Abstract
Background
Postmenopausal women with breast cancer (BC) receiving aromatase inhibitors are at increased risk for bone loss. The current study was undertaken to determine whether upfront versus delayed treatment with zoledronic acid (ZA) impacted bone loss. This report describes the 5-year follow-up results.
Methods
551 postmenopausal women with BC completing tamoxifen and undergoing daily letrozole treatment were randomized to upfront (274) or delayed (277) ZA 4 mg IV every 6 months. In the delayed arm, ZA was initiated for post-baseline bone mineral density (BMD) T-score < -2.0 or fracture.
Results
The incidence of a 5% decrease in total lumbar spine BMD at 5 years was 10.2% in the upfront arm versus 41.2% in the delayed arm, p < 0.0001. 41 patients in the delayed arm were eventually started on ZA. With the exception of increased grade 1/2 elevated creatinine and fever in the upfront arm and cerebrovascular ischemia in the delayed arm, there were no significant differences between arms with respect to the most common adverse events of arthralgia and back pain. Osteoporosis occurred less frequently in the upfront arm (2 versus 8 cumulative cases) though this difference was not statistically significant. Bone fractures occurred in 24 patients in the upfront arm versus 25 patients in the delayed arm.
Conclusions
Immediate treatment with ZA prevented bone loss compared with delayed treatment in postmenopausal women on letrozole and these differences were maintained at 5 years. The incidence of osteoporosis or fractures was not different between arms.
Keywords: zoledronic acid, bone loss, postmenopausal, breast cancer, letrozole, tamoxifen
Introduction
Aromatase inhibitors (AIs) are routinely incorporated in the adjuvant setting for postmenopausal women with hormone receptor-positive breast cancer (BC).1 Several trials have demonstrated that treatment with AIs leads to decreases in bone density2-8 and, with the exception of the MA.17 study2, lower bone density corresponded with an increased risk of fracture, although none of the studies were specifically designed to evaluate this endpoint.
The role of administering bisphosphonates to prevent bone pathology in women undergoing treatment with AIs remains controversial. Zoledronic acid (ZA) is an intravenous bisphosphonate approved for the prevention and treatment of osteoporosis in postmenopausal women. In the final results of the Z-fast trial, that randomized 602 patients with hormone receptor-positive BC receiving adjuvant letrozole to either upfront or delayed ZA for 5 years, the adjusted mean differences in lumbar spine and total hip bone mineral density (BMD) between the upfront and delayed treatment arms were 8.9% and 6.7%, respectively (P < .0001 for both), with improved bone density in the upfront ZA arm.9 Criteria for the delayed group to initiate ZA included a lumbar spine or total hip T-score less than -2 or a non-traumatic clinical fracture.
The current study had a similar study design to the Z-FAST trial.10 The early (one year) findings from our trial demonstrated that upfront treatment with ZA prevented bone loss among postmenopausal women with breast cancer starting letrozole after tamoxifen.11 Longer follow-up was essential to assess whether the observed effect was durable and if changes in BMD could serve as surrogates for fracture risk. Herein, the 5-year follow up results of the current study are described.
Patients and Methods
The N03CC trial was conducted by the North Central Treatment Group (NCCTG, now part of the Alliance for Clinical Trials in Oncology) and primarily funded by the National Cancer Institute (NCI), with supplemental funding from Novartis which, otherwise, had no involvement with the conduct of the study. Approval for the study was obtained from the local Institutional Review Board from participating sites and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The design of the N03CC trial has been previously described.11 Briefly, the study population consisted of post-menopausal women with an ECOG performance status of 0-2 and a history of stage I-IIIa estrogen and/or progesterone receptor positive breast cancer that had completed ≤ 6 years of tamoxifen with no evidence of recurrent or metastatic disease. At study entry, patients were required to have T scores ≥ -2. Key exclusion criteria included: history of fracture in absence of (or low-intensity) trauma; clinical/radiologic evidence of existing lumbar spine or total hip fracture; prior treatment with endocrine therapy, including estrogen or corticosteroids within the last 12 months; any prior treatment with AI or intravenous bisphosphonates; and prior exposure to anabolic steroids or growth hormone within the last six months.
In this open-label phase III trial, patients were randomly assigned to upfront versus delayed ZA. All patients received letrozole 2.5 mg daily, vitamin D 400 international units daily, and calcium 500 mg twice daily. The determinants for receipt of ZA in the delayed group included the development of a fracture or a T-score of < -2 at the lumbar spine or femoral neck at any time during the study. Lumbar and thoracic spine x-rays were performed at the treating physician's discretion during the course of participation to confirm evidence of a clinical fracture, or at month 36 if there was no clinical evidence of fracture. ZA 4 mg intravenously (with adjustments for creatinine clearance if necessary) was administered every six months for a duration of five years or until breast cancer recurrence; dual energy x-ray absorptiometry (DEXA) scans were obtained at baseline and at 12, 24, 36, 48, and 60 months.
Statistical analysis
A complete description of the statistical analysis has been published.11 Two-sample t-tests were used to compare the average change in the lumbar spine BMD at years 2-5, as well as the average change in the femoral neck and total hip BMD at years 1-5 between the two treatment arms. A clinically significant decrease in BMD, as well as the annual incidence rates of osteoporosis, bone fractures, and toxicity between the two arms was compared via chi-square testing. The primary and secondary endpoints were the mean intra-patient average percent change (g/cm2) in total lumbar spine BMD from baseline to 12 months, and from baseline to 24, 36, 48, and 60 months after study entry, respectively. Additional secondary endpoints included development of osteoporosis (defined as standardized BMD of at least 2.5 standard deviations [SD] below peak young values at any measured site), hip BMD, incidence of fractures, and toxicity. Patients randomized to the delayed treatment arm who subsequently crossed-over to receive ZA were analyzed with the delayed treatment group. The study was designed to have 90% power to detect a 2.9% difference in the average percent change from baseline in lumbar spine BMD between the arms with a 5% Type I error rate. A 5% difference in intra-patient BMD from baseline was defined as clinically significant. Stratification factors included duration of prior tamoxifen, use of adjuvant chemotherapy and baseline BMD T-scores. The lumbar spine BMD between the upfront and delayed treatment arms was compared by a repeated measures model of yearly BMD score, adjusting for patient characteristics such as race, duration of tamoxifen, performance status score, and prior chemotherapy.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies (https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FGovernance). Results analyzed were available in our database as of August 17, 2012.
Results
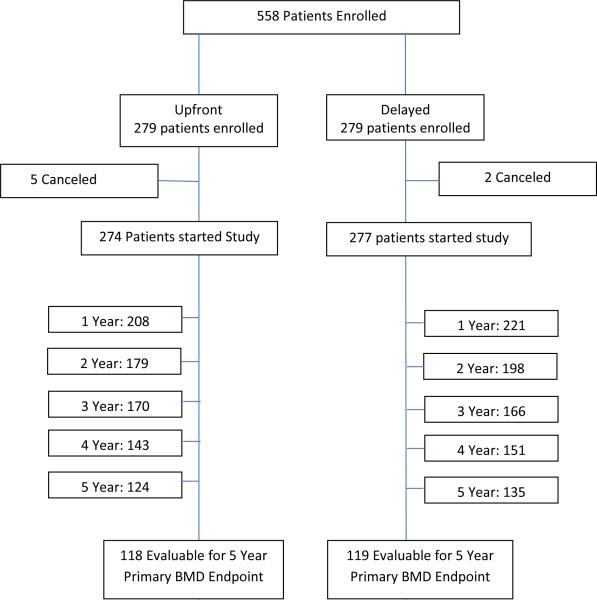
Between February 2005 and March 2006, 558 patients enrolled (Fig.1). Of these, 551 started treatment (274 on upfront ZA arm, 277 on delayed ZA arm). The number of patients evaluable for 5-year primary BMD endpoint was 237 (118 on upfront ZA arm, 119 on delayed ZA arm). Baseline patient characteristics were well balanced between arms11 (Table 1). Patients in the upfront and delayed treatment arms went off study for similar reasons, with the most common including patient refusal (n = 51 vs. 47), adverse events (n = 23 vs. 24), and disease progression (n = 13 vs. 13), respectively.
Figure 1.
CONSORT figure.
Table 1.
Baseline Patient Characteristics
| Upfront Zoledronate (N=274) | Delayed Zoledronate (N=277) | Total (N=551) | p value | |
|---|---|---|---|---|
| Age | 0.751 | |||
| N | 274 | 277 | 551 | |
| Mean (SD) | 59.2 (11.20) | 59.6 (10.25) | 59.4 (10.72) | |
| Median | 58.5 | 59.0 | 59.0 | |
| Q1, Q3 | 52.0, 68.0 | 52.0, 67.0 | 52.0, 67.0 | |
| Range | (0.0-82.0) | (0.0-83.0) | (0.0-83.0) | |
| Gender | 0.082 | |||
| female | 271 (98.9%) | 277 (100%) | 548 (99.5%) | |
| male | 3 (1.1%) | 0 (0%) | 3 (0.5%) | |
| Race | 0.502 | |||
| White | 269 (98.2%) | 268 (96.8%) | 537 (97.5%) | |
| Black or African American | 4 (1.5%) | 6 (2.2%) | 10 (1.8%) | |
| Asian | 1 (0.4%) | 0 (0%) | 1 (0.2%) | |
| American Indian or Alaska Native | 0 (0%) | 1 (0.4%) | 1 (0.2%) | |
| Not reported: patient refused or not available | 0 (0%) | 1 (0.4%) | 1 (0.2%) | |
| Unknown: Patient unsure | 0 (0%) | 1 (0.4%) | 1 (0.2%) | |
| Performance Score | 0.872 | |||
| 0 | 261 (95.3%) | 263 (94.9%) | 524 (95.1%) | |
| 1 | 13 (4.7%) | 14 (5.1%) | 27 (4.9%) | |
| Baseline BMD T Score | 0.952 | |||
| > −1 SD | 152 (55.5%) | 153 (55.2%) | 305 (55.4%) | |
| between −1 to −2 SD | 122 (44.5%) | 124 (44.8%) | 246 (44.6%) | |
| Prior Tamoxifen Duration | 0.952 | |||
| ≤ 2 years | 61 (22.3%) | 61 (22%) | 122 (22.1%) | |
| > 2 years | 213 (77.7%) | 216 (78%) | 429 (77.9%) | |
| Time Since Tamoxifen Ended | 0.982 | |||
| < 1 year | 264 (96.4%) | 267 (96.4%) | 531 (96.4%) | |
| ≥ 1 year | 10 (3.6%) | 10 (3.6%) | 20 (3.6%) | |
| Prior Chemotherapy | 0.852 | |||
| Yes | 190 (69.3%) | 190 (68.6%) | 380 (69%) | |
| No | 84 (30.7%) | 87 (31.4%) | 171 (31%) |
Kruskal Wallis
Chi-Square
Bone Mineral Density
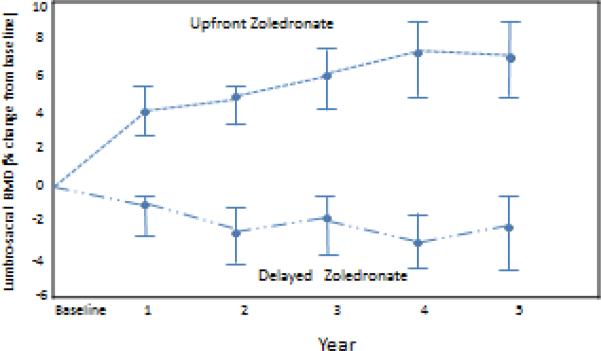
Total lumbar spine BMD data was evaluable from 429, 377, 336, 294, and 259 patients at 1, 2, 3, 4, and 5 years, respectively. Long-term follow-up of this randomized clinical trial demonstrated a gain in total lumbar spine BMD from baseline to 5 years of 0.58 (95% CI 0.45, 0.72) for the upfront ZA arm compared with a loss for the delayed arm of -0.24 (95% CI -0.36, -0.12); P < 0.001. The upfront ZA arm had statistically significantly higher values for both changes and mean percent change in lumbar spine total BMD from baseline compared to the delayed ZA arm (Fig. 2). The difference in mean percent change between arms was 5.3%, 7.34%, and 9.42% at 1, 2, and 5 years respectively. The incidence of a 3% decrease in lumbar spine BMD at 5 years was 12.7% (15/118) versus 47.9% (57/119) in the upfront and delayed treatment arms, respectively (p < 0.0001). A significant difference was maintained when a 5% decrease in lumbar spine BMD at 5 years was evaluated, with only slightly lower incidences seen (10.2% in the upfront arm versus 41.2% in the delayed arm, p < 0.0001). Additionally, comparing a 10% decrease in lumbar spine BMD at 5 years demonstrated significant differences between the arms (5.1% in upfront arm versus 16.8% in delayed arm, p<0.01). The mixed model of total lumbar spine score demonstrated that patients in the upfront zoledronic acid arm tended to have higher lumbar spine scores (p< 0.0001). Furthermore, race, duration of tamoxifen, performance status score, and prior chemotherapy significantly impacted the lumbar spine BMD (Table 2).
Figure 2.
Total Lumbar Spine BMD Percent Change. BMD, bone mineral density
Table 2.
Mixed Modeling of Total Lumbar Spine BMD
| Factors | Parameter Estimate | P value |
|---|---|---|
| Arm (Upfront vs. Delayed Zoledronic Acid) | 0.0394 | <.0001 |
| Age | 0.0004 | 0.2668 |
| Race (White vs. Other) | −0.1170 | <.0001 |
| Performance Status (0 vs. 1) | −0.0555 | 0.0010 |
| Prior Tamoxifen Duration (≤ 2 vs. >2 years) | 0.0316 | 0.0003 |
| Prior Chemotherapy (Yes vs. No) | −0.0235 | 0.0057 |
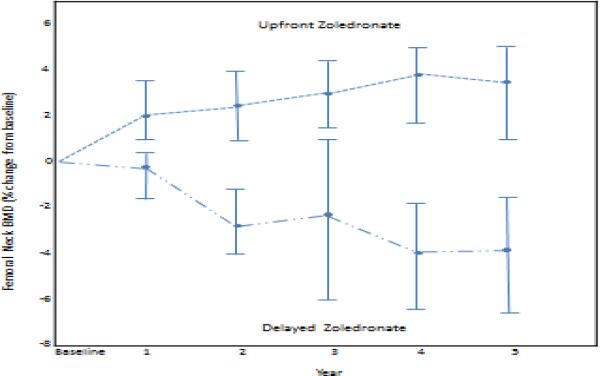
The mean change and mean percent change in the femoral neck BMD from baseline were significantly higher in the upfront ZA arm (Fig 3). Similar findings were encountered with the hip scores across all of these parameters. The incidence of a 5% decrease in femoral neck and total hip BMD at year 5 was 7.6% in the upfront ZA arm compared with 45.8% in the delayed arm (p < 0.0001).
Figure 3.
Femoral Neck BMD Percent Change. BMD, bone mineral density
At the end of one year, there were 221 patients in the delayed treatment arm, out of which 41 (18.6%) crossed over to treatment with ZA; this involved 10, 16, 8, 7, and 0 patients in the first through fifth years, respectively.
Osteoporosis/Fractures
Two patients developed osteoporosis (1 each at years 2 and 3) in the upfront ZA arm compared with 8 patients (3 in year 1, 2 in year 2, 1 in year 3, and 2 in year 4) in the delayed treatment arm. These differences in the incidence of osteoporosis between arms, however, were not statistically significant (P = 0.072). The number of bone fractures between arms was nearly the same, 24 for upfront zoledronic acid versus 25 for delayed zoledronic acid (P = 0.84).
Adverse Events
Adverse events occurring in > 10% of patients treated on either arm included: arthralgias, back pain, hot flashes, myalgias, nausea and vomiting, with no significant difference between arms noted. The incidence of fever (9% vs. 3%) and elevated creatinine (9% vs. 5%) was higher in the upfront treatment arm with p values of 0.004 and 0.041, respectively. The increased creatinine was grade 1/2 in 96% of cases in the upfront arm, with one case of grade 4. The incidence of grade 3/4 adverse events occurring at any time point from baseline through five years of follow-up was 24% (n = 68) in the delayed arm compared with 28% (n = 76) in the upfront arm. Three cases of life-threatening cerebrovascular ischemia occurred in the delayed ZA arm. Three patients in the upfront arm experienced grade 4 toxicities requiring hospitalization and were considered by the treating physician to be possibly related to treatment, including myocardial ischemia, pain, and decreased serum calcium. Osteonecrosis of the jaw occurred in 4 (2%) patients in the upfront arm and 2 (1%) patients in the delayed arm, after crossover (p = 0.40).
Breast Cancer Progression
Progression events occurred in 13 patients (4.7%) on each arm. No deaths occurred on either arm. There are insufficient data to examine the treatment impact on overall or progression-free survival.
Discussion
N03CC and the Zometa-Femara Adjuvant Synergy sister trials (Z-FAST, ZO-FAST, and E-ZO-FAST) were similarly designed to address the question as to whether early versus delayed initiation of bone-conserving therapy impacted BMD in post-menopausal women undergoing treatment with an AI.9, 12, 13 The absolute difference in lumbar spine BMD between arms in the current study is similar to those of the Z-FAST companion trials. At 5 years, the absolute differences in lumbar spine BMD were 8.9% and 10% for the Z-FAST and ZO-FAST trials, respectively, compared with 9.42% in the current study. Within the truly menopausal group of the ZO-FAST trial, the absolute difference was 9.5%. At 12 months of follow-up of the E-ZO-FAST study, the absolute difference in lumbar spine BMD was 5.43%, compared with 5.3% in the current study. In contrast to N03CC, which included treatment with tamoxifen prior to AI, patients in the Z-FAST trials received only AIs. Because tamoxifen mitigates bone loss in post-menopausal women, concern may be expressed that the current trial findings do not fully reflect the potential protective effects of ZA for individuals treated with AIs alone. However, the similar findings encountered in this study and the Z-FAST trials suggest that the bone loss associated with AIs is independent of preceding tamoxifen administration.
The lack of correlation between bone loss and the incidence of fracture seen in this study and other prevention trials9, 12 poses the question as to the definition of meaningful bone loss, particularly in a younger patient population whose baseline fracture risk is not high. A decline of 10-12% (one standard deviation) in BMD approximately doubles the risk of fracture.14 Given this association, this study defined a 5% difference in intra-patient BMD from baseline as clinically significant. At 5 years of follow-up, the incidence of a 5% decrease in lumbar spine BMD between arms was highly significant (41.2% in the delayed arm versus 10.2% in the upfront arm), yet no difference in the incidence of osteoporosis or fractures was demonstrated. As errors between any two BMD measurements of 2-6% have been described15, the Z-fast trial selected an 8% or greater decrease in BMD as being clinically significant.10 Although the Z-fast trial was not designed or adequately powered to detect a difference in fracture rates between groups, the slight increase in fractures demonstrated in the delayed versus upfront group at month 61 (33 versus 28) was not statistically significant.9 Whether a selected higher threshold for BMD loss may have corresponded to fracture risk in these studies is speculative, though one can conclude from the original adjuvant AI studies2, 4, 16 that a subset of patients may have benefited from bisphosphonates.
The MA-17 trial, which treated post-menopausal patients with 5 years of tamoxifen followed by letrozole versus placebo, did not detect a statistically significant difference in the clinical fracture rate between the arms (5.3% versus 4.6%, P = 0.25). However, the risk of fracture with letrozole compared with tamoxifen was increased in the BIG 1-98 trial (5.7% versus 4.0%, P < 0.0001) as well as in the ATAC trial (5.9% versus 3.7%, P < 0.0001).2, 4, 16 Notably, fracture rates decreased upon cessation of the AI in the ATAC trial.17 The incidence rate ratio (IRR) was 1.55 during active treatment and declined to 1.03 following treatment completion. These findings highlight the temporary and reversible impact of AIs on bone loss.
Because the incidence of osteoporosis and fracture is not different between the arms, these findings do not strongly support routine upfront administration of ZA. A meta-analysis of 7 studies including 7,967 patients demonstrated a fracture benefit with ZA (OR, 0.78; 95% CI, 0.63-0.96; however, subgroup analyses according to menopausal status were not performed and it is unclear whether a benefit would be seen in younger women.18 Although upfront use remains controversial from the standpoint of osteoporosis and fracture risk, the decision to start ZA in postmenopausal women receiving treatment with AIs may be based on a potential survival benefit.12, 18-20 A recently presented Oxford meta-analysis21 has provided more definitive evidence regarding the potential benefit of bisphosphonates such as zoledronate for improving outcomes in postmenopausal women with resected breast cancer. This new report influences clinical decisions regarding the use of zoledronate in clinical practice for postmenopausal women. The current study is limited in that it was not powered to adequately address survival differences, yet it provides insight into the feasibility of delaying treatment with zoledronic acid.
Acknowledgments
This study was conducted as a collaborative trial of the Alliance/North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35431, CA-35415, CA-35103, and CA-35269. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601).
Footnotes
Additional participating institutions include: Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith M. Rowland, Jr, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.); St. Vincent Regional Cancer Center CCOP, Green Bay, WI 54303 (Anthony J. Jaslowski, M.D.); Hematology & Oncology of Dayton, Inc, Dayton, OH 45415 (Howard M. Gross, M.D.); Sanford Cancer Center Oncology Clinic, Sioux Falls, SD 57105 (Miroslaw Mazurczak, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Christian S. Adonizio, M.D.) Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014 (Robert J. Behrens, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Lehigh Valley Hospital, Allentown, PA 18103 (Suresh Nair, M.D.); Upstate Carolina CCOP, Spartanburg, SC 29303 (James D. Bearden, III, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Rex B. Mowat, M.D.); Heartland Cancer Research CCOP, St. Louis, MO 63131 (Alan P. Lyss, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald J. Jurgens, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Mayo Clinic Arizona, Scottsdale, AZ 85259-5404 (William Wong, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 80224 (Deborah Weil Wilbur, M.D.); Colorado Cancer Research Program, Denver, CO 80224 (Keren Sturtz, M.D.); Essentia Health Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (David M. Anderson, M.D.); Illinois Oncology Research Assn. CCOP, Peoria, IL 61615-7828 (Nguyet Anh Le-Lindqwister, M.D.); Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald B. Wender, M.D.); Sanford Roger Maris Cancer Center, Fargo, ND 58122 (Preston D. Steen, M.D.)
Financial Disclosures:
NWJ has no relevant conflicts to disclose.
JAS has no relevant conflicts to disclose.
HL has no relevant conflicts to disclose.
AEK has no relevant conflicts to disclose.
SLH has no relevant conflicts to disclose.
SP has no relevant conflicts to disclose.
SRD has no relevant conflicts to disclose.
JML has no relevant conflicts to disclose.
EAP has no relevant conflicts to disclose.
CLL has no disclosures other than Novartis funding provided to Mayo for this trial.
References
- 1.Network NCC. [December 30, 2013];The NCCN Clinical Practice Guidelines in Oncology Bresat Cancer (Version1.2014) Available at: www.nccn.org.
- 2.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. Journal of the National Cancer Institute. 2005;97(17):1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 3.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 4.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. The New England journal of medicine. 2005;353(26):2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 5.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(5):486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. The New England journal of medicine. 2004;350(11):1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. The New England journal of medicine. 2003;349(19):1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(22):3629–35. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 9.Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 10.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(7):829–36. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 11.Hines SL, Mincey B, Dentchev T, et al. Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast cancer research and treatment. 2009;117(3):603–9. doi: 10.1007/s10549-009-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 13.Llombart A, Frassoldati A, Paija O, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-month analysis of the E-ZO-FAST trial. Clinical breast cancer. 2012;12(1):40–8. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnick SL, Johnston CC, Jr., Kleerekoper M, et al. Importance of precision in bone density measurements. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2001;4(2):105–10. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- 16.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 17.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. The lancet oncology. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 18.Valachis A, Polyzos NP, Coleman RE, et al. Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. The oncologist. 2013;18(4):353–61. doi: 10.1634/theoncologist.2012-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. The New England journal of medicine. 2011;365(15):1396–405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 20.Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. The lancet oncology. 2011;12(7):631–41. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 21.Coleman R, Gnant M, Paterson A, et al. Effects of bisphophonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomized trials. Cancer Res. 2013;73(24 suppl) Abstr S4-07. [Google Scholar]