006;3:311. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 43.Immordino ML, Dosio F, Cattel L. Int J Nanomed. 2006;1:297. [PMC free article] [PubMed] [Google Scholar]
- 44.Bartels JW, Cauet SI, Billings PL, Lin LY, Zhu J, Fidge C, Pochan DJ, Wooley KL. Macromolecules. 2010;43:7128. doi: 10.1021/ma1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DWY, Soutschek J, Toudjarska I, Vornlocher H-P, Zimmermann TS, Langer R, Anderson DG. Nat Biotechnol. 2008;26:561. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JE, Heo I, Tian Y, Simanshu DK, Chang H, Jee D, Patel DJ, Kim VN. Nature. 2011;475:201. doi: 10.1038/nature10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Röhl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky D, Limmer S, Manoharan M, Vornlocher HP. Nature. 2004;432:173. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 48.Peterson AM, Heemstra JM. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:282. doi: 10.1002/wnan.1309. [DOI] [PubMed] [Google Scholar]
- 49.Kedracki D, Chekini M, Maroni P, Schlaad H, Nardin C. Biomacromolecules. 2014;15:3375. doi: 10.1021/bm5008713. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen J, Szoka FC. Acc Chem Res. 2012;45:1153. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grijalvo S, Aviñó A, Eritja R. Expert Opin Ther Pat. 2014;24:801. doi: 10.1517/13543776.2014.915944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.